Tri(4-methoxybenzyloxyl)(pentamethylcyclopentadienyl) titanium/Modified MAO Catalyzed Polymerization of 1-Butene
2014-07-25WangJingZhouLuJiangWanheHuangQigu
Wang Jing; Zhou Lu; Jiang Wanhe; Huang Qigu
(State Key Laboratory of Chemical Resource Engineering, Key Laboratory of Carbon Fiber and Functional Polymers, Ministry of Education, Beijing University of Chemical Technology, Beijing 100029)
Tri(4-methoxybenzyloxyl)(pentamethylcyclopentadienyl) titanium/Modified MAO Catalyzed Polymerization of 1-Butene
Wang Jing; Zhou Lu; Jiang Wanhe; Huang Qigu
(State Key Laboratory of Chemical Resource Engineering, Key Laboratory of Carbon Fiber and Functional Polymers, Ministry of Education, Beijing University of Chemical Technology, Beijing 100029)
Tri(4-methoxy-1-benzyloxyl)(pentamethylcyclopentadienyl)titanium (Cp*Ti(OBzOCH3)3) in conjunction with modified methylalumoxane (mMAO) was an efficient catalyst for the living polymerization of butene-1. The steric and highly electron-releasing nature of the catalyst was probably responsible for the resulting polymers with high molecular weight and narrow molecular weight distribution (Mw/Mn=1.25 to 1.36). The effects of polymerization conditions on the catalytic activity, molecular weight and stereo-regularity of the products were investigated in detail. Especially, the content of TMA in MAO used in polymerization of butene-1 had a profound influence on polymer microstructure. The structural properties of the polybutene-1 product were characterized by13C NMR, GPC, DSC and WAXD. The results indicated that the polybutene-1 was isotacticity-rich (at 0 ℃, [mmmm] reached up to 60.3%) and the relative content of methylene pentad sequences [mmmm] of the polymer increased with a decreasing temperature.
half-metallocene; polybutene-1; narrow-dispersity; stereo-regularity
1 Introduction
Precise control over the architecture, stereochemistry, molecular weight and molecular weight distribution of the resultant polymer are important challenging goals in synthetic polymer chemistry, and living polymerization has provided a useful means for the synthesis of polymers featuring the property of monodisperse, terminally functional group and the morphology of block and star polymers. In recent decades, many efforts have been paid to the living copolymerization[1-2]and the living homogeneous polymerization of olefins, such as ethylene, propylene[3-11], and hexene-1[12-16]. Homogenous transition metal complexes were used as catalysts in the (co)polymerization. Wei Jia[17]reported that dinuclear bis-propagators were effective for the living coordinative chain transfer polymerization (LCCTP) of propylene starting with 20 equiv of ZnEt2. Norio Nakata[18]reported that poly(1-hexene) with perfect isotacticity ([mmmm]>95%) and high molecular weight (MW=74 800—421 000 g/mol) was obtained using hafnium complexes, and B(C6F5)3, (Ph3C)[B(C6F5)4], or dMAO (dried methylaluminoxane) as cocatalyst. Much attention has been attracted on the synthesis of polybutene-1 because of the important role for the application of plastic tube. Kaminsky[19]obtained polybutene-1 with a high molecular weight and good toluene-solubility by means of a two-component initiator system consisting of chiral [C2H4(4,5,6,7)-tetrahydro-1-indenyl]2ZrCl2and methylaluminoxane functioning as cocatalyst. Zambelli[20]reported the synthesis of isotactic, methanol-soluble polybutene-1 using the improved catalysts containing binary cyclopentadienyl ligands (identical or not identical). Rossi[21]used another metallocene precursor, rac-(dimethylsilyl) bis(4,5,6,7-tetrahydro-1-indenyl)zirconium dichloride, to produce isotactic polybutene-1. However, these polymerization reactions had no living behavior. Previously, we reported the living behavior of the formation of multi-stereo block polybutene-1 using Cp*Ti(OBz)3/mMAO catalyst[22]and atactic polybutene-1 with a high molecular weight produced by Cp*Ti(OCH2CH=CHC6H5)3/MAO catalyst[23]. In this paper, a new stereoregular polybutene-1 was synthesized with a novel catalytic system consisting of half-titanocene, tri(4-methoxybenzyloxyl)(pentamethylcyclopen-tadienyl) titanium/mMAO [Cp*Ti(OBzOCH3)3/mMAO] catalyst which can lead to the isotactic polybutene-1 in a living fashion.
2 Experimental
2.1 Materials
All operations of air- and moisture-sensitive materials were performed under the condition of rigorous repellency of oxygen and moisture in a flamed Schlenk-type glassware with a dual manifold Schlenk line in a nitrogen atmosphere. Toluene, benzene andn-hexane were further purified by refluxing over metallic sodium under nitrogen blanketing for 48 h and distilled before use. Triethylamine was dried by means of the molecular sieve 5A. Trimethylchlorosilane (purchased from Aldrich) was distilled after being treated by CaH2and degassed prior to use. TiCl4was freshly distilled in the presence of Cu turnings. Methylaluminoxane (MAO, white solid) containing 25 mol% of AlMe3was prepared according to the literature description[24]. The mMAO was prepared by dissolving methylalumoxane in toluene followed by removal of the solvent. The amount of TMA contained in MAO was decreased when the solvent was removed under vacuum, and the TMA content in MAO was determined by the pyridine titration method, with phenazine used as the indicator in benzene solution. The mMAO1 was defined as methylalumoxane which had been treated for one time, while mMAO2 denoted methylalumoxane which had been treated for two times. Pentamethylcyclopentadiene and p-methoxy-1-benzyl alcohol, which were purchased from Aldrich, were treated with molecular sieve for a week, and were further purified before use.
2.2 Preparation of the catalyst Cp*Ti(OBzOCH3)3
Pentamethylcyclopentadiene (12.4 mL, 79.4 mmol) was treated in a 500 mL Schlenk flask with metallic K (3.1 g, 79.5 mmol) at room temperature until the metal completely disappeared in hexane solution (with 250 mL of hexane), and then the mixture reacted upon trimethylchlorosilane (10.1 mL, 80.0 mmol) at room temperature to form the trimethylsilyl derivative. Pentamethylcyclopentadienyltrichlorotitanium (Cp*TiCl3) was prepared by the reaction of the trimethylsilyl derivative on TiCl4fed dropwise from a syringe cooled in an ice-water bath. The mixture was warmed to room temperature and kept at that temperature for 4 hr to remove the solvent. The crude product was sublimated at 115—120 ℃ under vacuum to give red needle crystal. Cp*TiCl3weighed 15.5 g with a putiry of 68% (1H NMR:δ=2.06, s 15H, Cp*). Microanalysis of Cp*TiCl3showed that it contained 41.8 % of C and 5.32% of H, while the calculated C content was 41.6% and the H content was 5.19%. Cp*TiCl3(0.81 g, 28 mmol) was esterified with 4-methoxy-1-benzyl alcohol (8.8 mL, 70.9 mmol) in benzene (20 mL) solution in the presence of triethylamine (1.2 mL, 84.5 mmol) at ambient temperature for 24 h to obtain the metallocene catalysts. The1H NMR (CDCl3) analysis of tri(4-methoxybenzyloxyl)(pentamethylcyclopentadienyl)titanium (Scheme 1) showed the following data:δ6.73—7.19 (s, 12H, Ph), 3.70 (s, 9H, O—CH3), 1.90 (s, 15H, Cp*), 4.46 (s, 6H, O—CH2). Analysis of Cp*Ti(OCH2C6H4OCH3)3showed that it contained 68.7% of carbon and 7.72% of hydrogen, while the calculated C content was 68.5% and the H content was 7.55%.
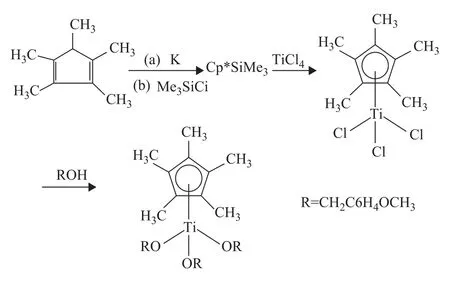
Scheme 1 The synthesis course of Cp*Ti(OBzOCH3)3catalyst
2.3 Polymerization
All polymerization reactions were carried out in a 100 mL glass flask equipped with a magnetic stirrer. After purging out all moisture and oxygen through evacuation, the desired amounts of MAO, Cp*Ti(OBzOCH3)3and 30 mL of toluene were added in sequence. After the solvent was saturated with butene-1, the gas pressure was maintained at 130 kPa throughout the course of the reaction. The monomer consumption was measured with a flowmeter. The reaction was terminated with addition of 10% of HClin alcohol after 1 h, and then the polymer was precipitated. The insoluble polymer was stirred overnight, and then washed with alcohol and water. The product was dried in vacuum until a constant weight was obtained and weighed to calculate the catalytic activity.
2.4 Characterization
The average molecular weight and molecular weight distribution was measured by a PL-GPC200 instrument using standard polystyrene as the reference and 1,2,4-trichlorobenzene as the solvent at 150 ℃. The13C NMR spectra of samples in 1,2,4-trichlorobenzene (d3) solvent were determined at 130 ℃ by a Varian Inova 500 spectrometer. Chemical shifts were referenced using internal solvent resonance and reported as being related to tetramethylsilane. The DSC thermograms were recorded with a Perkin-Elmer DSC-7 instrument at a heating rate of 10 K/min. The melting temperature of polybutene-1 was determined at the second heating scanning operation. Elemental analyses were performed on a PE-2400 spectrometer. The WAXD analysis was carried out with a Rigaku D/max-3A instrument, using a Ni-filtered Cu Kα radiation (λ=1.540 5×10-10m). The WAXD intensity was recorded from 5° to 40° at a continuous scanning speed.
3 Results and Discussion
3.1 Polymerization of butene-1
Reaction conditions for butene-1 polymerization in the presence of Cp*Ti(OBzOCH3)3/mMAO2 catalyst were varied (Table 1). We noticed that the catalytic activity and the number average molecular weight of the polymer increased within the range of 0—30 ℃(runs 1 and 2 in Table 1) with a maximum value of 65.2 kgP/molTi·h and Mnof 51.7×104g/mol at 30 ℃, respectively. However, they decreased obviously at 50 ℃(entry 3 in Table 1) because of both the lower monomer concentration and the fast deactivation rate of the active species at a relatively high temperature. Moreover, entries 2, 4—8 indicated that the catalyst showed high activity and generated high molecular weight of the polymer at an Al/Ti molar ratio ranging from 150 to 300, which reached a maximum value at an Al/Ti molar ratio of 200. However, the catalytic activity obviously decreased at higher or lower ratios of Al/Ti, 33.4 kgP/molTi·h and 31.7 kgP/molTi·h at Al/Ti molar ratios of 100 and 400, respectively (runs 4 and 8 in Table 1). Beyond this Al/Ti molar ratio in the range of from 150 to 300, the molecular weight of the polymer decreased, which indicated that toosmall amount of mMAO in the system was not favorable for the polymerization, since more mMAO added in the system might act as a chain transfer agent to some extent. Entries 2, 9—11 showed that the catalyst exhibited the highest activity at a Ti concentration of 1.0 mmol/L (run 2 in Table 1). The molecular weight and molecular weight distribution of polymers given in Table 1 had also been reported. The polymer exhibited a narrow molecular weight distribution (Mw/Mn=1.25—1.33) at polymerization temperature in the range of from 0 ℃ to 50 ℃, indicating that the polymerization was in a living manner.
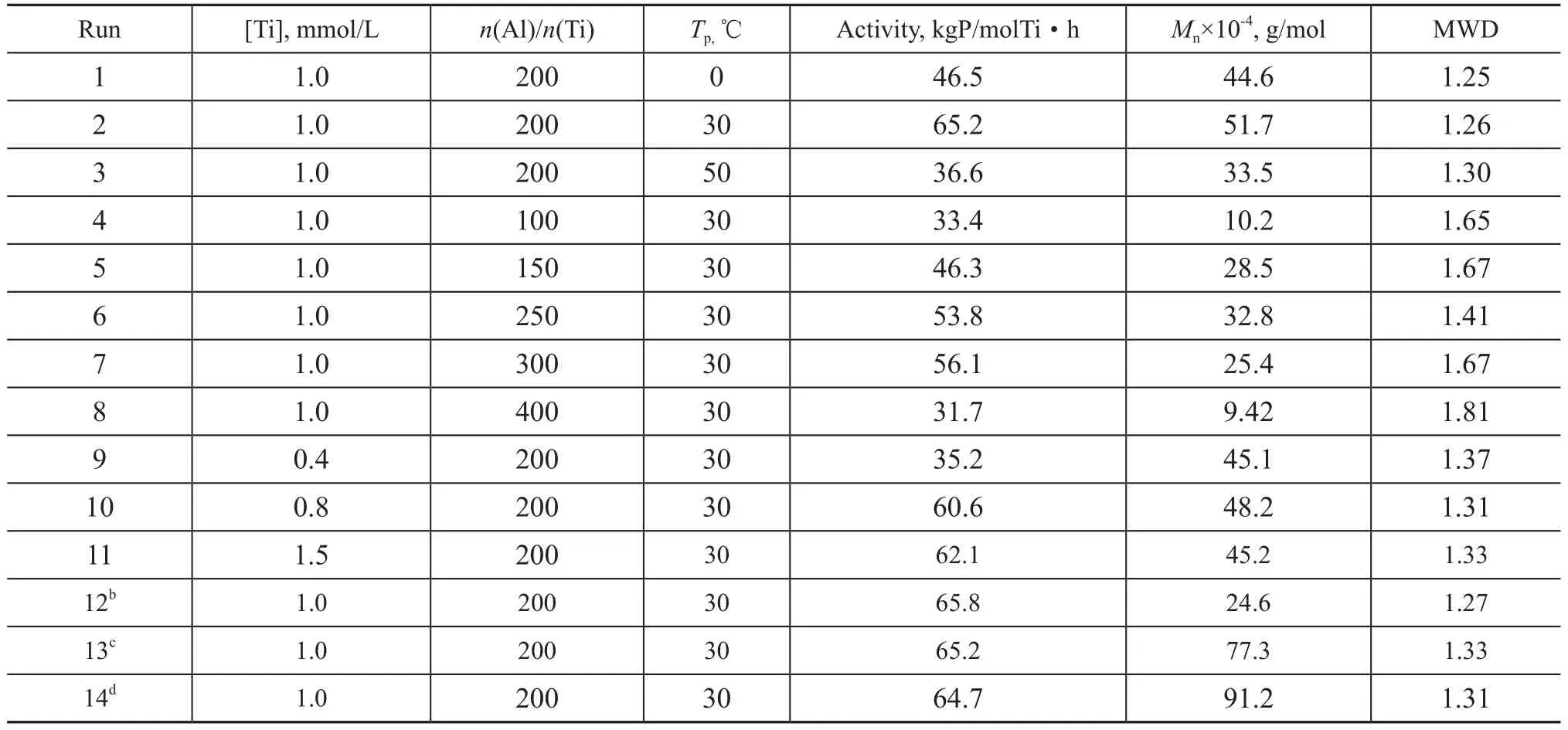
Table 1 The effect of conditions on the polymerization of butene-1 over the Cp*Ti(OBzOCH3)3/mMAOacatalyst
Time-course plots of the number average molecular weights (Mn) and theMw/Mnvalues are shown in Figure 1. TheMnvalue increased proportionally with polymerization time, and theMw/Mnvalue was kept in a narrow range from 1.26 to 1.33 for each entry, which suggested that the polymerization was characterized as the living polymerization. This result was also consistent with the GPC data of the polymer obtained at different polymerization time, as shown in Figure 2. Along with the increase of polymerization time, the peaks of GPC curves moved to the direction of lower retention time, which indicated that theMnvalue increased with an increasing polymerization time. Remarkably, polymers withMn=51.7×104g/mol could be synthesized with a fairly narrow molecular weight distribution (Mw/Mn=1.26).
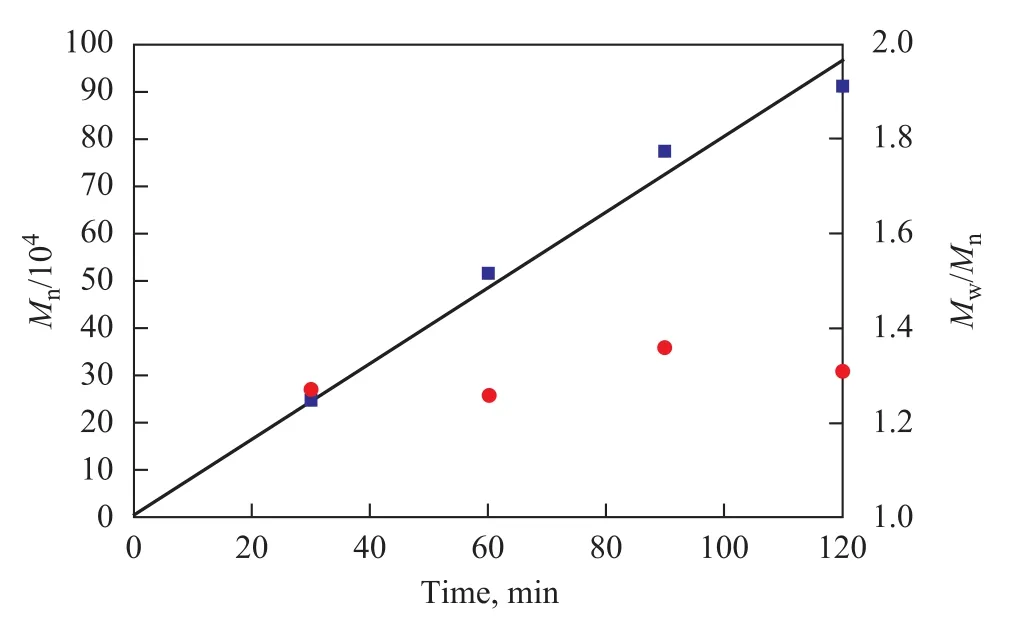
Figure 1 The plot of Mnand Mw/Mnvs. polymerization time with Cp*Ti(OBzOCH3)3/mMAO2 catalyst
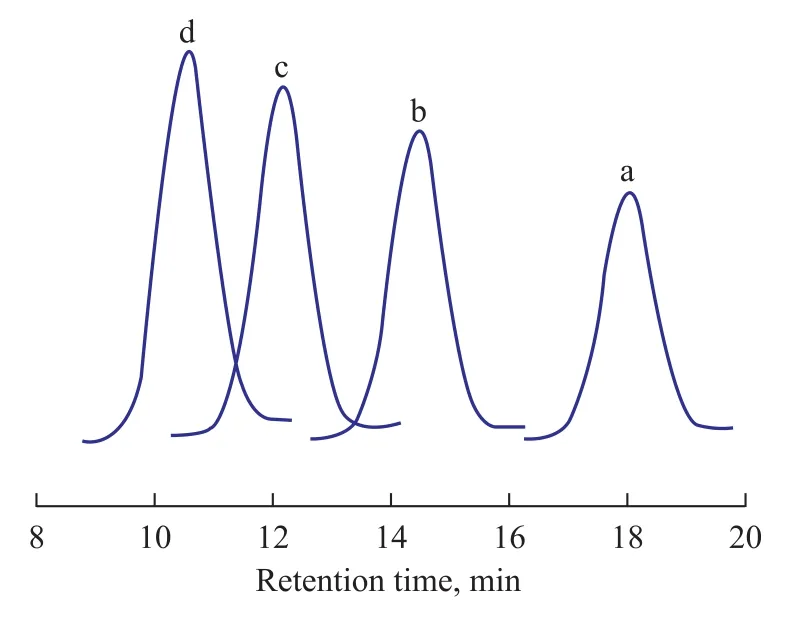
Figure 2 GPC curves of the obtained polymer catalyzed by Cp*Ti(OBzOCH3)3/mMAO2at different polymerization time
3.2 Characterization of the Polymer
The residual trimethylaluminium (TMA) in MAO has been known to have significant effect on catalytic activity of metallocene complexes and on molecular weight and structure of polyolefins[25]. Therefore, in this work, the effect of TMA content in MAO on polymerization of butene-1 catalyzed by Cp*Ti(OBzOCH3)3/mMAO was investigated in detail. The residual TMA content in MAO was determined by pyridine titration[26]. According to Table 2, it was clear that as the TMA content in MAO was decreased, Ti (IV) active species that were favorable for butene-1 polymerization was significantly increased with the TMA content reaching 21.0% in MAO, and the catalytic activity was only 27.5 kgP/molTi·h (run 16 in Table 2) which was up to 65.2 kgP/molTi·h at 17.8% of TMA in MAO (run 2 in Table 2). What impressed us most was that at 21.0% of TMA in MAO the molecular weight of polymer was only 5.3×104(run 16 in Table 2), and the molecular weight reached 51.7×104at 17.8% of TMA in MAO (run 2 in Table 2), which was more than ten times as many as run 16. It can be seen from Table 2 that the molecular weight of polymer remarkably increased with an decreasing TMA content in MAO.
The13C NMR spectra (Figure 3) also exhibited that the polymer microstructure greatly depended upon the amount of TMA in MAO. The resonance of the methylstereochemical pentads [mmmm] were at 27.10 and were marked by stars in the spectra. The result of13C NMR spectroscopic analysis of polymers, formed over different amount of TMA in MAO, demonstrated that upon using the MAO1 (at a TMA content of 21.0 %) coctalyst to promote Cp*Ti(OBzOCH3)3complex for producing polybutene-1 the [mmmm] (atδ=27.10) of the polymer was lower, as compared with that of polymers, which were catalyzed by Cp*Ti(OBzOCH3)3/MAO2 (at a TMA content of 17.8%).
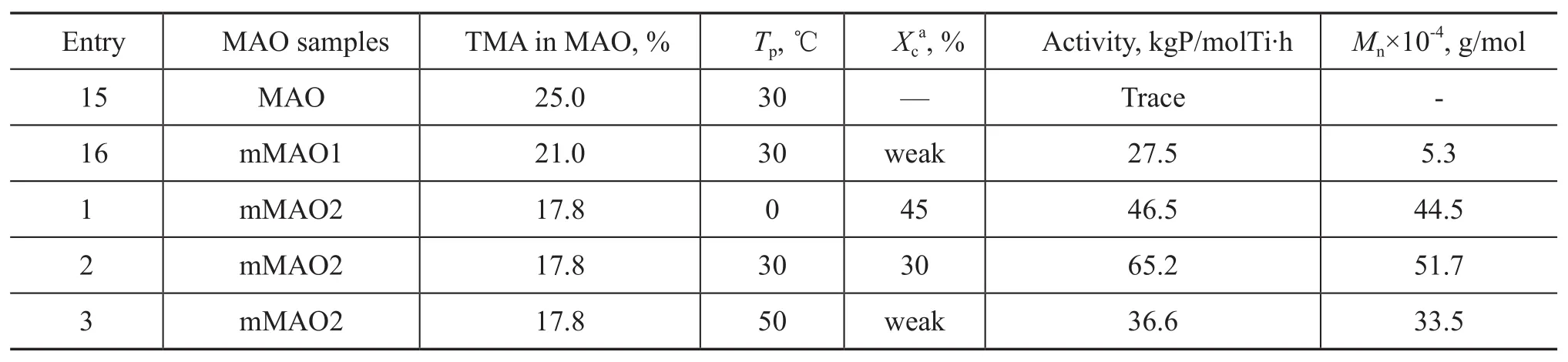
Table 2 The effects of TMA in MAO on the polymerization of butene-1 over Cp*Ti(OBzOCH3)3/mMAO catalyst
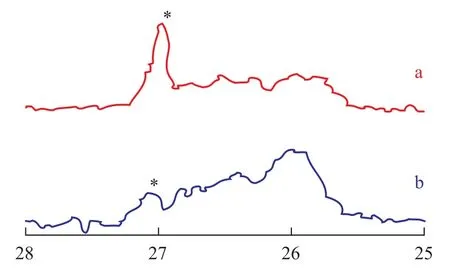
Figure 3 The13C NMR spectra of polybutene-1 catalyzed by Cp*Ti(OBzOCH3)3/mMAO
The polybutene-1 obtained in this catalytic system was an elastomeric thermoplastic with higher molecular weight than most of those formed from the metallocenes mentioned in the introduction. The higher molecular weight of the stereoregular polybutene-1 formed over the catalyst Cp*Ti(OR)3/mMAO could be mainly attributed to the electronic effect caused by the presence of the more electron-releasing Cp*and OR ligands, resulting in an increase in electron density at the metal center and also at the β-carbon sites. Consequently, the elimination of β-hydrogen and chain transfer to monomer reactions, which were the dominant chain termination reactions during the polymerization over most metallocene catalyst systems, diminished[27-28]. Additionally, the bulk of the Cp*ligand had a certain steric effect on the elimination of β-hydrogen atoms, causing an increase in energy for the transition state that needed the polymer chain to rotate about the Cα—Cβbond so that the filled β-H б orbital overlapped with the vacant orbital of the Ti atom.
Figure 4 shows the13C NMR spectra of polybutene-1. A trace amount of resonance due to head-to-tail sequences atδ=10.6, 25.5—27.3, 33.9, and 39.2, corresponding to methyl, methylene (pendant), methine, and methylene of head-to-tail sequences, calculated by Grant-Paul method[29], were observed in all spectra. This appeared to be the chain propagation of butene-1 involved in primarily the head-to-tail 1,2-insertions. While these were not negligible proportions with polybutene-1 units arranged in head-to-head and tail-to-tail sequences at a temperature of above 30 ℃, methyl, methylene (pendant), methine, and methylene in head-to-head, and tail-to-tail sequences were observed at 12.9, 23.2, 35.0 and 36.5, respectively.
The microstructure of polybutene-1 changed significantly with temperature derived from several lines. The13C NMR spectra of polybutene-1 are shown in Figure 4, indicating that [mmmm] (atδ=27.10) was sensitive to polymerization temperature and increased with a decreasing temperature. It can be observed that the content of methylene pentad-sequences [mmmm] was 60.3% and 42.6% at 0 ℃ and 30℃, respectively. But the [mmmm] of the polybutene-1 decreased to 15.9% as the polymerization temperature increased to 50 ℃. The stereoregularity of the polymers was also observed from13C NMR spectra.
The polybutene-1 with different isotacticity under variouspolymerization temperatures were confirmed by WAXD analyses (Figure 5). At polymerization temperatures equating to 0 ℃ and 30 ℃, five diffraction peaks of polybutene-1 obtained thereby appeared at 2θ=11.6°, 12.1°, 17.3°, 18.6°, and 20.3°, respectively, indicating to the existence of long sequences of isotactic segments. But when the polymerization temperature reached up to 50 ℃, the diffraction peaks of the polybutene-1 were weak, which indicated that the copolymers contained only minor portion of shorter isotactic segments.
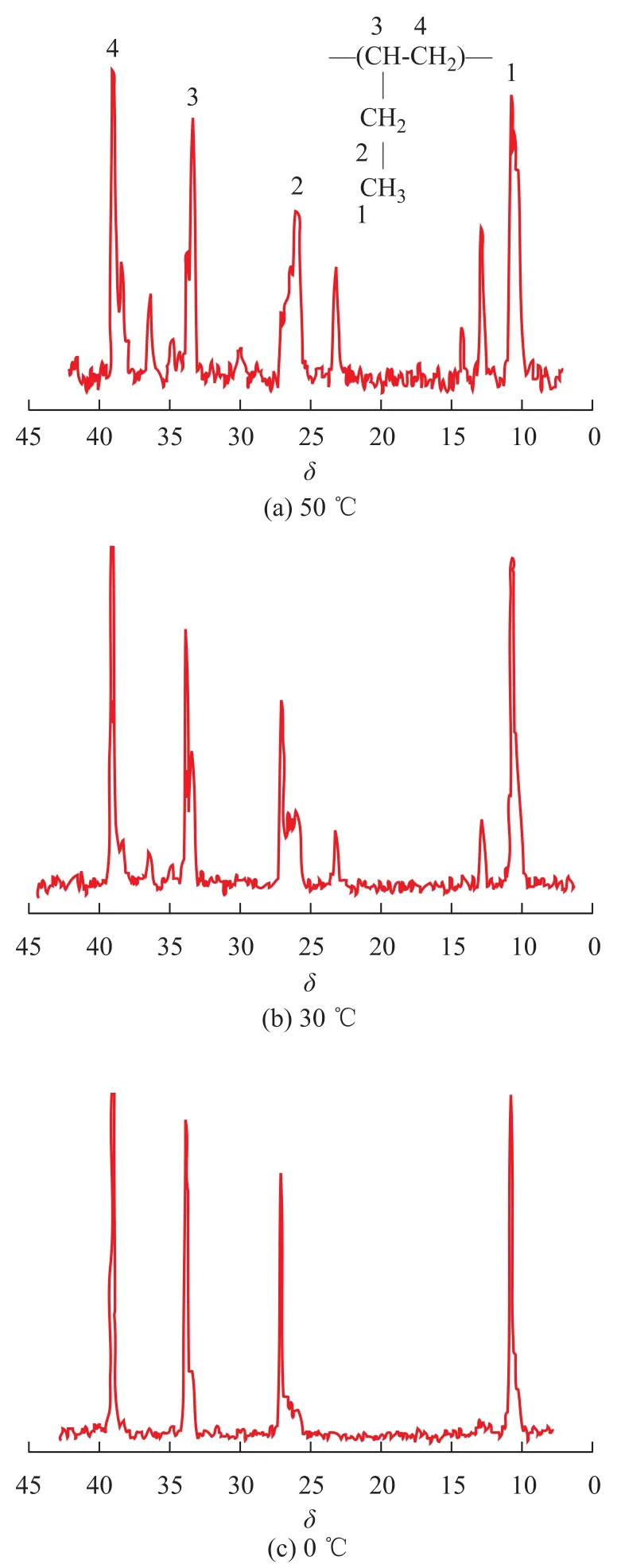
Figure 4 The13C NMR spectra of polybutene-1 catalyzed by Cp*Ti(OBzOCH3)3/mMAO2 at different temperatures
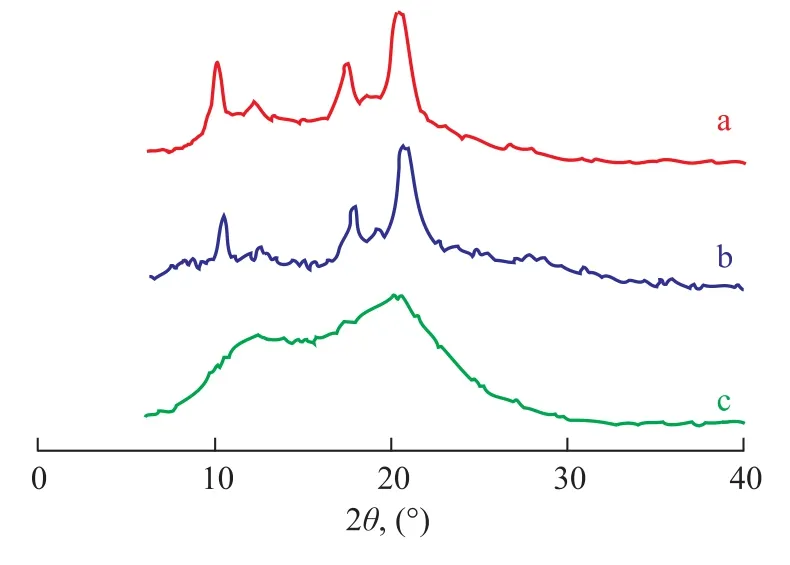
Figure 5 WAXD diagrams of polybutene-1 catalyzed by Cp*Ti(OBzOCH3)3/mMAO2 at different temperatures
Stereoregular polybutene-1 products formed over the Cp*Ti(OBzOCH3)3/mMAO2 catalyst system at 0—50 ℃were characterized by differential scanning calorimetry (DSC). For the sample prepared at 0 ℃, two melting temperatures at 54.4 ℃ and 85.7 ℃ were identified. But the DSC data of the sample produced at 30 ℃ exhibited three melting temperatures at 36.5 ℃, 43.7 ℃, and 87.3 ℃, respectively. These results implied that the obtained polybutene-1 could be considered to have a multi-stereo block microstructure involving isotactic and atactic segments. The melting temperature of polymer would depend on the multi-stereoblock number[30]. The samples with higher content of [mmmm] showed higher melting points. But their melting points were less than those of conventional isotactic polybutene-1 (iPB-1). The results also showed that the polybutene-1 was a stereoregular one, not purely iPB-1 or aPB-1. However, as the polymerization temperature increased, the obtained polybutene-1 had no crystallization, so that the DSC analysis of the sample produced at 50 ℃ did not show a melting peak, indicating that the polymer possessed a low tacticity.
4 Conclusions
The Cp*Ti(OBzOCH3)3/mMAO catalytic system showed a living behavior for producing isotacticity-rich polybutene-1 with monodispersity and high molecular weight. As demonstrated by butene-1 polymerization, the changes in Al/Ti (mol/mol) ratios, polymerization temperatures, Ti concentrations, and TMA contents in MAO significantly affected the catalytic activity and the polymer properties including molecular weight and stereo-regularity. Theresults of13C NMR spectroscopic analysis of polymers, obtained at different temperatures, demonstrated that the chain propagation of butene-1 involved primarily the head-to-tail 1,2-insertions and the relative contents of methylene pentad sequences [mmmm] of the polymer increased with a decreasing temperature as indicated by DSC and WAXD analyses.
Acknowledgement:We sincerely thank the National Natural Science Foundation of China (No. 21174011) and the Natural Science Foundation of Beijing, China (No. 2102036).
[1] Zhang H, Nomura K. Living copolymerization of ethylene with styrene catalyzed by (cyclopentadienyl)(ketimide) titanium (IV) complex-MAO catalyst system[J]. J Am Chem Soc, 2005, 127(26): 9364-9365
[2] Yoshida Y, Mohri J, Ishii S, et al. Living copolymerization of ethylene with norbornene catalyzed by bis (pyrrolideimine) titanium complexes with MAO[J]. J Am Chem Soc, 2004, 126(38): 12023-12032
[3] Rose J M, Cherian A E, Coates G W. Living polymerization of α-olefins with an α-diimine Ni (II) catalyst: Formation of well-defined ethylene-propylene copolymers through controlled chain-walking[J]. J Am Chem Soc, 2006, 128(13): 4186-4187
[4] Cherian A E, Rose J M, Lobkovsky E B, et al. AC2-symmetric, living α-diimine Ni (II) catalyst: Regioblock copolymers from propylene[J]. J Am Chem Soc, 2005, 127(40): 13770-13771
[5] Tian J, Hustad P D, Coates G W. A new catalyst for highly syndiospecific living olefin polymerization: Homopolymers and block copolymers from ethylene and propylene[J]. J Am Chem Soc, 2001, 123(21): 5134-5135
[6] Mitani M, Mohri J, Fujita T, et al. Living polymerization of ethylene catalyzed by titanium complexes having fluorinecontaining phenoxy-imine chelate ligands[J]. J Am Chem Soc, 2002, 124(13): 3327-3336
[7] Saito J, Mitani M, Fujita T, et al. Living polymerization of ethylene with a titanium complex containing two phenoxyimine chelate ligands[J]. Angew Chem Int Ed, 2001, 40(15): 2918-2920
[8] Coates G W, Hustad P D, Reinartz S. Catalysts for the living insertion polymerization of alkenes: access to new polyoleif n architectures using Ziegler–Natta chemistry[J]. Angew Chem Int Ed, 2002, 41(13): 2236-2257
[9] Zai S B, Liu F S, Gao H. Y, et al. Longstanding living polymerization of ethylene: Substituent effect on bridging carbon of 2-pyridinemethanamine nickel catalysts[J]. Chem Commun, 2010, 46(24): 4321-4323
[10] Gao H Y, Hu H B, Zhu F M, et al. A thermally robust amine–imine nickel catalyst precursor for living polymerization of ethylene above room temperature[J]. Chem Commun, 2012, 48(12): 3312-3314
[11] Sheng Y P, Huang H B, Huang Q G, et al. Synthesis of 4-arms hydroxy-functionalized PMMA-b-PE through combining free radical polymerization with coordination polymerization [J]. J Ind Eng Chem, 2014, 20(2): 419-425
[12] Mitani M, Furuyama R, Mohri J, et al. Syndiospeci fic living propylene polymerization catalyzed by titanium complexes having fluorine-containing phenoxy-imine chelate ligands[J]. J Am Chem Soc, 2003, 125(14): 4293-4305
[13] Scollard J D, McConville D H. Living polymerization of α-ole fins by chelating diamide complexes of titanium[J]. J Am Chem Soc, 1996, 118(41): 10008-10009
[14] Tshuva E Y, Goldberg I, Kol M. Isospeci fic living polymerization of 1-hexene by a readily available nonmetallocene C2-symmetrical zirconium catalyst[J]. J Am Chem Soc, 2000, 122(43): 10706-10707
[15] Jayaratne K C, Sita L R. Stereospeci fic living Ziegler-Natta polymerization of 1-hexene[J]. J Am Chem Soc, 2000, 122(5): 958-959
[16] Baumann R, Davis WM, Schrock R R. Synthesis of titanium and zirconium complexes that contain the tridentate diamido ligand, [((t-Bu-d6)N-o-C6H4)2O]2-([NON]2-) and the living polymerization of 1-hexene by activated [NON] ZrMe2[J]. J Am Chem Soc, 1997, 119(16): 3830-3831
[17] Wei J, Wonseok H, Zhang W, et al. Dinuclear bis-propagators for the stereoselective living coordinative chain transfer polymerization of propene[J]. J Am Chem Soc, 2013, 135(6): 2132-2135
[18] Nakata N, Toda T, Matsuo T, et al. Controlled isospeci fic polymerization of α-ole fins by hafnium complex incorporating with a trans-cyclooctanediyl-bridged [OSSO]-type bis(phenolate) ligand [J]. Macromolecules, 2013, 46(17): 6758-6764
[19] Kaminsky W, Külper K, Brintzinger H H, et al. Polymerisation von propen und buten mit einem chiralen zircono-cen und methylaluminoxan als cokatalysator [J]. Angew Chem Int Ed, 1985, 97(6): 507-508
[20] Longo P, Grassi A, Pellecchia C, Zambelli A. Carbon-13 enriched end groups of isotactic polypropylene and poly(1-butene) prepared in the presence of ethylenediindenyldimethyltitanium and methylalumoxane[J]. Macromolecules, 1987, 20(5): 1015-1018
[21] Rossi A, Odian G, Zhang J B. End groups in 1-butene polymerization via methylaluminoxane and zirconocene catalyst[J]. Macromolecules, 1995, 28(6): 1739-1749
[22] Zhu F M, Huang Q G, Lin S. Syntheses of multi-stereoblock polybutene-1 using novel monocyclopentadienyltitanium and modified methylaluminoxane catalysts[J]. Journal of Polymer Science. Part A, Polymer Chemistry, 1999, 37(24): 4497-4501
[23] Huang Q G, Wu Q, Zhu F M, et al. Synthesis and characterization of high molecular weight atactic polybutene-1 with a monotitanocene/methylaluminoxane catalyst system[J]. Journal of Polymer Science. Part A, Polymer Chemistry, 2001, 39(23): 4068-4073
[24] Wu Q, Ye Z, Lin S. Syndiotactic polymerization of styrene with cyclopentadienyltribenzyloxytitanium/methylaluminoxane catalyst[J]. Macromol Chem Phys, 1997, 198(6): 1823-1828
[25] (a) Tritto I, Sacchi M C, Locatelli P, et al. Low-temperature 1H and13C NMR investigation of trimethylaluminium contained in methylaluminoxane cocatalyst for metallocene-based catalysts in olefin polymerization[J]. Macromol Chem Phys, 1996, 197(4), 1537-1544. (b) Barron AR. New method for the determination of the trialkylaluminum content in alumoxanes[J]. Organometallics, 1995, 14(7): 3581-3583
[26] Jordan D E. Visual titrimetric determination of total reactivity and differentiation of trialkylaluminum and dialkylaluminum hydride in mixtures[J]. Analytical Chemistry, 1968, 40(14): 2150-2153
[27] Fierro R, Chien J C W, Rausch M D. Asymmetric zirconocene precursors for catalysis of propylene polymerization[J]. Journal of Polymer Science. Part A, Polymer Chemistry, 1994, 32(15): 2817-2824
[28] Lee I M, Gauthier W J, Ball J M, et al. Electronic effects of Ziegler-Natta polymerization of propylene and ethylene using soluble metallocene catalysts[J]. Organometallics, 1992, 11(6): 2115-2122
[29] Grant D M, Paul E G. Carbon-13 magnetic resonance. II. Chemical shift data for the alkanes[J]. J Am Chem Soc, 1964, 86(15): 2984
[30] Edson J B, Wang Z, Kramer E J, et al. Fluorinated bis(phenoxyketimine)titanium complexes for the living, isoselective polymerization of propylene: Multiblock isotactic polypropylene copolymers via sequential monomer addition[J]. J Am Chem Soc, 2008, 130(14): 4968-4977
Received date: 2014-03-25; Accepted date: 2014-08-05.
Dr. Huang Qigu, E-mail: huangqg@ mail.buct.edu.cn.
杂志排行
中国炼油与石油化工的其它文章
- Spray Characteristics Study of Combined Trapezoid Spray Tray
- Effect of Stirring on Oil-Water Separation in Rare Earth Mixer-Settler
- Synergetic Effect of Y Zeolite and ZSM-5 Zeolite Ratios on Cracking, Oligomerization and Hydrogen Transfer Reactions
- Preparation of Tungsten Film and Its Tribological Properties under Boundary Lubrication Conditions
- Solvothermal Synthesis of V2O3Catalysts for Oxidative Desulfurization of Dibenzothiophene
- Preparation of Spherical MgCl2/SiO2/THF-Supported Late-Transition Metal Catalysts for Ethylene Polymerization
