水热法制备NiS/Cd1-xZnxS及其高效光催化产氢性能
2015-01-04林彩芳陈小平上官文峰上海交通大学燃烧与环境技术中心上海200240
林彩芳 陈小平 陈 澍 上官文峰(上海交通大学燃烧与环境技术中心,上海200240)
水热法制备NiS/Cd1-xZnxS及其高效光催化产氢性能
林彩芳 陈小平 陈 澍 上官文峰*
(上海交通大学燃烧与环境技术中心,上海200240)
利用水热法制备NiS负载的Cd1-xZnxS光催化剂.结果表明:在0.35 mol·L-1Na2SO3和0.25 mol·L-1Na2S牺牲剂下,0.5%(摩尔分数,y)NiS/Cd0.3Zn0.7S(1840 μmol·h-1)获得最好活性,是Cd0.3Zn0.7S(884 μmol·h-1)的2.1倍,高于0.5%(质量分数,w)Pt(1390 μmol·h-1)的产氢活性.测得其在λ=420 nm附近的表观量子效率为36.8%.X射线衍射(XRD)、紫外-可见漫反射光谱(UV-Vis DRS)、透射电子显微镜(TEM)以及X射线光电子能谱(XPS)的表征结果表明,NiS作为产氢活性位,转移光生电子,因此提高了光催化产氢活性.
Cd1-xZnxS;NiS;水热法;光催化活性;产氢
1 Introduction
As the increasing global energy and environmental problems, hydrogen energy as the future energy carrier has attracted great attention.1-3Compared with traditional hydrogen production methods,4photocatalytic hydrogen production from water splitting using semiconductors is more clean and economic.5-7Since the first report about photocatalytic hydrogen production using titanium dioxide by Fujishima and Honda in 1972,8extensive works have been devoted to the research of photocatalytic hydrogen production from water over semiconductor.6,9-11Especially,the research under visible light has been paid more attention due to its various advantages.9-11
Among the visible-light-sensitive photocatalysts reported,CdS-based photocatalysts have been extensively investigated,owing to its relatively narrow band gap with good visible light absorption properties and more negative conduction band edge than theH/H2reduction potential. However,CdS alone exhibits low activities and inherent photocorrosion,which limits its application.16,17Numerous efforts have been made to solve these problems,such as modifying CdS with cocatalysts,18-20fabricating CdS/ oxide heterostructures,21,22incorporating CdS particles into an interlayer,23doping CdS with another wide band gap semiconductor.24,25Recently,the photocatalytic properties of CdS mixed with ZnS,a kind of wide band gap semiconductor,to form Cd1-xZnxS solid solution have been paid a great deal of attention, owning to the significantly improved photocatalytic activity and reduced usage of toxic Cd.26,27Further,the band position and light absorption of a certain Cd1-xZnxS solid solution can be controlled by adjusting the value of x,28which consequently enhances H2production activity.Besides,the x value of Cd1-xZnxS with the highest H2production activity can be affected by the synthesis method.It has been reported that the certain Cd1-xZnxS solid solution exhibits the optimal H2production activity,when x was 0.9,290.65,300.6,310.5,320.4,33respectively.Further,the recombination of photogenerated electron-hole pairs of Cd1-xZnxS is still inevitable,and always plays the main role in a photocatalytic reaction. Loading cocatalysts can promote separating and transferring the photogenerated carriers,thus notably improving the photocatalytic activity.34,35However,in most work reported so far,the employed cocatalysts are usually noble metals or their oxides.36,37Practical applications of noble metals in large scale are greatly limited by their high cost.Since the first report of MoS2loaded CdS for effective photocatalytic H2evolution,18great efforts have been undertaken to develop noble metal free cocatalysts.19,38,39Among them,it has been reported that Ni,either in the form of a metallic or oxidized state,is served as a cocatalyst enhancing photocatalytic H2evolution.20,39,40Recently,NiS loaded on Cd1-xZnxS by insitu synthesis method was reported by Wang31and Li et al.,40respectively.
©Editorial office ofActa Physico-Chimica Sinica
In this paper,we report a simple synthesis of Cd1-xZnxS solid solution photocatalysts by a hydrothermal method.Then,NiS was loaded on Cd1-xZnxS by a hydrothermal method for further improving its photocatalytic hydrogen evolution activity.And the possible mechanism for the enhanced photocatalytic activity is also discussed.
2 Experimental
2.1 Preparation of Cd1-xZnxS solid solutions
All of the reagents used were of analytical purity and used without further purification.Cd1-xZnxS solid solutions were prepared by a co-precipitation and hydrothermal method.In a typical synthesis,appropriate amounts of Cd(CH3COO)2·2H2O(≥98.5%, Sinopharm Chemical Reagent Co.,Ltd.)and Zn(CH3COO)2·2H2O (≥99.0%,Sinopharm Chemical Reagent Co.,Ltd.)were dissolved in 50 mL deionized water in a 100 mL stainless Teflon-lined autoclave.After magnetic stirring for 30 min,a certain amount of Na2S·9H2O(≥98.0%,Sinopharm Chemical Reagent Co.,Ltd.) was added.Then the autoclave was sealed and heated at 200°C for 12 h,cooled down to room temperature naturally.The resulting precipitates were separated by centrifugation and washed using deionized water several times,followed by drying at 80°C for 10 h in an oven.
2.2 Preparation of NiS modified Cd0.3Zn0.7S
Typically,the as-prepared Cd0.3Zn0.7S was dispersed in 30 mL deionized water in a 100 mL stainless Teflon-lined autoclave,followed by a drop-wise addition of a certain volume of Ni(CH3COO)2(0.05 mol·L-1,≥98.0%,Sinopharm Chemical Reagent Co.,Ltd.).After magnetic stirring for 30 min,a certain amount of Na2S·9H2O was added.Then the autoclave was sealed and heated at 180°C for 5 h.After cooling,the product was separated by centrifugation,washed with deionized water several times and dried at 80°C for 10 h in an oven.
2.3 Characterization
X-ray diffraction(XRD)patterns were confirmed by X-ray diffraction(Rigaku D/max-2200/PC Japan)with Cu Kαradiation (40 kV,20 mA).The UV-Vis diffuse reflection spectra(DRS) were measured on a UV-Vis spectrophotometer UV-2450(Shimadzu,Japan)and were converted to absorbance by the Kubelka-Munk method.The transmission electron microscopy(TEM)and high-resolution transmission electron microscopy(HRTEM) images were taken on a JEM-2100F(Japan).The surface area of the prepared samples were analyzed by BET measurement (Quanta Chrome NOVA1000,USA).The surface electronic state was analyzed by X-ray photoelectron spectroscopy(XPS,Shimadzu-Kratos,Axis Ultra DLD,Japan).All the binding energy (EB)values were referenced to the standard EBvalue of contaminant carbon(EB(C 1s)=284.6 eV).The amounts of metal elements were determined by inductively coupled plasma analyzer(ICAP 6000 Radial,Thermo).
2.4 Photocatalytic H2production activities test
Photocatalytic reactions were conducted in a Pyrex reaction cell connected to a closed gas circulation.0.1 g as-prepared photocatalysts were suspended in 100 mL of aqueous solution containing 0.35 mol·L-1Na2SO3(≥97.0%,Sinopharm Chemical Reagent Co.,Ltd.)and 0.25 mol·L-1Na2S.The suspension was then thoroughly degassed and irradiated by a Xe lamp(300 W) equippedwithachemical liquidfilter(λ>400nm,containing1mol· L-1NaNO2).Pt as a cocatalyst was photodeposited on the photocatalyst from the precursor of H2PtCl6·6H2O.The reaction cell was connected to a vacuum system,and the rate of H2generation was analyzed using an online gas chromatography.The apparent quantum efficiency(QE)was measured under the photocatalytic reaction condition with irradiation light at 420 nm by using combined band-pass(Kenko)and cut-off filters(HOYA)and 500 W Xe lamp.The QE was calculated as following equations:

3 Results and discussion
3.1 Cd1-xZnxS solid solution
The various Cd1-xZnxS solid solutions were synthesized by a simple hydrothermal method as described in the experimental section.The successive transitions of XRD patterns and UV-Vis spectra were shown in Fig.1 and Fig.2 respectively,which can confirm the feature of the Cd1-xZnxS solid solution.From Fig.1 it can be found that there were three predominant peaks of Cd1-xZnxS,and the diffraction peaks of the photocatalysts shifted to a higher-angle side from the hexagonal CdS(JCPDF No.411049)to the cubic ZnS(JCPDF No.050566)as the value of x increased.The successive shifts of the XRD patterns indicate that the crystals obtained were not a simple mixture of ZnS and CdS,but Cd1-xZnxS solid solution,in well agreement with the literature.28,32,40
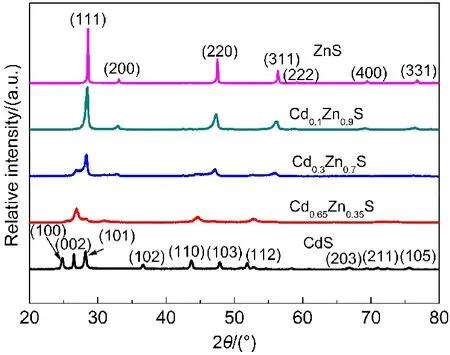
Fig.1 XRD patterns of Cd1−xZnxS solid solutions with different x values
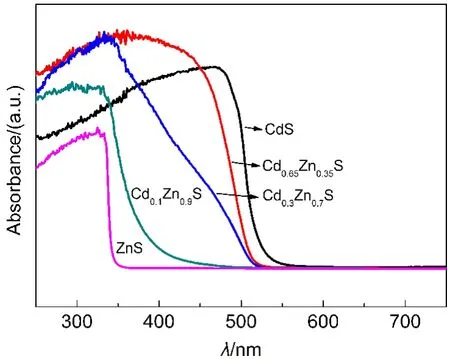
Fig.2 UV-Vis spectra of Cd1−xZnxS with different x values
As shown in Fig.2,the absorption edge of each solid solution was gradually red shifted as the amount of zinc decreased and distributed between CdS and ZnS.Surprisingly,the absorption edge of Cd0.3Zn0.7S sample was not so smooth,which indicated that Cd1-xZnxS samples prepared by this method were not quite homogeneous solid solutions between ZnS and CdS.And the similar absorption features of Cd1-xZnxS samples synthesized can also be found in literature.30,31
The photocatalytic H2production activities of the various Cd1-xZnxS solid solutions in an aqueous solution containing 0.25 mol·L Na2S and 0.35 mol·L Na2SO3under visible light irradiation(λ>400 nm)were compared in Fig.3.It could be found from Fig.3 that all of the solid solutions had much better activities of H2evolution than pure CdS.And the Cd0.3Zn0.7S exhibited the optimal H2production activity(885.4 μmol·h-1),three times of the one of pure CdS under visible light irradiation.It was known that the band gap would continuously increase as the amount of Zn ions increased in Cd1-xZnxS solid solutions.The increase of band gap would lead to the decrease in visible light absorption,which was undesired for photocatalytic reactions under visible light. However,the increase of band gap contributed to a higher conduction band,beneficial to the enhancement of the reduction ability for H+to be reduced to H2.Therefore,the optimal hydrogen evolution activity of Cd0.3Zn0.7S might attribute to the delicate balance between an appropriate band gap width and a suitable conduction band edge position.41

Fig.3 Photocatalytic H2production activities of Cd1−xZnxS solid solution in an aqueous solution containing 0.25 mol·L-1Na2S and 0.35 mol·L-1Na2SO3under visible light irradiation(λ>400 nm)

Fig.4 UV-Vis spectra of as prepared Cd0.3Zn0.7S and NiS/Cd0.3Zn0.7S photocatalysts
3.2 NiS modified Cd0.3Zn0.7S
3.2.1 Characterization of NiS modified Cd0.3Zn0.7S
UV-Vis diffuse reflectance spectra of Cd0.3Zn0.7S solid solutions with different NiS contents were shown in Fig.4.There was no obvious shift in the absorption edge of the NiS/Cd0.3Zn0.7S samples in comparison to naked Cd0.3Zn0.7S,implying that NiS was just deposited on the surface instead of being incorporated into thelattice of Cd0.3Zn0.7S.
A series of the NiS modified Cd0.3Zn0.7S samples were studied by XRD to identify the crystal structure,as shown in Fig.5.It is worth noting that characteristic peaks of NiS can not be observed in these XRD patterns,which may be due to the low concentration and the high dispersion of NiS particles on the surface of Cd0.3Zn0.7S.Besides,the intensity and the peak position of the XRD diffraction peaks of all as-prepared samples changes little, indicating that NiS surface modification has no clear influence on the crystal structure.No lattice deformation is obtained from the XRD data,suggesting that NiS particles are highly dispersed on the surface but not doped into the lattice of Cd0.3Zn0.7S.
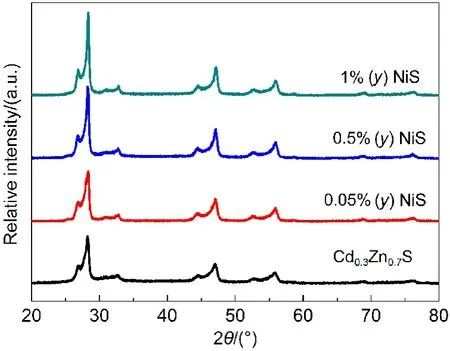
Fig.5 XRD patterns of as prepared Cd0.3Zn0.7S and NiS/Cd0.3Zn0.7S photocatalysts
As shown in Table 1,the BET surface area and pore volume change a little with loading the NiS,which may be attributed to the formation of NiS depositing on the surface of Cd0.3Zn0.7S.The atomic ratio of Zn/Cd obtained from ICP was also displayed, which suggested that the chemical compositions measured were very close to theoretical values.However,the loaded NiS content measured by ICP was relatively lower than theoretical values.It may attribute to the loss of NiS during the loading process,and NiS was not fully loaded on the surface of Cd0.3Zn0.7S.

Table 1 Nickel contents,BET surface area,and pore structure of samples
TEM and HRTEM images of the prepared NiS/Cd0.3Zn0.7S were presented in Fig.6.As shown in Fig.6,the sample was composed of numerous nanoparticles of about 30-50 nm in diameter and the HRTEM image clearly showed lattice fringes suggesting a welldefined crystal structure.It can be seen from these images that a layer of NiS was deposited on the surface of Cd0.3Zn0.7S.The HRTEM image shows fringes with lattice spacing of 0.200 and 0.320 nm,corresponding to the(111)plane of NiS and the(111) plane of Cd0.3Zn0.7S,respectively.A more detailed observation indicates that an intimate contact between NiS and Cd0.3Zn0.7S lattices was formed during the hydrothermal loading process of NiS in this work,and thus enhancing the charge separation and photocatalytic activity.18
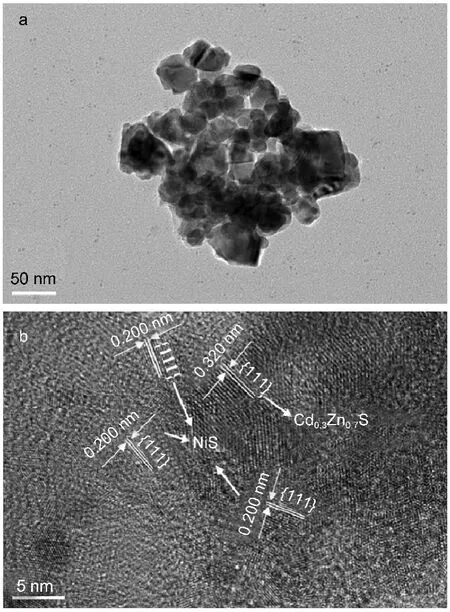
Fig.6 Images of NiS/Cd0.3Zn0.7S sample

Fig.7 (a)XPS survey spectrum and(b)high-resolution XPS spectrum of Ni 2p
XPS survey spectra of NiS/Cd0.3Zn0.7S sample was shown in Fig.7.The existences of Cd,S,Ni,and O elements were shown in Fig.7a.From Fig.7b,the observed binding energy of Ni 2p3/2at 856.8 eV indicated the existence of NiS,31which was consistent with TEM results.
3.2.2 Photocatalytic performance of NiS modified Cd0.3Zn0.7S
The influence of the amount of NiS on the H2evolution rate over NiS/Cd0.3Zn0.7S samples was shown in Fig.8.The H2evolution rate of Cd0.3Zn0.7S was 884 μmol·h-1.After a small amount of NiS loaded on the surface of Cd0.3Zn0.7(the mole ratio of Ni2+/ (Cd2++Zn2+)was 0.05%),the H2evolution rate increased to 1243 μmol·h-1.Further increasing the amount of NiS(the mole ratio of Ni2+/(Cd2++Zn2+)was 0.5%)led to the highest H2evolution rate of 1840 μmol·h-1with a quantum efficiency of 36.8%at 420 nm when 0.35 mol·L-1Na2SO3and 0.25 mol·L-1Na2S as sacrifice agent,even higher than the one of Cd0.3Zn0.7S loaded with 0.5% (w)Pt(1390 μmol·h-1).Further increasing the content of NiS,the activity decreased,probably because of shielding the incident light by covering the surface of Cd0.3Zn0.7S.

Fig.8 Photocatalytic hydrogen production activity of samples under visible light irradiation
Based on above discussions,a proposed role of NiS for enhancing the photocatalytic H2evolution activity is inferred.As shown in Fig.9,photogenerated charges are produced when the Cd0.3Zn0.7S was irradiated under visible light.The holes are consumed by the S/SO3during the photocatalytic process and the photogenerated electrons in the conduction band of Cd0.3Zn0.7S are easily transferred to the surface of NiS due to the intimate contact between the two phases,respectively.The p-type NiS intimately loaded on the surface of n-type Cd0.3Zn0.7S could form a large number of p-n junctions,which could effectively reduce the recombination rate of photogenerated electrons and holes,thus greatly enhancing the photocatalytic activity.Besides,it was suggested that the H+could be easily adsorbed on the surface of NiS and the NiS cocatalyst was as the active site for H2evolution,42which was beneficial to transferring electrons quickly,hence leading to the enhancement of photocatalytic activity.

Fig.9 Proposed role of NiS for enhancing the photocatalytic H2evolution activity
4 Conclusions
NiS surface modified Cd0.3Zn0.7S photocatalysts can be easily prepared by a simple hydrothermal method.The highest H2production rate reaches 1840 μmol·h-1,when Cd0.3Zn0.7S loaded with 0.5%(y)NiS and Na2SO3and Na2S as sacrificial agent,even higher than the one of Cd0.3Zn0.7S modified with 0.5%(w)Pt.The NiS cocatalyst is as active sites for H2evolution,which is more beneficial to transfer electron quickly,thus leading to the enhancement of photocatalytic activity.This work suggests the possibility to use the low cost NiS as a substitute for noble metals in photocatalytic hydrogen evolution.
(1) Andrews,J.;Shabani,B.Int.J.Hydrog.Energy2012,37, 1184.doi:10.1016/j.ijhydene.2011.09.137
(2) Lewis,N.S.Science2007,315,798.doi:10.1126/ science.1137014
(3) Cortright,R.D.;Davda,R.R.;Dumesic,J.A.Nature2002,418,964.doi:10.1038/nature01009
(4) Turner,J.A.Science2004,305,972.doi:10.1126/ science.1103197
(5) Maeda,K.;Teramura,K.;Lu,D.;Takata,T.;Saito,N.;Inoue, Y.;Domen,K.Nature2006,440,295.doi:10.1038/440295a
(6) Kudo,Y.;Miseki,Y.Chem.Soc.Rev.2009,38,253.doi: 10.1039/b800489g
(7) Chen,X.B.;Shen,S.H.;Guo,L.J.;Mao,S.S.Chem.Rev.2010,110,6503.doi:10.1021/cr1001645
(8) Fujishima,A.;Honda,K.Nature1972,238,37.doi:10.1038/ 238037a0
(9) Navarro,Y.R.;Álvarez,G.M.;Del,V.F.;José,A.V.M.;José, L.G.F.ChemSusChem2009,2,471.doi:10.1002/cssc.v2:6
(10) Zhang,K.;Guo,L.Catal.Sci.Technol.2013,3,1672.doi: 10.1039/c3cy00018d
(11) Dong,L.L.;Wang,Y.Y.;Tong,X.L.;Jin,G.Q.;Guo,X.Y.Acta Phys.-Chim.Sin.2014,30,135.[董莉莉,王英勇,童希立,靳国强,郭向云.物理化学学报,2014,30,135.]doi: 10.3866/PKU.WHXB201311052
(12) Buehler,N.;Meier,K.;Reber,J.F.J.Phys.Chem.1984,88, 3261.doi:10.1021/j150659a025
(13) Bao,N.;Shen,L.;Takata,T.;Domen,K.Chem.Mat.2008,20, 110.doi:10.1021/cm7029344
(14) Li,Y.X.;Hu,Y.F.;Peng,S.Q.;Lu,G.X.;Li,S.B.J.Phys. Chem.C2009,113,9352.
(15) Chen,X.P.;Shangguan,W.F.Front.Energy2013,7,111.doi: 10.1007/s11708-012-0228-4
(16) Meissner,D.;Memming,R.;Kastening,B.J.Phys.Chem.1988,92,3476.doi:10.1021/j100323a032
(17) Acharya,K.P.;Khnayzer,R.S.;O'Connor,T.;Diederich,G.; Kirsanova,M.;Klinkova,A.;Roth,D.;Kinder,E.;Imboden, M.;Zamkov,M.Nano Lett.2011,11,2919.
(18)Zong,X.;Yan,H.J.;Wu,G.B.;Ma,G.J.;Wen,F.Y.;Wang,L.; Li,C.J.Am.Chem.Soc.2008,130,7176.doi:10.1021/ ja8007825
(19) Lin,P.B.;Yang,Y.;Chen,W.;Gao,H.Y.;Chen,X.P.;Yuan,J.; Shangguan,W.F.Acta Phys.-Chim.Sin.2013,29(6),1313. [林培宾,杨 俞,陈 威,高寒阳,陈小平,袁 坚,上官文峰.物理化学学报,2013,29(6),1313.]doi:10.3866/PKU. WHXB201303141
(20) Chen,X.P.;Chen,W.;Lin,P.B.;Yang,Y.;Gao,H.Y.;Yuan,J.; Shangguan,W.F.Catal.Commun.2013,36,104.doi:10.1016/j. catcom.2013.03.016
(21) Wang,X.W.;Liu,G.;Chen,Z.G.;Li,F.;Wang,L.Z.;Lu,G. Q.;Cheng,H.M.Chem.Commun.2009,3452.
(22) Li,W.;Xie,S.L.;Li,M.Y.;Ouyang,X.W.;Cui,G.F.;Lu,X. H.;Tong,Y.X.J.Mater.Chem.A2013,1,4190.doi:10.1039/ c3ta10394c
(23) Shangguan,W.F.;Yoshida,A.J.Phys.Chem.B2002,106, 12227.doi:10.1021/jp0212500
(24) Jang,J.S.;Kim,H.G.;Joshi,U.A.;Janga,J.W.;Lee,J.S.Int. J.Hydrog.Energy2008,33,5975.doi:10.1016/j. ijhydene.2008.07.105
(25) Wu,G.;Tian,M.;Chen,A.J.Photochem.Photobiol.2012,233, 65.doi:10.1016/j.jphotochem.2012.02.021
(26) Villoria,J.A.;Navarro,Y.R.;Al-Zahrani,S.M.;Fierro,J.L.G.Ind.Eng.Chem.Res.2010,49,6854.doi:10.1021/ie901718r
(27) Shi,J.Y.;Cui,H.N.;Liang,Z.X.;Lu,X.H.;Tong,Y.X.;Su, C.Y.;Liu,H.Energy Environ.Sci.2011,4,466.doi:10.1039/ c0ee00309c
(28) Xing,C.;Zhang,Y.;Yan,W.;Guo,L.Int.J.Hydrog.Energy2006,31,2018.doi:10.1016/j.ijhydene.2006.02.003
(29) Zhang,X.H.;Jing,D.W.;Liu,M.C.;Guo,L.J.Catal. Commun.2008,9,1720.doi:10.1016/j.catcom.2008.01.032
(30) Zhang,W.;Xu,R.Int.J.Hydrog.Energy2009,34,8495.doi: 10.1016/j.ijhydene.2009.08.041
(31) Wang,J.;Li,B.;Chen,J.Z.;Li,N.;Zheng,J.F.;Zhao,J.H.; Zhua,Z.P.Appl.Surf.Sci.2012,259,118.
(32) Li,Q.;Meng,H.;Zhou,P.;Zheng,Y.Q.;Wang,J.;Yu,J.G.; Gong,J.R.ACS Catal.2013,3,882.doi:10.1021/cs4000975
(33) Yu,Y.G.;Chen,G.;Hao,L.X.Chem.Commun.2013,49, 10142.doi:10.1039/c3cc45568h
(34) Yan,H.J.;Yang,J.H.;Ma,G.J.;Wu,G.P.;Zong,X.;Lei,Z. B.;Shi,J.Y.;Li,C.J.Catal.2009,266,165.doi:10.1016/j. jcat.2009.06.024
(35) Ran,J.R.;Zhang,J.;Yu,J.G.;Jaronie,M.;Zhang,S.Chem. Soc.Rev.2014,doi:10.1039/c3cs60425j
(36) Maeda,K.;Teramura,K.;Lu,D.;Saito,N.;Inoue,Y.;Domen, K.Angew.Chem.Int.Edit.2006,45,7806.
(37) Wang,Y.B.;Wang,Y.S.;Xu,R.J.Phys.Chem.C2013,117, 783.
(38) Ran,J.R.;Yu,J.G.;Jaroniec,M.Green Chem.2011,13, 2708.doi:10.1039/c1gc15465f
(39) Zhang,W.;Wang,Y.B.;Wang,Z.;Zhong,Z.Y.;Xu,R.Chem. Commun.2010,46,7631.doi:10.1039/c0cc01562h
(40) Li,N.X.;Zhou,B.Y.;Guo,P.H.;Zhou,J.C.;Jing,D.W.Int.J. Hydrog.Energy2013,38,11268.doi:10.1016/j. ijhydene.2013.06.067
(41) Valle,F.D.;Ishikawa,A.;Domen,K.;Villoria,J.A.;Mano,D. L.;Sánchez-Sánchez,M.C.;González,I.D.;Herreras,S.; Mota,N.;Rivas,M.E.;Álvarez,G.M.C.;Fierro,J.L.G.; Navarro,R.M.Catal.Today2009,143,51.doi:10.1016/j. cattod.2008.09.024
(42) Assuncao,N.A.;Giz,M.J.;Tremiliosi,G.;Gonzalez,E.R.J.Electrochem.Soc.1997,144,2794.doi:10.1149/1.1837897
Preparation of NiS-Modified Cd1-xZnxS by a Hydrothermal Method and Its Use for the Efficient Photocatalytic H2Evolution
LIN Cai-Fang CHEN Xiao-Ping CHEN Shu SHANGGUAN Wen-Feng*
(Research Center for Combustion and Environment Technology,Shanghai Jiao Tong University,Shanghai 200240,P.R.China)
NiS-modified Cd1-xZnxS has been prepared using a simple hydrothermal method.Notably,the H2evolution rate of 0.5%(y,molar fraction)NiS/Cd0.3Zn0.7S(1840 μmol·h-1)was found to be 2.1-and 1.3-fold greater than those of Cd0.3Zn0.7S(884 μmol·h-1)and 0.5%(w,mass fraction)Pt(1390 μmol·h-1),respectively,when 0.35 mol·L-1Na2SO3and 0.25 mol·L-1Na2S were used as sacrificial agents.The apparent quantumefficiency of 0.5% (y)NiS/Cd0.3Zn0.7S at 420 nm was 36.8%.The characterization of this material by X-ray diffraction(XRD), ultraviolet-visible diffuse reflection spectroscopy(UV-Vis DRS),transmission electron microscopy(TEM),and X-ray photoelectron spectroscopy(XPS)showed that the NiS particles provided the active sites required for H2evolution and transferring the photo generated electrons,and therefore enhanced the photocatalytic activity of the catalyst towards H2production.
Cd1-xZnxS;NiS;Hydrothermal method;Photocatalytic activity;Hydrogen production
O643
10.3866/PKU.WHXB201411175www.whxb.pku.edu.cn
Received:October 8,2014;Revised:November 17,2014;Published on Web:November 17,2014.
∗Corresponding author.Email:shangguan@sjtu.edu.cn;Tel/Fax:+86-21-34206020.
The project was supported by the National High Technology Research and Development Program of China(863)(2012AA051501)and International Cooperation Project of Shanghai Municipal Science and Technology Commission,China(12160705700).
国家高技术研究发展计划项目(863)(2012AA051501)和上海市科委国际合作项目(12160705700)资助
