基于高通量测序的全基因组关联研究策略
2014-05-10周家蓬裴智勇陈禹保陈润生
周家蓬,裴智勇,2,陈禹保,陈润生
1. 北京市计算中心,北京 100094;
2. 中国科学院北京基因组研究所,北京 100101;
3. 中国科学院生物物理研究所,北京 100101
全基因组关联研究(Genome-wide association study,GWAS)是对多个个体在全基因组范围的遗传变异多态性进行检测,获得基因型,进而将基因型与可观测的性状,即表型,进行群体水平的统计学分析,根据统计量或P值筛选出最有可能影响该性状的遗传变异。GWAS的主要方法学依据是归纳法中的共变法,是探究复杂因果关系的最主要的思想和方法。因此,GWAS特别适用于遗传机理不明的复杂疾病或性状。得到高密度、高可信的遗传变异是GWAS的基础,这些遗传变异包括单核苷酸多态性(Single nucleotide polymorphisms,SNP)、插入缺失(Insertion and deletion,InDel)及拷贝数变异(Copy number variation,CNV)等,其中最主要的是SNP,占标记总量的90%以上。目前获得高密度SNP的方法主要是SNP芯片(Array),因其高效、易用、廉价等特点在近些年被广泛使用。而第二代测序技术(Next-generation sequencing,NGS)的快速发展,为GWAS的相关研究工作提供了新的技术和思路。NGS可以获得大量低频甚至稀有的遗传变异,一些无法由芯片的高频或常规变异(即 MAF ≥ 5%的变异)检测到的表型关联,有望通过基于NGS的GWAS方法得到有意义的结果。本文对基于高通量测序的GWAS的原理、策略及相关研究进展进行了阐述,并对其如何应用于个体化医疗(Personalized medicine,PM)进行了展望。
1 GWAS概述
GWAS的研究思路最早于1996 年由Risch等[1]提出,目的是将人类复杂疾病的研究从候选基因转向全基因组水平,以期用更大规模的检测得到与疾病相关的每一个基因。近年来,已有多篇报道对GWAS研究进展进行了综述[2~4]。多年来,在人类疾病相关研究中,关于年龄相关性黄斑变性[5]、冠心病[6,7]、皮肤病[8,9]、2 型糖尿病[10~13]、癌症[14,15]、精神分裂症[16~18]、阿尔茨海默氏症[19]等 GWAS成果相继被报道。据NHGRI GWA Catalog网站(www.genome.gov/GWAStudies)的统计,截至 2013年底,在人类疾病或重要性状研究方面,共有1778篇高质量的GWAS研究工作被收录,累计发现12123个SNP位点,影响癌症、心血管系统、免疫系统、神经系统等17大类800多种重大疾病或其相关性状。GWAS不仅在人类医学研究中被广泛应用,在动植物遗传选育等方面也在逐渐兴起。在动物育种方面,利用GWAS方法研究了奶牛[20~24]、猪[25,26]、肉鸡[27,28]等经济动物的数量性状。GWAS在植物上也有较多报道,如玉米的开花时间[29]、叶片结构[30]、抗枯萎病[31,32],还有水稻的十几种农艺性状的研究[33]等。以上研究主要采用基于芯片的GWAS方法。
2 基于NGS的GWAS
2.1 “合成关联”假设
传统GWAS是基于“Common disease-common variation”(CD-CV)的假设,该假设比较符合诸如“身高”等数量性状的遗传模式。Allen等[34]收集了约18万例样本进行分析,发现了180个与身高显著关联的基因座(Loci)在生物学通路上高度富集,并且这些基因均与骨骼生长缺陷有关,累计可解释约10%~16%的表型变异。另一种观点认为,一个常见变异的“致病”效应可能是从一个稀有变异的致病效应“稀释”而来,即“Common disease-rare variation”(CD-RV)假设。Dickson等[35]首次提出“合成关联”(Synthetic association)的概念来描述这一现象,利用模拟数据证实合成关联普遍存在于常见变异与稀有变异之间。越来越多的研究证实“合成关联”模型的真实性[36~38]。Yang等[39]对近4000个无关个体的295 K芯片基因型数据和身高表型数据进行关联分析,发现全部SNP可解释高达45%的表型变异;然而,尚有35%的表型变异无法通过芯片上已有的SNP解释,这主要由常见变异和稀有变异(Rare variation)间的连锁不平衡所导致。千人基因组计划(1000 Genomics Project,http://www.1000genomes.org/)提供了大量可供研究的人群遗传变异数据,统计研究发现,人类基因区域的遗传变异一般在进化上是近期发生的,且具有稀有性和人群特异性的特点[40~42]。目前认为,常见变异和稀有变异都在致病效应上有所贡献[34,39,43](图1),效应的大小可能与频率成反比[44,45],符合进化和选择的观点。随着研究的不断深入,稀有变异所占的份量可能会越来越重,如Fu等[42]对6500例非、欧裔美国人外显子组进行测序发现,在多达1.1 M的外显子区域变异中,73%在进化上是近期发生,且频率较低;而在可能致病的变异中,这个比例高达86%。以上研究表明,NGS技术可以为“缺失的遗传力”[44]问题提供新的解决方案,而随着NGS技术的不断成熟与实验成本的降低,NGS- GWAS的研究和应用可能会逐渐兴起。
2.2 基于NGS的GWAS新策略和方法
围绕CD-CV假设而设计的GWAS芯片主要面向高频SNP,国际人类基因组单体型图计划(International HapMap Project,http://www.hapmap.org)的数据库主要基于芯片技术构建,因而也以常见变异为主,目前 HapMap III期共收录约 10 M 个 SNP。传统GWAS对 MAF<0.05的 SNP很少研究。随着 NGS技术的兴起,特别是千人基因组计划的实施及其第Ⅰ阶段工作的完成,获得了37 M SNP,此外还检测出1.4 M插入缺失(InDel)和14 K结构变异(SV);2014年发布的第Ⅲ阶段结果,遗传变异位点总量已高达79 M。此外,外显子组测序和转录组测序也累积了大量的遗传变异。
基于NGS的GWAS可以验证CD-RV假设。该假设认为,复杂疾病是由低频或稀有变异引起的,且这些携带较大遗传效应的变异往往不与常规 SNP紧密连锁,这可能是造成芯片GWAS的所谓“缺失的遗传力”问题[44,45]的主要原因。NGS技术采用高通量的平行测序方式,可以快速地获取高密度的SNP。随着该技术的完善和成熟,以及实验成本的降低,研究者开始尝试进行基于NGS技术的GWAS工作,一些新的策略和方法也应运而生。
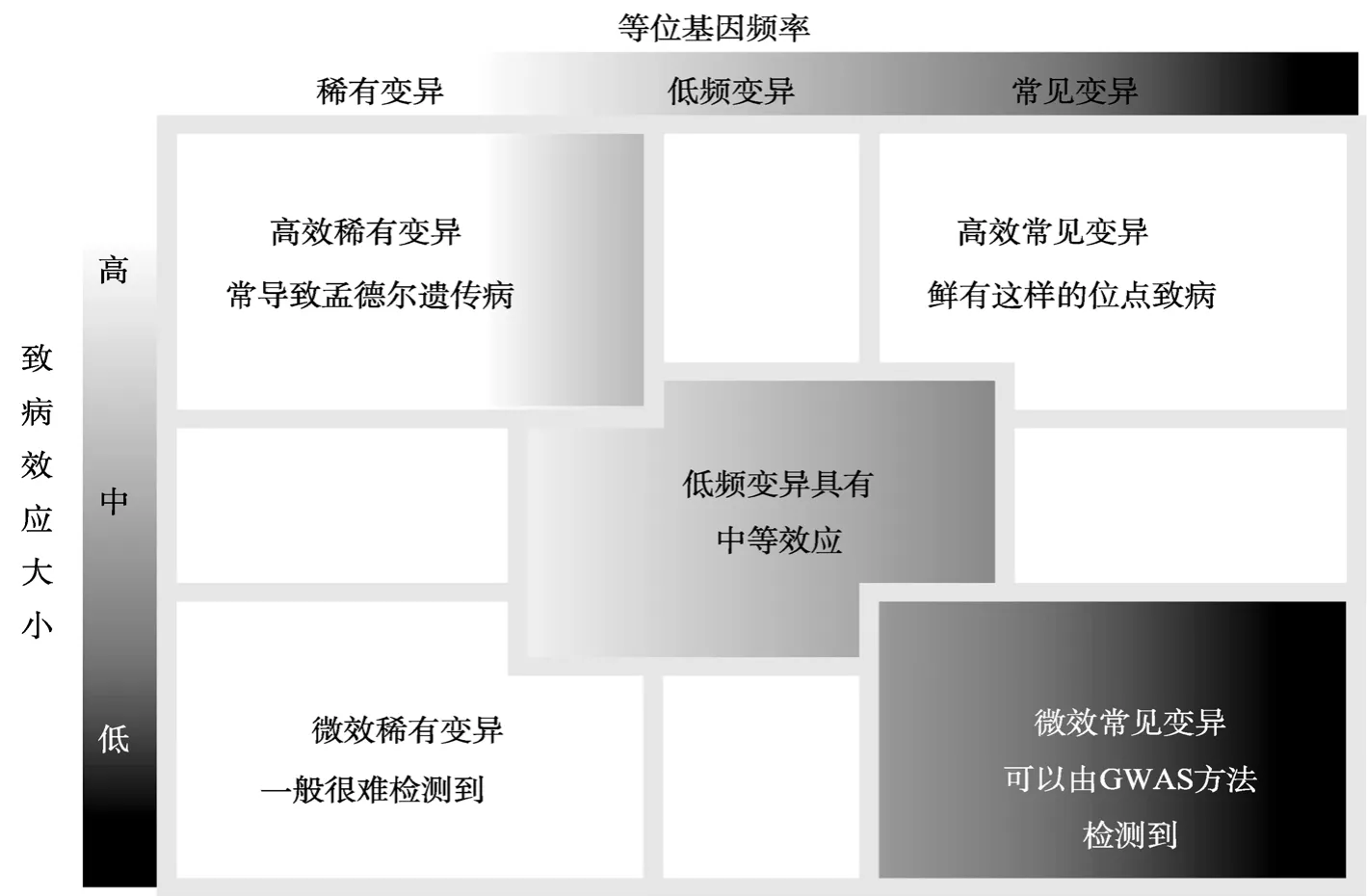
图1 基于等位基因频率的复杂疾病遗传假设
2.2.1 外显子组测序
根据对孟德尔遗传病的研究发现,外显子突变是其主要病因,而复杂疾病很可能是由与其功能相关孟德尔遗传病的致病变异所影响。因此,外显子组测序相当于对基因组水平的致病变异进行了浓缩,只考虑外显子组,这样较易于解释生物学功能,且易于取得医学上的应用,是一种合理的优化策略。一些稀有变异与复杂性状的关联关系,可以通过外显子组测序的方法进行研究[46]。
在一些疾病研究中,如帕金森氏综合征,基于外显子测序的关联分析可在一定程度上得到更加丰富的数据[47]。Ng等[48]对4个Freeman-Sheldon综合征患者和 8个正常对照进行了外显子组测序,在 4个患者上发现的致病基因变异位点,不存在于任一对照个体中,也未在dbSNP数据库中发现,表明该策略在发掘致病变异研究中是可靠的。外显子组测序同样可用于检测病因未知的疾病,Ng等[49]通过对4个未知病因的Miller综合征患者和8个正常对照进行外显子组测序,通过对非同义突变、InDel、可变剪切变异等筛查,检测到DHODH基因在4个病例中存在上述变异而在对照个体中不存在。外显子组测序还可用于疾病辅助诊断和治疗。在皮肤病的相关研究中,Tang等[50]对781名银屑病患者以及676名健康对照个体的样本进行了外显子组测序,并在第二阶段扩大样本总量至 21309例。通过 GWAS分析,科研人员在IL23R和GJB2基因上检测到了2个低频错义突变,在LCE3D、ERAP1、CARD14 和ZNF816A基因上检测到了5个常见错义突变,都与银屑病的发生显着关联。Leslie等[51]通过对2005个个体进行外显子组测序及GWAS分析,发现PCSK9、LDLR和 APOB基因与人体内低密度脂蛋白胆固醇的含量相关。这两项研究在GWAS分析中均采用了BURDEN test的方法对稀有变异进行检测,该方法在近些年的相关研究中应用较为普遍。Choi等[52]通过检测致病突变位点,间接确认某患者的疾病症状。他们识别出一些纯合同义突变,这些突变从无脊椎动物到人类都高度保守。检测这些突变可能引发的疾病类型和症状后,发现其中一个突变位于导致先天性失氯性腹泻(Congenital chloridediarrhea,CCD)的基因上。外显子组测序也在复杂疾病研究中得以应用,如 Bowden等[53]对浆乙二腈水平无显著性差别的2个家系中的3个患者进行外显子组测序,发现ADIPOQ基因上的1个频率为1.1%的低频变异,能解释 17%的西班牙裔美国人的血浆乙二腈水平,63%的家族存在该突变。Bilguvar等[54]对1列脑皮质发育异常患者进行测序,发现WDR62基因与该疾病相关,结合功能分析可以解释一系列的严重皮质畸形,如小头畸形、胼胝体发育不全等;WDR62基因突变的某些患者还会发生脑裂畸形、小脑发育不全等。
基于外显子测序进行GWAS的计算和统计方法[55]包括:(1)沿用传统的单SNP位点模型,由于低频突变样本数少,可选用Fisher精确检验;(2)使用多因素模型,将单SNP位点的效应加和及校正,计算过程也需要借助一些降维的方法如Lasso[56]等,以减少运算量;(3)折叠法(Collapsing methods),其原理是将同一个功能元件上的变异合并,根据功能元件的不同,分为 CAST[57]和 CMC[58]两种主要检测方法,前者考虑全部稀有变异,后者则关注非同义稀有突变,前文提到的BURDEN test即属于此方法,此外还有“变量阈值”法[59]和 RareCover[60]等方法;(4)聚合法(Aggregation methods),相当于对折叠法中稀有变异以及常规GWAS中的常规变异进行加权,可分为“加权和”法[61]和 KBAC[62]两种。
由于外显子组测序只关注外显子及其剪切位点,因而对某些类型的致病变异无能为力,如线粒体基因中的突变、结构变异、内含子中的基因、调控序列、CNV、表观遗传学改变、“单亲二倍体”、基因之间的相互作用等;另外,有些外显子藏在染色体末端的重复区域内,因而无法被外显子测序所检测。
2.2.2 低覆盖度测序结合基因型填充
低覆盖度测序结合基因型填充的策略,是利用已有的公共基因组数据,如千人基因组数据,来填充覆盖度较低的测序数据,使之达到有效进行GWAS研究的数据量。该策略的有效性已有许多研究报道。该策略方案中应当主要注重两点:一是检测效力;二是计算速度。
检测效力的高低主要取决于数据量,因此,关键要找到SNP数量与样本量的均衡点。SNP数据量要足以涵盖致病突变;样本数量也应充足,以期检测到致病效应较小的变异,获得更多的缺失遗传力。Zheng等[63]对153例样本分别用3种芯片(317 K、610K和1 M)进行基因分型,分别用HapMap2和千人基因组预实验(1000G pilot)数据作为参考,进行缺失基因型填充。HapMap2填充的准确性大约 94%,1000G pilot约84%。对于MAF介于0.3%~5%的稀有SNP,三款芯片数据的填充准确性分别为49%,60%和69%。值得一提的是,尽管1000G pilot的准确性比HapMap2低,但其填充SNP的数据量(约8.5 M)要远高于后者(约2.5 M)。
对原始数据的产出量的选择问题,即如何控制测序覆盖度以达到SNP分型目的,千人基因组研究[64]给出了参考,即2~4×测序深度即可获得个人基因组约 85%的区域,数据产出和测序成本的比例最优。而这个最优解是针对个人基因组而非群体基因组测序而估计的,随着参考数据的不断累积,个体测序的覆盖度可以降得更低,如Pasaniuc等[65]采用极低覆盖度的策略(平均~0.24×)依然可以获得较好的填充效果。
由于NGS的数据量大,基因型填充的过程运算量大、耗时长,因而一些研究者开发出了加快运算的优化算法。Howie等[66]开发的Pre-phasing填充方法,通过对GWAS样本进行连锁相构建,进而利用参考库的单倍型进行缺失基因型填充。该方法可以在很大程度上缩短运算时间,在大样本中效果更加明显。Howie等使用MaCH、IMPUTE2软件,利用WTCCC2、GAIN、WHI以及 1000G数据,对该方法进行了测试,结果显示Pre-phasing方法的效率明显高于常规方法。

表1 高密度芯片与低覆盖度测序技术对比
因此,低覆盖度全基因组重测序结合缺失基因型填充的方法应当是一种可行的策略。高密度 SNP芯片与低覆盖度测序的技术参数比较见表1。
Rohland等[67]发明了一种廉价高效的建库方法,一个技术人员可以在一天内构建 192个测序库,使建库成本降至每样本15美元。这些库不仅可以用于低覆盖度测序,还可以在多达 100例加标签样本(Barcoded samples)混池的条件下进行有效测序。他们用极低覆盖度的外显子组测序数据(0.1~0.5×)结合千人基因组基因型数据做填充,证明了该方法的有效性。这使得在成本降低的情况下,捕获或填充的SNP的数目、分型的准确性都有所增加,检测效力也得到提高[65]。目前该策略的缺点是对稀有变异的基因型推断与填充效果不够理想。填充策略的准确性和有效性取决于实验样本和参考数据库的数据样本量的多少,随着测序技术不断提高、成本不断降低,以及公共数据库数据量的快速增加,低覆盖度测序的策略可能将会更多地被采用。此外,测序的准确性对于科学研究十分重要,测序错误来源有许多因素[68],应当在研究中注意。
2.2.3 家系病例或极端性状个体重测序
由于全基因组测序的费用比较高,要求对样本进行选择性测序。在这一策略中,可挑选有多个发病个体的家系进行测序,也可以挑选表型比较极端的个体进行测序。Yang等[69]使用发病个体家系的设计,对发病家系的19个发病个体和27个正常对照进行选择性测序,最终识别出11个风险CNV位点。之后对其中4个CNV在大群体进行验证,发现其确实在发病人群中高度富集。Sobreira等[70]使用类似的设计发现了混合性软骨瘤的致病变异。Lander等[71]在 1989年就提出选择极端表型个体进行分析可以降低实验成本,同时保证检测效力不会过多丧失。在高密度芯片兴起之后,Manolio等[72]根据HapMap高密度SNP数据讨论了这种策略的可行性;Verlaan等[73]进一步对其进行了检测效力的研究。Cirulli等[74]首先筛选出一般人群中的极端表型个体,然后对这些个体进行高覆盖度测序,找出明显高于一般人群等位基因频率的SNP位点,再对这些位点区域进行目标区域测序或分型。
基于医院病例(Hospital-based)数据的实验设计属于该策略[50,51],例如高血压患者就是由极端表型个体(160/100 mmHg)转化为病例的,其他种类疾病的诊断过程也可看作是极端表型的筛选,即以某指标为阈值进行病例筛选。该策略目前主要的问题是有可能会因为抽样偏差或忽略某些重要的协变量产生假阳性结果。如基于医院病例的数据,一般只重视对症状的诊断,而忽略患者的发病影响因素。
2.2.4 其他策略
除了以上3种主要的策略,还有其他研究策略或方法,如目标区域捕获测序[75]、混池测序[76]等。目标区域捕获测序的原理和外显子组测序一样,而目标更加明确,通常将几个至几十个疾病风险基因的外显子、内含子、上下游序列进行测序,从而降低实验成本。混池测序则是将多个样本混合成一个样本进行测序,该策略适用于病例对照设计,而不太适合于连续型表型;若结合加标签(Barcoding)技术则可区分个体,可用于连续型表型。另外,随着Illumina Hiseq X Ten和Nextseq 500等新的测序平台的应用,实验成本将进一步降低。
3 结语与展望
NGS技术在实验成本和速度上优于传统的Sanger测序,在数据类型和通量等方面优于芯片技术。目前,在植物育种方面已经有多篇NGS-GWAS的文章发表,如采用低覆盖度测序结合基因型填充策略对水稻14个农艺性状的研究[77],利用RNA-seq数据对玉米产油量性状的 eQTL研究[78]等。然而,对关联分析显著性结果的解释及功能发掘,需要进一步研究。例如,利用生物信息学的工具可以发掘出许多新的功能作用元件。此外,用变异位点解释复杂疾病的机理有一定难度。目前,传统GWAS主要使用单SNP位点模型,显得过于单薄,因此需要开发更加复杂和精密的模型,例如针对外显子组测序数据的 Lasso回归、折叠法、聚合法,及针对生物调控网络的互作模型等。随着大量新的遗传变异类型及其变异位点被发现,对变异的注释和使用方式将面临新的挑战。NGS将产生海量的新变异,不仅包括SNP、InDel、SV,还包括cSNP、表达量变异、可变剪切、甲基化变异等数据,使分析变得更加复杂。
关于人类基因组研究,目前基于NGS的GWAS策略多是围绕降低成本而设计,但不同策略中需要考虑的问题是相同的,即如何更全面系统地检测出致病变异并有效应用于医药。因此,不断积累并共享的数据必不可少,系统性的生物信息学挖掘也至关重要。由于NGS和GWAS两种技术成本高,因此人们需要将其策略和实验设计进行优化,在保证不过多丧失检测效力和准确性的情况下,极大提高研究实施的可行性。目前,随着公共数据库的不断累积和共享,外显子组测序、极低覆盖度重测序结合基因型填充策略,可能会在医学健康研究领域被广泛采用。随着基于NGS的RNA-seq、ChIP-seq等功能学方面数据不断积累,以及非编码RNA(ncRNA)等新型功能数据库的发展,有必要对前人的一些结果进行重新注释,并尝试在医学上加以实际应用。
运用GWAS方法对复杂疾病的研究、早期预警及个性化医疗方面已开始起步。以高血压为例,结合遗传变异信息的降压治疗方法已有多篇文献报道[79]。高血压的传统疗法是服用抗高血压类药物,如噻嗪类利尿剂、β-受体阻滞剂、ACE抑制剂、血管紧张素受体阻滞剂和钙通道阻滞剂等。全世界范围内约有30%的患者只服用一种药物,40%服用两种,30%服用三种或以上。但是这类药物对收缩压或舒张压的控制率不到35%[80]。其根本原因之一在于个体遗传变异对药物反应的特异性,因此开展药物基因组学研究具有重要意义。药物基因组学研究药物反应的遗传机制及药物反应的个体差异性,是功能基因组学和分子药物学的结合。早期的研究主要围绕单个候选基因与降压药物的作用关系,如ACE、ADD1、NEDD4L、ADRB1和KCNMB1基因。2008年,第1篇基于GWAS的药物基因组学研究被报道,发现人类12号染色体YEATS4基因附近区段影响噻嗪类利尿剂的治疗效果[81]。之后,越来越多的采用GWAS方法的药物基因组学工作被相继报道,如抗高血压药物氢氯噻嗪的治疗效果[82]等。未来的疾病防治工作,应该是治疗向预防前移,防大于治,并且应该结合遗传因素、环境因素、生活方式、药物反应等,对患者或潜在患者进行全方位、个体化的评估、预警、诊断和治疗。基于不断发展的 NGS新技术的GWAS策略将在人类医学研究领域发挥重要作用。
[1]Risch N,Merikangas K. The future of genetic studies of complex human diseases. Science,1996,273(5281):1516–1517.
[2]韩建文,张学军. 全基因组关联研究现状. 遗传,2011,33(1): 25–35.
[3]严卫丽. 复杂疾病全基因组关联研究进展——研究设计和遗传标记. 遗传,2008,30(4): 400–406.
[4]许睿玮,严卫丽. 原发性高血压全基因组关联研究进展.遗传,2012,34(7): 793–809.
[5]Klein RJ,Zeiss C,Chew EY,Tsai JY,Sackler RS,Haynes C,Henning AK,Sangiovanni JP,Mane SM,Mayne ST,Bracken MB,Ferris FL,Ott J,Barnstable C,Hoh J. Complement factor H polymorphism in age-related macular degeneration. Science,2005,308(5720): 385–389.
[6]Lettre G,Palmer CD,Young T,Ejebe KG,Allayee H,Benjamin EJ,Bennett F,Bowden DW,Chakravarti A,Dreisbach A,Farlow DN,Folsom AR,Fornage M,Forrester T,Fox E,Haiman CA,Hartiala J,Harris TB,Hazen SL,Heckbert SR,Henderson BE,Hirschhorn JN,Keating BJ,Kritchevsky SB,Larkin E,Li M,Rudock ME,Mckenzie CA,Meigs JB,Meng YA,Mosley TH,Newman AB,Newton-Cheh CH,Paltoo DN,Papanicolaou GJ,Patterson N,Post WS,Psaty BM,Qasim AN,Qu L,Rader DJ,Redline S,Reilly MP,Reiner AP,Rich SS,Rotter JI,Liu Y,Shrader P,Siscovick DS,Tang WH,Taylor HA,Tracy RP,Vasan RS,Waters KM,Wilks R,Wilson JG,Fabsitz RR,Gabriel SB,Kathiresan S,Boerwinkle E. Genome-wide association study of coronary heart disease and its risk factors in 8,090 African Americans: the NHLBI CARe Project. PLoS Genet,2011,7(2): e1001300.
[7]Barbalic M,Reiner AP,Wu CY,Hixson JE,Franceschini N,Eaton CB,Heiss G,Couper D,Mosley T,Boerwinkle E.Genome-wide association analysis of incident coronary heart disease (CHD) in African Americans: a short report.PLoS Genet,2011,7(8): e1002199.
[8]Wang LD,Zhou FY,Li XM,Sun LD,Song X,Jin Y,Li JM,Kong GQ,Qi H,Cui J,Zhang LQ,Yang JZ,Li JL,Li XC,Ren JL,Liu ZC,Gao WJ,Yuan L,Wei W,Zhang YR,Wang WP,Sheyhidin I,Li F,Chenbp,Ren SW,Liu B,Li D,Ku JW,Fan ZM,Zhou SL,Guo ZG,Zhao XK,Liu N,Ai YH,Shen FF,Cui WY,Song S,Guo T,Huang J,Yuan C,Huang J,Wu Y,Yue WB,Feng CW,Li HL,Wang Y,Tian JY,Lu Y,Yuan Y,Zhu WL,Liu M,Fu WJ,Yang X,Wang HJ,Han SL,Chen J,Han M,Wang HY,Zhang P,Li XM,Dong JC,Xing GL,Wang R,Guo M,Chang ZW,Liu HL,Guo L,Yuan ZQ,Liu H,Lu Q,Yang LQ,Zhu FG,Yang XF,Feng XS,Wang Z,Li Y,Gao SG,Qige Q,Bai LT,Yang WJ,Lei GY,Shen ZY,Chen LQ,Li EM,Xu LY,Wu ZY,Cao WK,Wang JP,Bao ZQ,Chen JL,Ding GC,Zhuang X,Zhou YF,Zheng HF,Zhang Z,Zuo XB,Dong ZM,Fan DM,He X,Wang J,Zhou Q,Zhang QX,Jiao XY,Lian SY,Ji AF,Lu XM,Wang JS,Chang FB,Lu CD,Chen ZG,Miao JJ,Fan ZL,Lin RB,Liu TJ,Wei JC,Kong QP,Lan Y,Fan YJ,Gao FS,Wang TY,Xie D,Chen SQ,Yang WC,Hong JY,Wang L,Qiu SL,Cai ZM,Zhang XJ. Genome-wide association study of esophageal squamous cell carcinoma in Chinese subjects identifies susceptibility loci at PLCE1 and C20orf54. Nat Genet,2010,42(9): 759–763.
[9]Sun LD,Xiao FL,Li Y,Zhou WM,Tang HY,Tang XF,Zhang H,Schaarschmidt H,Zuo XB,Foelster-Holst R,He SM,Shi M,Liu Q,Lv YM,Chen XL,Zhu KJ,Guo YF,Hu DY,Li M,Li M,Zhang YH,Zhang X,Tang JP,Guo BR,Wang H,Liu Y,Zou XY,Zhou FS,Liu XY,Chen G,Ma L,Zhang SM,Jiang AP,Zheng XD,Gao XH,Li P,Tu CX,Yin XY,Han XP,Ren YQ,Song SP,Lu ZY,Zhang XL,Cui Y,Chang J,Gao M,Luo XY,Wang PG,Dai X,Su W,Li H,Shen CP,Liu SX,Feng XB,Yang CJ,Lin GS,Wang ZX,Huang JQ,Fan X,Wang Y,Bao YX,Yang S,Liu JJ,Franke A,Weidinger S,Yao ZR,Zhang XJ. Genome-wide association study identifies two new susceptibility loci for atopic dermatitis in the Chinese Han population. Nat Genet,2011,43(7): 690–694.
[10]Cho YS,Chen CH,Hu C,Long J,Ong RT,Sim X,Takeuchi F,Wu Y,Go MJ,Yamauchi T,Chang YC,Kwak SH,Ma RC,Yamamoto K,Adair LS,Aung T,Cai Q,Chang LC,Chen YT,Gao Y,Hu FB,Kim HL,Kim S,Kim YJ,Lee JJ,Lee NR,Li Y,Liu JJ,Lu W,Nakamura J,Nakashima E,Ng DP,Tay WT,Tsai FJ,Wong TY,Yokota M,Zheng W,Zhang R,Wang C,So WY,Ohnaka K,Ikegami H,Hara K,Cho YM,Cho NH,Chang TJ,Bao Y,Hedman AK,Morris AP,Mccarthy MI,Consortium D,Mu TC,Takayanagi R,Park KS,Jia W,Chuang LM,Chan JC,Maeda S,Kadowaki T,Lee JY,Wu JY,Teo YY,Tai ES,Shu XO,Mohlke KL,Kato N,Han BG,Seielstad M. Meta-analysis of genome-wide association studies identifies eight new loci for type 2 diabetes in east Asians. Nat Genet,2012,44(1): 67–72.
[11]Kooner JS,Saleheen D,Sim X,Sehmi J,Zhang WH,Frossard P,Been LF,Chia KS,Dimas AS,Hassanali N,Jafar T,Jowett JB,Li X,Radha V,Rees SD,Takeuchi F,Young R,Aung T,Basit A,Chidambaram M,Das D,Grundberg E,Hedman AK,Hydrie ZI,Islam M,Khor CC,Kowlessur S,Kristensen MM,Liju S,Lim WY,Matthews DR,Liu J,Morris AP,Nica AC,Pinidiyapathirage JM,Prokopenko I,Rasheed A,Samuel M,Shah N,Shera AS,Small KS,Suo C,Wickremasinghe AR,Wong TY,Yang M,Zhang F,Diagram,Muther,Abecasis GR,Barnett AH,Caulfield M,Deloukas P,Frayling TM,Froguel P,Kato N,Katulanda P,Kelly MA,Liang J,Mohan V,Sanghera DK,Scott J,Seielstad M,Zimmet PZ,Elliott P,Teo YY,Mccarthy MI,Danesh J,Tai ES,Chambers JC. Genome-wide association study in individuals of South Asian ancestry identifies six new type 2 diabetes susceptibility loci. Nat Genet,2011,43(10): 984–989.
[12]Hanson RL,Muller YL,Kobes S,Guo T,Bian L,Ossowski V,Wiedrich K,Sutherland J,Wiedrich C,Mahkee D,Huang K,Abdussamad M,Traurig M,Weil EJ,Nelson RG,Bennett PH,Knowler WC,Bogardus C,Baier LJ. A genome-wide association study in American Indians implicates DNER as a susceptibility locus for type 2 diabetes.Diabetes,2014,63(1): 369–376.
[13]Li HX,Gan W,Lu L,Dong X,Han XY,Hu C,Yang Z,Sun L,Bao W,Li PT,He MN,Sun LD,Wang YQ,Zhu JW,Ning QQ,Tang Y,Zhang R,Wen J,Wang D,Zhu XL,Guo KQ,Zuo XB,Guo XH,Yang HD,Zhou XH,Consortium D,Consortium A-TD,Zhang XJ,Qi L,Loos RJ,Hu FB,Wu TC,Liu Y,Liu LQ,Yang Z,Hu RM,Jia WP,Ji LN,Li YX,Lin X. A genome-wide association study identifies GRK5 and RASGRP1 as type 2 diabetes loci in Chinese Hans. Diabetes,2013,62(1): 291–298.
[14]Pharoah PD,Tsai YY,Ramus SJ,Phelan CM,Goode EL,Lawrenson K,Buckley M,Fridley BL,Tyrer JP,Shen H,Weber R,Karevan R,Larson MC,Song H,Tessier DC,Bacot F,Vincent D,Cunningham JM,Dennis J,Dicks E,Australian Cancer S,Australian Ovarian Cancer Study G,Aben KK,Anton-Culver H,Antonenkova N,Armasu SM,Baglietto L,Bandera EV,Beckmann MW,Birrer MJ,Bloom G,Bogdanova N,Brenton JD,Brinton LA,Brooks-Wilson A,Brown R,Butzow R,Campbell I,Carney ME,Carvalho RS,Chang-Claude J,Chen YA,Chen Z,Chow WH,Cicek MS,Coetzee G,Cook LS,Cramer DW,Cybulski C,Dansonka-Mieszkowska A,Despierre E,Doherty JA,Dork T,Du Bois A,Durst M,Eccles D,Edwards R,Ekici AB,Fasching PA,Fenstermacher D,Flanagan J,Gao YT,Garcia-Closas M,Gentry-Maharaj A,Giles G,Gjyshi A,Gore M,Gronwald J,Guo Q,Halle MK,Harter P,Hein A,Heitz F,Hillemanns P,Hoatlin M,Hogdall E,Hogdall CK,Hosono S,Jakubowska A,Jensen A,Kalli KR,Karlan BY,Kelemen LE,Kiemeney LA,Kjaer SK,Konecny GE,Krakstad C,Kupryjanczyk J,Lambrechts D,Lambrechts S,Le ND,Lee N,Lee J,Leminen A,Lim BK,Lissowska J,Lubinski J,Lundvall L,Lurie G,Massuger LF,Matsuo K,Mcguire V,Mclaughlin JR,Menon U,Modugno F,Moysich KB,Nakanishi T,Narod SA,Ness RB,Nevanlinna H,Nickels S,Noushmehr H,Odunsi K,Olson S,Orlow I,Paul J,Pejovic T,Pelttari LM,Permuth-Wey J,Pike MC,Poole EM,Qu X,Risch HA,Rodriguez-Rodriguez L,Rossing MA,Rudolph A,Runnebaum I,Rzepecka IK,Salvesen HB,Schwaab I,Severi G,Shen H,Shridhar V,Shu XO,Sieh W,Southey MC,Spellman P,Tajima K,Teo SH,Terry KL,Thompson PJ,Timorek A,Tworoger SS,Van Altena AM,Van Den Berg D,Vergote I,Vierkant RA,Vitonis AF,Wang-Gohrke S,Wentzensen N,Whittemore AS,Wik E,Winterhoff B,Woo YL,Wu AH,Yang HP,Zheng W,Ziogas A,Zulkifli F,Goodman MT,Hall P,Easton DF,Pearce CL,Berchuck A,Chenevix-Trench G,Iversen E,Monteiro AN,Gayther SA,Schildkraut JM,Sellers TA. GWAS meta-analysis and replication identifies three new susceptibility loci for ovarian cancer. Nat Genet,2013,45(4): 362–370.
[15]Turner AM. Fifty years on: GWAS confirms the role of a rare variant in lung disease. PLoS Genet,2013,9(8):e1003768.
[16]Yue WH,Wang HF,Sun LD,Tang FL,Liu ZH,Zhang HX,Li WQ,Zhang YL,Zhang Y,Ma CC,Du B,Wang LF,Ren YQ,Yang YF,Hu XF,Wang Y,Deng W,Tan LW,Tan YL,Chen Q,Xu GM,Yang GG,Zuo XB,Yan H,Ruan YY,Lu TL,Han X,Ma XH,Wang Y,Cai LW,Jin C,Zhang HY,Yan J,Mi WF,Yin XY,Ma WB,Liu Q,Kang L,Sun W,Pan CY,Shuang M,Yang FD,Wang CY,Yang JL,Li KQ,Ma X,Li LJ,Yu X,Li QZ,Huang X,Lv LX,Li T,Zhao GP,Huang W,Zhang XJ,Zhang D. Genome-wide association study identifies a susceptibility locus for schizophrenia in Han Chinese at 11p11. 2. Nat Genet,2011,43(12):1228–1231.
[17]Ripke S,O'Dushlaine C,Chambert K,Moran JL,Kähler AK,Akterin S,Bergen SE,Collins AL,Crowley JJ,Fromer M,Kim Y,Lee SH,Magnusson PK,Sanchez N,Stahl EA,Williams S,Wray NR,Xia K,Bettella F,Borglum AD,Bulik-Sullivan BK,Cormican P,Craddock N,De Leeuw C,Durmishi N,Gill M,Golimbet V,Hamshere ML,Holmans P,Hougaard DM,Kendler KS,Lin K,Morris DW,Mors O,Mortensen PB,Neale BM,O'neill FA,Owen MJ,Milovancevic MP,Posthuma D,Powell J,Richards AL,Rileybp,Ruderfer D,Rujescu D,Sigurdsson E,Silagadze T,Smit AB,Stefansson H,Steinberg S,Suvisaari J,Tosato S,Verhage M,Walters JT,Multicenter Genetic Studies of Schizophrenia Consortium,Levinson DF,Gejman PV,Kendler KS,Laurent C,Mowry BJ,O'Donovan MC,Owen MJ,Pulver AE,Rileybp,Schwab SG,Wildenauer DB,Dudbridge F,Holmans P,Shi J,Albus M,Alexander M,Campion D,Cohen D,Dikeos D,Duan J,Eichhammer P,Godard S,Hansen M,Lerer FB,Liang KY,Maier W,Mallet J,Nertney DA,Nestadt G,Norton N,O'Neill FA,Papadimitriou GN,Ribble R,Sanders AR,Silverman JM,Walsh D,Williams NM,Wormley B,Psychosis Endophenotypes International Consortium,Arranz MJ,Bakker S,Bender S,Bramon E,Collier D,Crespo-Facorro B,Hall J,Iyegbe C,Jablensky A,Kahn RS,Kalaydjieva L,Lawrie S,Lewis CM,Lin K,Linszen DH,Mata I,Mcintosh A,Murray RM,Ophoff RA,Powell J,Rujescu D,Van Os J,Walshe M,Weisbrod M,Wiersma D,Wellcome Trust Case Control Consortium 2,Donnelly P,Barroso I,Blackwell JM,Bramon E,Brown MA,Casas JP,Corvin AP,Deloukas P,Duncanson A,Jankowski J,Markus HS,Mathew CG,Palmer CN,Plomin R,Rautanen A,Sawcer SJ,Trembath RC,Viswanathan AC,Wood NW,Spencer CC,Band G,Bellenguez C,Freeman C,Hellenthal G,Giannoulatou E,Pirinen M,Pearson RD,Strange A,Su Z,Vukcevic D,Donnelly P,Langford C,Hunt SE,Edkins S,Gwilliam R,Blackburn H,Bumpstead SJ,Dronov S,Gillman M,Gray E,Hammond N,Jayakumar A,Mccann OT,Liddle J,Potter SC,Ravindrarajah R,Ricketts M,Tashakkori-Ghanbaria A,Waller MJ,Weston P,Widaa S,Whittaker P,Barroso I,Deloukas P,Mathew CG,Blackwell JM,Brown MA,Corvin AP,Mccarthy MI,Spencer CC,Bramon E,Corvin AP,O'Donovan MC,Stefansson K,Scolnick E,Purcell S,Mccarroll SA,Sklar P,Hultman CM,Sullivan PF. Genome-wide association analysis identifies 13 new risk loci for schizophrenia. Nat Genet,2013,45(10): 1150–1159.
[18]Yu H,Bi W,Liu C,Zhao Y,Zhang JF,Zhang D,Yue W.Protein-interaction-network-based analysis for genomewide association analysis of schizophrenia in Han Chinese population. J Psychiatr Res,2014,50: 73-78.
[19]Cruchaga C,Kauwe JSK,Harari O,Jin SC,Cai Y,Karch CM,Benitez BA,Jeng AT,Skorupa T,Carrell D,Bertelsen S,Bailey M,Mckean D,Shulman JM,De Jager PL,Chibnik L,Bennett DA,Arnold SE,Harold D,Sims R,Gerrish A,Williams J,Van Deerlin VM,Lee VMY,Shaw LM,Trojanowski JQ,Haines JL,Mayeux R,Pericak-Vance MA,Farrer LA,Schellenberg GD,Peskind ER,Galasko D,Fagan AM,Holtzman DM,Morris JC,GERAD Consortium,Alzheimer's Disease Neuroimaging Initiative(ADNI),Alzheimer Disease Genetic Consortium (ADGC),Goate AM. GWAS of cerebrospinal fluid tau levels identifies risk variants for Alzheimer's disease. Neuron,2013,78(2): 256–268.
[20]Feugang JM,Kaya A,Page GP,Chen L,Mehta T,Hirani K,Nazareth L,Topper E,Gibbs R,Memili E. Two-stage genome-wide association study identifies integrin beta 5 as having potential role in bull fertility. BMC Genomics,2009,10: 176.
[21]Snelling WM,Allan MF,Keele JW,Kuehn LA,Mcdaneld T,Smith TPL,Sonstegard TS,Thallman RM,Bennett GL.Genome-wide association study of growth in crossbred beef cattle. J Anim Sci,2010,88(3): 837–848.
[22]Hayes BJ,Bowman PJ,Chamberlain AJ,Savin K,Van Tassell CP,Sonstegard TS,Goddard ME. A validated genome wide association study to breed cattle adapted to an environment altered by climate change. PLoS ONE,2009,4(8): e6676.
[23]Pryce JE,Bolormaa S,Chamberlain AJ,Bowman PJ,Savin K,Goddard ME,Hayes BJ. A validated genome-wide association study in 2 dairy cattle breeds for milk production and fertility traits using variable length haplotypes. J Dairy Sci,2010,93(7): 3331–3345.
[24]Jiang L,Liu JF,Sun DX,Ma PP,Ding XD,Yu Y,Zhang Q.Genome wide association studies for milk production traits in Chinese Holstein population. PLoS ONE,2010,5(10): e13661.
[25]Duijvesteijn N,Knol EF,Merks JW,Crooijmans RP,Groenen MA,Bovenhuis H,Harlizius B. A genome-wide association study on androstenone levels in pigs reveals a cluster of candidate genes on chromosome 6. BMC Genet,2010,11: 42.
[26]Onteru SK,Fan B,Du ZQ,Garrick DJ,Stalder KJ,Roth-schild MF. A whole-genome association study for pig reproductive traits. Anim Genet,2012,43(1): 18–26.
[27]Abasht B,Lamont SJ. Genome-wide association analysis reveals cryptic alleles as an important factor in heterosis for fatness in chicken F2 population. Anim Genet,2007,38(5): 491–498.
[28]Gu XR,Feng CG,Ma L,Song C,Wang YQ,Da Y,Li HF,Chen KW,Ye SH,Ge CR,Hu XX,Li N. Genome-wide association study of body weight in chicken F2 resource population. PLoS ONE,2011,6(7): e21872.
[29]Buckler ES,Holland JB,Bradbury PJ,Acharya CB,Brown PJ,Browne C,Ersoz E,Flint-Garcia S,Garcia A,Glaubitz JC,Goodman MM,Harjes C,Guill K,Kroon DE,Larsson S,Lepak NK,Li HH,Mitchell SE,Pressoir G,Peiffer JA,Rosas MO,Rocheford TR,Romay MC,Romero S,Salvo S,Sanchez Villeda H,Da Silva HS,Sun Q,Tian F,Upadyayula N,Ware D,Yates H,Yu JM,Zhang ZW,Kresovich S,Mcmullen MD. The genetic architecture of maize flowering time. Science,2009,325(5941): 714–718.
[30]Tian F,Bradbury PJ,Brown PJ,Hung H,Sun Q,Flint-Garcia S,Rocheford TR,Mcmullen MD,Holland JB,Buckler ES. Genome-wide association study of leaf architecture in the maize nested association mapping population. Nat Genet,2011,43(2): 159–162.
[31]Kump KL,Bradbury PJ,Wisser RJ,Buckler ES,Belcher AR,Oropeza-Rosas MA,Zwonitzer JC,Kresovich S,Mcmullen MD,Ware D,Balint-Kurti PJ,Holland JB. Genome-wide association study of quantitative resistance to southern leaf blight in the maize nested association mapping population. Nat Genet,2011,43(2): 163–168.
[32]Poland JA,Bradbury PJ,Buckler ES,Nelson RJ. Genome-wide nested association mapping of quantitative resistance to northern leaf blight in maize. Proc Natl Acad Sci USA,2011,108(17): 6893–6898.
[33]Zhao KY,Tung CW,Eizenga GC,Wright MH,Ali ML,Price AH,Norton GJ,Islam MR,Reynolds A,Mezey J,Mcclung AM,Bustamante CD,Mccouch SR. Genome-wide association mapping reveals a rich genetic architecture of complex traits in Oryza sativa. Nat Commun,2011,2: 467.
[34]Lango Allen H,Estrada K,Lettre G,Berndt SI,Weedon MN,Rivadeneira F,Willer CJ,Jackson AU,Vedantam S,Raychaudhuri S,Ferreira T,Wood AR,Weyant RJ,Segrè AV,Speliotes EK,Wheeler E,Soranzo N,Park JH,Yang J,Gudbjartsson D,Heard-Costa NL,Randall JC,Qi L,Vernon Smith A,Mägi R,Pastinen T,Liang LM,Heid IM,Luan JA,Thorleifsson G,Winkler TW,Goddard ME,Sin Lo K,Palmer C,Workalemahu T,Aulchenko YS,Johansson A,Zillikens MC,Feitosa MF,Esko T,Johnson T,Ketkar S,Kraft P,Mangino M,Prokopenko I,Absher D,Albrecht E,Ernst F,Glazer NL,Hayward C,Hottenga JJ,Jacobs KB,Knowles JW,Kutalik Z,Monda KL,Polasek O,Preuss M,Rayner NW,Robertson NR,Steinthorsdottir V,Tyrer JP,Voight BF,Wiklund F,Xu J,Zhao JH,Nyholt DR,Pellikka N,Perola M,Perry JR,Surakka I,Tammesoo ML,Altmaier EL,Amin N,Aspelund T,Bhangale T,Boucher G,Chasman DI,Chen C,Coin L,Cooper MN,Dixon AL,Gibson Q,Grundberg E,Hao K,Juhani Junttila M,Kaplan LM,Kettunen J,Konig IR,Kwan T,Lawrence RW,Levinson DF,Lorentzon M,Mcknight B,Morris AP,Muller M,Suh Ngwa J,Purcell S,Rafelt S,Salem RM,Salvi E,Sanna S,Shi J,Sovio U,Thompson JR,Turchin MC,Vandenput L,Verlaan DJ,Vitart V,White CC,Ziegler A,Almgren P,Balmforth AJ,Campbell H,Citterio L,De Grandi A,Dominiczak A,Duan J,Elliott P,Elosua R,Eriksson JG,Freimer NB,Geus EJ,Glorioso N,Haiqing S,Hartikainen AL,Havulinna AS,Hicks AA,Hui J,Igl W,Illig T,Jula A,Kajantie E,Kilpelainen TO,Koiranen M,Kolcic I,Koskinen S,Kovacs P,Laitinen J,Liu J,Lokki ML,Marusic A,Maschio A,Meitinger T,Mulas A,Pare G,Parker AN,Peden JF,Petersmann A,Pichler I,Pietilainen KH,Pouta A,Ridderstrale M,Rotter JI,Sambrook JG,Sanders AR,Schmidt CO,Sinisalo J,Smit JH,Stringham HM,Bragi Walters G,Widen E,Wild SH,Willemsen G,Zagato L,Zgaga L,Zitting P,Alavere H,Farrall M,Mcardle WL,Nelis M,Peters MJ,Ripatti S,Van Meurs JB,Aben KK,Ardlie KG,Beckmann JS,Beilby JP,Bergman RN,Bergmann S,Collins FS,Cusi D,Den Heijer M,Eiriksdottir G,Gejman PV,Hall AS,Hamsten A,Huikuri HV,Iribarren C,Kahonen M,Kaprio J,Kathiresan S,Kiemeney L,Kocher T,Launer LJ,Lehtimaki T,Melander O,Mosley TH,Jr.,Musk AW,Nieminen MS,O'donnell CJ,Ohlsson C,Oostra B,Palmer LJ,Raitakari O,Ridker PM,Rioux JD,Rissanen A,Rivolta C,Schunkert H,Shuldiner AR,Siscovick DS,Stumvoll M,Tonjes A,Tuomilehto J,Van Ommen GJ,Viikari J,Heath AC,Martin NG,Montgomery GW,Province MA,Kayser M,Arnold AM,Atwood LD,Boerwinkle E,Chanock SJ,Deloukas P,Gieger C,Gronberg H,Hall P,Hattersley AT,Hengstenberg C,Hoffman W,Lathrop GM,Salomaa V,Schreiber S,Uda M,Waterworth D,Wright AF,Assimes TL,Barroso I,Hofman A,Mohlke KL,Boomsma DI,Caulfield MJ,Cupples LA,Erdmann J,Fox CS,Gudnason V,Gyllensten U,Harris TB,Hayes RB,Jarvelin MR,Mooser V,Munroe PB,Ouwehand WH,Penninx BW,Pramstaller PP,Quertermous T,Rudan I,Samani NJ,Spector TD,Volzke H,Watkins H,Wilson JF,Groop LC,Haritunians T,Hu FB,Kaplan RC,Metspalu A,North KE,Schlessinger D,Wareham NJ,Hunter DJ,O'connell JR,Strachan DP,Wichmann HE,Borecki IB,Van Duijn CM,Schadt EE,Thorsteinsdottir U,Peltonen L,Uitterlinden AG,Visscher PM,Chatterjee N,Loos RJ,Boehnke M,Mccarthy MI,Ingelsson E,Lindgren CM,Abecasis GR,Stefansson K,Frayling TM,Hirschhorn JN. Hundreds of variants clustered in genomic loci and biological pathways affect human height. Nature,2010,467(7317): 832–838.
[35]Dickson SP,Wang K,Krantz I,Hakonarson H,Goldstein DB. Rare variants create synthetic genome-wide associations. PLoS Biol,2010,8(1): e1000294.
[36]Nejentsev S,Walker N,Riches D,Egholm M,Todd JA.Rare variants of IFIH1,a gene implicated in antiviral responses,protect against type 1 diabetes. Science,2009,324(5925): 387–389.
[37]Sanna S,Jackson AU,Nagaraja R,Willer CJ,Chen WM,Bonnycastle LL,Shen HQ,Timpson N,Lettre G,Usala G,Chines PS,Stringham HM,Scott LJ,Dei M,Lai S,Albai G,Crisponi L,Naitza S,Doheny KF,Pugh EW,Ben-Shlomo Y,Ebrahim S,Lawlor DA,Bergman RN,Watanabe RM,Uda M,Tuomilehto J,Coresh J,Hirschhorn JN,Shuldiner AR,Schlessinger D,Collins FS,Davey Smith G,Boerwinkle E,Cao A,Boehnke M,Abecasis GR,Mohlke KL. Common variants in the GDF5-UQCC region are associated with variation in human height. Nat Genet,2008,40(2): 198–203.
[38]Weedon MN,Lango H,Lindgren CM,Wallace C,Evans DM,Mangino M,Freathy RM,Perry JR,Stevens S,Hall AS,Samani NJ,Shields B,Prokopenko I,Farrall M,Dominiczak A,Diabetes Genetics Initiative,The Wellcome Trust Case Control Consortium,Johnson T,Bergmann S,Beckmann JS,Vollenweider P,Waterworth DM,Mooser V,Palmer CN,Morris AD,Ouwehand WH,Cambridge GEM Consortium,Zhao JH,Li S,Loos RJ,Barroso I,Deloukas P,Sandhu MS,Wheeler E,Soranzo N,Inouye M,Wareham NJ,Caulfield M,Munroe PB,Hattersley AT,Mccarthy MI,Frayling TM. Genome-wide association analysis identifies 20 loci that influence adult height. Nat Genet,2008,40(5): 575–583.
[39]Yang J,Benyamin B,Mcevoybp,Gordon S,Henders AK,Nyholt DR,Madden PA,Heath AC,Martin NG,Montgomery GW,Goddard ME,Visscher PM. Common SNPs explain a large proportion of the heritability for human height. Nat Genet,2010,42(7): 565–569.
[40]1000 Genomes Project Consortium,Abecasis GR,Auton A,Brooks LD,Depristo MA,Durbin RM,Handsaker RE,Kang HM,Marth GT,Mcvean GA. An integrated map of genetic variation from 1,092 human genomes. Nature,2012,491(7422): 56–65.
[41]Tennessen JA,Bigham AW,O'Connor TD,Fu WQ,Kenny EE,Gravel S,Mcgee S,Do R,Liu XM,Jun G,Kang HM,Jordan D,Leal SM,Gabriel S,Rieder MJ,Abecasis G,Altshuler D,Nickerson DA,Boerwinkle E,Sunyaev S,Bustamante CD,Bamshad MJ,Akey JM,Broad GO,Seattle GO,on behalf of the NHLBI Exome Sequencing Project. Evolution and functional impact of rare coding variation from deep sequencing of human exomes. Science,2012,337(6090): 64–69.
[42]Fu W,O'Connor TD,Jun G,Kang HM,Abecasis G,Leal SM,Gabriel S,Rieder MJ,Altshuler D,Shendure J,Nickerson DA,Bamshad MJ,NHLBI Exome Sequencing Project,Akey JM. Analysis of 6,515 exomes reveals the recent origin of most human protein-coding variants. Nature,2013,493(7431): 216–220.
[43]Wray NR,Purcell SM,Visscher PM. Synthetic associations created by rare variants do not explain most GWAS results. PLoS Biol,2011,9(1): e1000579.
[44]Manolio TA,Collins FS,Cox NJ,Goldstein DB,Hindorff LA,Hunter DJ,Mccarthy MI,Ramos EM,Cardon LR,Chakravarti A,Cho JH,Guttmacher AE,Kong A,Kruglyak L,Mardis E,Rotimi CN,Slatkin M,Valle D,Whittemore AS,Boehnke M,Clark AG,Eichler EE,Gibson G,Haines JL,Mackay TF,Mccarroll SA,Visscher PM.Finding the missing heritability of complex diseases. Nature,2009,461(7265): 747–753.
[45]Mccarthy MI,Abecasis GR,Cardon LR,Goldstein DB,Little J,Ioannidis JPA,Hirschhorn JN. Genome-wide association studies for complex traits: consensus,uncertainty and challenges. Nat Rev Genet,2008,9(5): 356–369.
[46]Kiezun A,Garimella K,Do R,Stitziel NO,Neale BM,Mclaren PJ,Gupta N,Sklar P,Sullivan PF,Moran JL,Hultman CM,Lichtenstein P,Magnusson P,Lehner T,Shugart YY,Price AL,De Bakker PI,Purcell SM,Sunyaev SR. Exome sequencing and the genetic basis of complex traits. Nat Genet,2012,44(6): 623–630.
[47]Bras JM,Singleton AB. Exome sequencing in Parkinson's disease. Clin Genet,2011,80(2): 104–109.
[48]Ng SB,Turner EH,Robertson PD,Flygare SD,Bigham AW,Lee C,Shaffer T,Wong M,Bhattacharjee A,Eichler EE,Bamshad M,Nickerson DA,Shendure J. Targeted capture and massively parallel sequencing of 12 human exomes. Nature,2009,461(7261): 272–276.
[49]Ng SB,Buckingham KJ,Lee C,Bigham AW,Tabor HK,Dent KM,Huff CD,Shannon PT,Jabs EW,Nickerson DA,Shendure J,Bamshad MJ. Exome sequencing identifies the cause of a mendelian disorder. Nat Genet,2010,42(1):30–35.
[50]Tang HY,Jin X,Li Y,Jiang H,Tang XF,Yang X,Cheng H,Qiu Y,Chen G,Mei JP,Zhou FS,Wu RH,Zuo XB,Zhang Y,Zheng XD,Cai Q,Yin XY,Quan C,Shao HJ,Cui Y,Tian FZ,Zhao X,Liu H,Xiao FL,Xu FP,Han JW,Shi DM,Zhang AP,Zhou C,Li QB,Fan X,Lin LY,Tian HQ,Wang ZX,Fu HL,Wang F,Yang BQ,Huang SW,Liang B,Xie XF,Ren YQ,Gu QQ,Wen GD,Sun YL,Wu XL,Dang L,Xia M,Shan JJ,Li TH,Yang L,Zhang XY,Li YZ,He CD,Xu A,Wei LP,Zhao XH,Gao XH,Xu JH,Zhang FR,Zhang JZ,Li YR,Sun LD,Liu JJ,Chen RS,Yang S,Wang J,Zhang XJ. A large-scale screen for coding variants predisposing to psoriasis. Nat Genet,2014,46(1): 45–50.
[51]Lange LA,Hu YN,Zhang H,Xue CY,Schmidt EM,Tang ZZ,Bizon C,Lange EM,Smith JD,Turner EH,Jun G,Kang HM,Peloso G,Auer P,Li KP,Flannick J,Zhang J,Fuchsberger C,Gaulton K,Lindgren C,Locke A,Manning A,Sim XL,Rivas MA,Holmen OL,Gottesman O,Lu YC,Ruderfer D,Stahl EA,Duan Q,Li Y,Durda P,Jiao S,Isaacs A,Hofman A,Bis JC,Correa A,Griswold ME,Jakobsdottir J,Smith AV,Schreiner PJ,Feitosa MF,Zhang Q,Huffman JE,Crosby J,Wassel CL,Do R,Franceschini N,Martin LW,Robinson JG,Assimes TL,Crosslin DR,Rosenthal EA,Tsai M,Rieder MJ,Farlow DN,Folsom AR,Lumley T,Fox ER,Carlson CS,Peters U,Jackson RD,Van Duijn CM,Uitterlinden AG,Levy D,Rotter JI,Taylor HA,Gudnason V Jr,Siscovick DS,Fornage M,Borecki IB,Hayward C,Rudan I,Chen YE,Bottinger EP,Loos RJ,Saetrom P,Hveem K,Boehnke M,Groop L,Mccarthy M,Meitinger T,Ballantyne CM,Gabriel SB,O'donnell CJ,Post WS,North KE,Reiner AP,Boerwinkle E,Psaty BM,Altshuler D,Kathiresan S,Lin DY,Jarvik GP,Cupples LA,Kooperberg C,Wilson JG,Nickerson DA,Abecasis GR,Rich SS,Tracy RP,Willer CJ,NHLBI Grand Opportunity Exome Sequencing Project.Whole-exome sequencing identifies rare and low-frequency coding variants associated with LDL cholesterol.Am J Hum Genet,2014,94(2): 233–245.
[52]Choi M,Scholl UI,Ji WZ,Liu TW,Tikhonova IR,Zumbo P,Nayir A,Bakkaloğlu A,Özen S,Sanjad S,Nelson-Williams C,Farhi A,Mane S,Lifton RP. Genetic diagnosis by whole exome capture and massively parallel DNA sequencing. Proc Natl Acad Sci USA,2009,106(45):19096–19101.
[53]Bowden DW,An SS,Palmer ND,Brown WM,Norris JM,Haffner SM,Hawkins GA,Guo XQ,Rotter JI,Chen YD,Wagenknecht LE,Langefeld CD. Molecular basis of a linkage peak: exome sequencing and family-based analysis identify a rare genetic variant in the ADIPOQ gene in the IRAS Family Study. Hum Mol Genet,2010,19(20):4112–4120.
[54]Bilgüvar K,Öztürk AK,Louvi A,Kwan KY,Choi M,Tatli B,Yalnizoğlu D,Tüysüz B,Çağlayan AO,Gökben S,Kaymakçalan H,Barak T,Bakircioğlu M,Yasuno K,Ho W,Sanders S,Zhu Y,Yilmaz S,Dincer A,Johnson MH,Bronen RA,Koçer N,Per H,Mane S,Pamir MN,Yalçinkaya C,Kumandaş S,Topçu M,Özmen M,Šestan N,Lifton RP,State MW,Günel M. Whole-exome sequencing identifies recessive WDR62 mutations in severe brain malformations. Nature,2010,467(7312): 207–210.
[55]Stitziel NO,Kiezun A,Sunyaev S. Computational and statistical approaches to analyzing variants identified by exome sequencing. Genome Biol,2011,12(9): 227.
[56]Wu TT,Chen YF,Hastie T,Sobel E,Lange K. Genome-wide association analysis by lasso penalized logistic regression. Bioinformatics,2009,25(6): 714–721.
[57]Morgenthaler S,Thilly WG. A strategy to discover genes that carry multi-allelic or mono-allelic risk for common diseases: a cohort allelic sums test (CAST). Mutat Res,2007,615(1–2): 28–56.
[58]Li BS,Leal SM. Methods for detecting associations with rare variants for common diseases: application to analysis of sequence data. Am J Hum Genet,2008,83(3): 311–321.
[59]Price AL,Kryukov GV,De Bakker PI,Purcell SM,Staples J,Wei LJ,Sunyaev SR. Pooled association tests for rare variants in exon-resequencing studies. Am J Hum Genet,2010,86(6): 832–838.
[60]Bhatia G,Bansal V,Harismendy O,Schork NJ,Topol EJ,Frazer K,Bafna V. A covering method for detecting genetic associations between rare variants and common phenotypes. PLoS Comput Biol,2010,6(10): e1000954.
[61]Madsen BE,Browning SR. A groupwise association test for rare mutations using a weighted sum statistic. PLoS Genet,2009,5(2): e1000384.
[62]Liu DJ,Leal SM. A novel adaptive method for the analysis of next-generation sequencing data to detect complex trait associations with rare variants due to gene main effects and interactions. PLoS Genet,2010,6(10): e1001156.
[63]Zheng HF,Ladouceur M,Greenwood CMT,Richards JB.Effect of genome-wide genotyping and reference panels on rare variants imputation. J Genet Genomics,2012,39(10): 545–550.
[64]1000 Genomes Project Consortium,Abecasis GR,Altshuler D,Auton A,Brooks LD,Durbin RM,Gibbs RA,Hurles ME,Mcvean GA. A map of human genome variation from population-scale sequencing. Nature,2010,467(7319):1061–1073.
[65]Pasaniuc B,Rohland N,Mclaren PJ,Garimella K,Zaitlen N,Li H,Gupta N,Neale BM,Daly MJ,Sklar P,Sullivan PF,Bergen S,Moran JL,Hultman CM,Lichtenstein P,Magnusson P,Purcell SM,Haas DW,Liang LM,Sunyaev S,Patterson N,De Bakker PI,Reich D,Price AL. Extremely low-coverage sequencing and imputation increases power for genome-wide association studies. Nat Genet,2012,44(6): 631–635.
[66]Howie B,Fuchsberger C,Stephens M,Marchini J,Abecasis GR. Fast and accurate genotype imputation in genome-wide association studies through pre-phasing. Nat Genet,2012,44(8): 955–959.
[67]Rohland N,Reich D. Cost-effective,high-throughput DNA sequencing libraries for multiplexed target capture.Genome Res,2012,22(5): 939–946.
[68]Robasky K,Lewis NE,Church GM. The role of replicates for error mitigation in next-generation sequencing. Nat Rev Genet,2014,15(1): 56–62.
[69]Yang SZ,Wang K,Gregory B,Berrettini W,Wang LS,Hakonarson H,Bucan M. Genomic landscape of a three-generation pedigree segregating affective disorder.PLoS ONE,2009,4(2): e4474.
[70]Sobreira NLM,Cirulli ET,Avramopoulos D,Wohler E,Oswald GL,Stevens EL,Ge DL,Shianna KV,Smith JP,Maia JM,Gumbs CE,Pevsner J,Thomas G,Valle D,Hoover-Fong JE,Goldstein DB. Whole-genome sequencing of a single proband together with linkage analysis identifies a Mendelian disease gene. PLoS Genet,2010,6(6): e1000991.
[71]Lander ES,Botstein D. Mapping mendelian factors underlying quantitative traits using RFLP linkage maps. Genetics,1989,121(1): 185–199.
[72]Manolio TA,Brooks LD,Collins FS. A HapMap harvest of insights into the genetics of common disease. J Clin Invest,2008,118(5): 1590–1605.
[73]Verlaan DJ,Ge B,Grundberg E,Hoberman R,Lam KC,Koka V,Dias J,Gurd S,Martin NW,Mallmin H,Nilsson O,Harmsen E,Dewar K,Kwan T,Pastinen T. Targeted screening of cis-regulatory variation in human haplotypes.Genome Res,2009,19(1): 118–127.
[74]Cirulli ET,Goldstein DB. Uncovering the roles of rare variants in common disease through whole-genome sequencing. Nat Rev Genet,2010,11(6): 415–425.
[75]Sikkema-Raddatz B,Johansson LF,De Boer EN,Almomani R,Boven LG,Van Den Berg MP,Van Spaendonck-Zwarts KY,Van Tintelen JP,Sijmons RH,Jongbloed JDH,Sinke RJ. Targeted next-generation sequencing can replace Sanger sequencing in clinical diagnostics. Hum Mutat,2013,34(7): 1035–1042.
[76]Kofler R,Pandey RV,Schlötterer C. PoPoolation2: identifying differentiation between populations using sequencing of pooled DNA samples (Pool-Seq). Bioinformatics,2011,27(24): 3435–3436.
[77]Huang XH,Wei XH,Sang T,Zhao Q,Feng Q,Zhao Y,Li CY,Zhu CR,Lu TT,Zhang ZW,Li M,Fan DL,Guo YL,Wang AH,Wang L,Deng LW,Li WJ,Lu YQ,Weng QJ,Liu KY,Huang T,Zhou TY,Jing YF,Li W,Lin Z,Buckler ES,Qian Q,Zhang QF,Li JY,Han B. Genome-wide association studies of 14 agronomic traits in rice landraces.Nat Genet,2010,42(11): 961–967.
[78]Li H,Peng ZY,Yang XH,Wang WD,Fu JJ,Wang JH,Han YJ,Chai YC,Guo TT,Yang N,Liu J,Warburton ML,Cheng YB,Hao XM,Zhang P,Zhao JY,Liu YJ,Wang GY,Li JS,Yan JB. Genome-wide association study dissects the genetic architecture of oil biosynthesis in maize kernels.Nat Genet,2013,45(1): 43–50.
[79]Johnson JA. Pharmacogenomics of antihypertensive drugs:past,present and future. Pharmacogenomics,2010,11(4):487–491.
[80]Thoenes M,Neuberger HR,Volpe M,Khan BV,Kirch W,Böhm M. Antihypertensive drug therapy and blood pressure control in men and women: an international perspective. J Hum Hypertens,2010,24(5): 336–344.
[81]Turner ST,Bailey KR,Fridley BL,Chapman AB,Schwartz GL,Chai HS,Sicotte H,Kocher JP,Rodin AS,Boerwinkle E. Genomic association analysis suggests chromosome 12 locus influencing antihypertensive response to thiazide diuretic. Hypertension,2008,52(2): 359–365.
[82]Johnson JA,Boerwinkle E,Zineh I,Chapman AB,Bailey K,Cooper-Dehoff RM,Gums J,Curry RW,Gong Y,Beitelshees AL,Schwartz G,Turner ST. Pharmacogenomics of antihypertensive drugs: rationale and design of the Pharmacogenomic Evaluation of Antihypertensive Responses (PEAR) study. Am Heart J,2009,157(3): 442–449.
