Clinical, radiological and molecular diagnosis correlation in serum samples from patients with osteoarticular tuberculosis
2014-03-23GuadalupeGarcElorriagaOlgaMartnezElizondoGuillermodelReyPinedasarGonzlezBonilla
Guadalupe García-Elorriaga, Olga Martínez-Elizondo, Guillermo del Rey-Pineda, César González-Bonilla
1Unidad de Investigación Médica en Inmunología e Infectología, Hospital de Infectología, Centro Médico Nacional La Raza (CMNR), Instituto Mexicano del Seguro Social (IMSS), Mexico City, Mexico (Medical Research Unit in Immunology and Infectious Disease, Hospital for Infectious Disease, “La Raza”National Medical Center (CMNR), Mexican Social Security Institute (IMSS), Mexico City, Mexico)
2Hospital de Ortopedia “Dr. Victorio de la Fuente Narváez”, Instituto Mexicano del Seguro Social (IMSS), Mexico City, Mexico (Orthopedics Hospital“Dr. Victorio de la Fuente Narváez”, Mexican Social Security Institute (IMSS), Mexico City, Mexico)
3Banco Central de Sangre, CMNR, IMSS, and Departamento de Infectología, Hospital Infantil de México Federico Gómez. Secretaría de Salud (SSA), Mexico City, Mexico (Central Blood Bank, CMNR, IMSS and Department of Infectious Disease “Federico Gómez” Children’s Hospital. Health Ministry (SSA), Mexico City, Mexico)
Clinical, radiological and molecular diagnosis correlation in serum samples from patients with osteoarticular tuberculosis
Guadalupe García-Elorriaga1*, Olga Martínez-Elizondo2, Guillermo del Rey-Pineda3, César González-Bonilla1
1Unidad de Investigación Médica en Inmunología e Infectología, Hospital de Infectología, Centro Médico Nacional La Raza (CMNR), Instituto Mexicano del Seguro Social (IMSS), Mexico City, Mexico (Medical Research Unit in Immunology and Infectious Disease, Hospital for Infectious Disease, “La Raza”National Medical Center (CMNR), Mexican Social Security Institute (IMSS), Mexico City, Mexico)
2Hospital de Ortopedia “Dr. Victorio de la Fuente Narváez”, Instituto Mexicano del Seguro Social (IMSS), Mexico City, Mexico (Orthopedics Hospital“Dr. Victorio de la Fuente Narváez”, Mexican Social Security Institute (IMSS), Mexico City, Mexico)
3Banco Central de Sangre, CMNR, IMSS, and Departamento de Infectología, Hospital Infantil de México Federico Gómez. Secretaría de Salud (SSA), Mexico City, Mexico (Central Blood Bank, CMNR, IMSS and Department of Infectious Disease “Federico Gómez” Children’s Hospital. Health Ministry (SSA), Mexico City, Mexico)
PEER REVIEW
Peer reviewer
Dr. Narayan D. Chaurasiya, National Center for Natural Products Research, Research Institute of Pharmaceutical Sciences, School of Pharmacy, University of Mississippi, University, MS 38677, USA.
Tel: 662-202-6317 (Cell), 662-915-1364 (Office)
E-mail: narayan.chaurasiya@gmail. com
Comments
This is a very useful study, in which authors have evaluated the diagnosis techniques for detection of TB patients. The results obtained in this work clearly suggested that diagnosis technique has been designed to detect early stage TB patients.
Details on Page 584
Objective:To assess the role of polymerase chain reaction (PCR) in serum samples, in the diagnosis of osteoarticular tuberculosis (OTB) in a setting where only clinical and imaging diagnoses determine the treatment.
Osteoarticular tuberculosis, Molecular diagnosis, Nested polymerase chain reaction, Sensitivity, Specificity
1. Introduction
Osteoarticular tuberculosis (OTB) accounts for about 10%-15% of all tuberculosis (TB) notifications in the nonindustrialised world. However, in the Western world, OTB tends to be uncommon and accounts for about only 1%-2% of all cases of TB and about 10%-15% of extrapulmonary TB[1]. Tuberculous spondylodiscitis or Pott’s disease is the most common form of osteoarticular involvement[2]. The infection begins in sub-chondral bone and slowly spreads to theintervertebral disk space and the adjacent vertebral bodies, leading to signs of destruction and inflammation. Tuberculous spondylodiscitis affects about 50% of all patients with OTB. Tuberculous lesions often involves the intervertebral disc and the endplates of the adjacent superior and inferior vertebral bodies, although the posterior column is usually spared; a kyphotic deformity is often the result of severe destruction[3,4]. In most cases of tuberculous spondylodiscitis, only one vertebral segment is affected, particularly the last dorsal and the lumbar vertebrae. The natural history of articular disease evolves over several years from localized synovitis to joint destruction and the prognosis is related to the stage of disease at presentation. Lumbar pain is the major symptom of tuberculous spondylitis. The symptoms of tuberculous bone and joint infections are nonspecific, and the clinical course is often indolent, which usually lead to significant delays in diagnosis and resultant bone or joint destruction[5].
The microbiological diagnosis is a problem in some parts of the world. TB is a multi-faceted disease, the key to diagnosing OTB hinges on a strong clinical suspicion. Although some typical radiological findings exist, TB may be an excellent imitator and no single pathognomonic finding can differentiate it from other pathologies[6].
TB, a systemic disease, should always be kept in mind among the differential diagnoses of any unusual radiographic spinal abnormality, especially when other organs are involved. Fine needle aspiration cytology may provide the correct diagnosis[7]. Diagnostic reasoning is a process by which we confirm or exclude a diagnosis. The traditional medical model includes a clinical history, examination, investigation and treatment, but reality tends to differ. What we really do is to propose a diagnostic hypothesis which is refined through a process of exclusion and confirmation in order to reach a final diagnosis. Radiological assessment is often the first step in the diagnostic workup of patients with OTB and further investigations are determined by the findings of radiography. Both the radiologist and the clinician should be aware of this diagnostic possibility. Magnetic resonance imaging (MRI) is a very sensitive and useful ancillary technique in the differential diagnosis of spondylodiscitis, but in order to confirm whether it is tuberculous in origin, either the microorganism or the typical tuberculous granulomatous lesion need to be identified[8].
Extraspinal OTB favors large joints (hip and knee)[9]. TB of the hip joint accounts for about 15% of cases[10]. The knee is affected in approximately 8% of cases[11]. The nonspecific, often indolent, clinical presentation of extra spinal OTB whose low prevalence and low index of suspicion among clinicians may result in diagnostic delay. However, prompt diagnosis and treatment of this curable disease remains critical for initiation of proper management and prevention of joint deformity and permanent bone destruction.Mycobacterium tuberculosis (M. tuberculosis)is the main causative organism and only a few cases are attributable toMycobacterium bovis[9]. OTB mostly results from hematogenous dissemination of mycobacteria or lymphatic spread from a primary or reactivated focus of infection[12].
Treatment of tuberculous spondylodiscitis is fundamentally medical and should be initiated as early as possible. Treatment consists of bed rest, placement of a corset at the affected vertebral segment and the administration of the usual tuberculostatic drugs used in pulmonary tuberculous infection: isoniazid (300 mg/d), rifampicin (600 mg/d), pyrazinamide (1 500 mg/d) and/or ethambutol (1 200 mg/d), if no resistance or hypersensitivity to these drugs is detected[2,13]. There are different recommendations for treatment duration ranging from 6 to 12 and even 15 months[13].
The diagnosis of OTB in endemic areas is based on clinical experience and radiography. Not every patient presentthe classical picture. OTB is a paucibacillary disease, so bacteriological diagnosis is possible in only 10%-30% of cases. In order to contribute our experience and better understand this disease, we conducted a prospective study of all OTB cases diagnosed in the Hospital de Ortopedia in about four years (May, 2004 to February, 2008). This study was undertaken to correlate clinical and radiological diagnoses with molecular diagnosis in serum samples. Since most patients had bone destruction, it was not possible to carry out invasive procedures and obtain biological material.
2. Material and methods
Forty four consecutive serum specimens were collected from patients diagnosed with OTB clinically and radiologically [X-ray or MRI/computed tomography (CT)], with involvement of the dorsal spine (n=33), hip (n=7) and knee (n=4) (Table 1). Thirty nine specimens collected from patients suffering from other bone diseases of nontuberculous origin were included as negative controls. They were screened by in-house nested polymerase chain reaction (PCR), and only a few specimens obtained from OTB patients such as tissue/pus were examined by Gram and acid fast bacilli (AFB) stain, histopathology and routine bacterial culture, since most cases had associated with bone destruction [37/44 (84%)]; therefore, it was not possible to conduct invasive procedures and obtain biological material in most cases. Biopsies were performed in a few cases to confirm OTB, especially by the presence of caseation necrosis, epithelioid cell granulomas and multinuclear giant cells.
Referred symptoms and laboratory markers included localized pain (with or without neurologic impairment), joint stiffness and swelling, low grade fever, sweating, chills, weight/appetite loss, malaise, cough, breathlessness, tenderness, effusion, restriction of movements, osseous vertebral destruction, caseum necrosis and an elevated erythrocyte sedimentation rate; a positive family history of pulmonary TB, a positive history of previous TB diagnosed by X-ray or MRI/(CT) and possible contact information were also recorded.
All serum specimens were stored at 4 °C for PCR, and the rest were immediately processed as follows:
(1) Smears: Bacterial smears (i.e., Gram stain, AFB) were obtained in a few cases, and aerobic and anaerobic cultures were processed according to standard procedures.
(2) PCR: DNA extraction. PCR: the mycobacterial strain used as a positive control wasM. tuberculosisH37Rv. DNA was isolated with guanidine isothiocyanate and phenol, utilizing 500 µL of the TRIzol reagent (Gibco, BRL) according to the procedure described by Chomczynski[14]. Specimens were processed in the same way. DNA was resuspended in 50 µL of distilled water after precipitation with 75% ethanol. This solution was heated at 55 °C for 20 min. We determined its absorbance at 260/280 nm and took 5 µL for gene amplification by PCR, coding for the 32-kDa protein[15], the MTP40 species-specific protein[16], and the IS6110 sequence insertion[17]. The initiator sequences employed (Accesolab, Invitrogen. Life Technologies) to amplify the species-specific gene included prothrombin time (PT)1 (5′CAA CGC GCC GTC GGT GG) and PT2 (5′CCC CCC ACG GCA CCG CCG GG), with a resulting fragment of 396 bp[18]. For IS6110 insertion-element amplification, the specific MercatoBenzoThiazole-complex initiators were IS5 (5’CGG AGA CGG TGC GTA AGT GG) and IS6 (5′GAT GGA CCG CCA GGG CTT GC), with a 984-bp amplification fragment. The specific initiators amplifying the gene coding for the α 32-kDa antigen, present in all described mycobacteria (genusspecific) were MT1 (5′TTC CTG ACC AGC GAG CTG CCG) and MT2 (5′CCC CAG TAC TCC CAG CTG TGC), with a 506-bp amplification fragment[19-22]. All reactions were taken to a final volume of 50 µL containing 100 ng of purified DNA of the reference strain as well as of each clinical specimen, reaction buffer 1×, 2.5 IU ofTaqpolymerase (Promega), 0.2 mmol/L of each triphosphate deoxynucleotide, and 20 pmol/L of each of the three pairs of initiators. The reaction was carried out in a thermocycler (Biometra). Controls were included in every batch. Cycles included initial denaturation at 94 °C for 5 min, followed by 35 repeated denaturation cycles at 94 °C for 1 min, annealed at 71 °C for 2 min, and extended at 72 °C for 3 min. Subsequently, a final extension was carried out at 72 °C for 10 min.
To increase amplification sensitivity, we conducted nested PCR, amplifying an internal segment of the specific gene designed by Del Portillo[18]; internal initiators in the second PCR corresponded to nucleosides 44-65 (PT3, 5′-CAC CAC GTT AGG GAT GCA CTG C-3′) and 244-265 (PT4, 5′-CTG ATG GTC TCC GAC ACG TTC G-3′), which amplified the 223-bp internal region[23]. For this second step, we took 5 µL of the multiplex PCR product and transferred it into 45 µL of the pre-mixed solution containing the PCR reagents at the same previously described concentration[15]. Controls were included in every batch. Amplification was repeated for 30 cycles with the same time and temperature parameters as described previously, except for annealing at 75 °C for 2 min, an extension at 72 °C for 2 min, and a final extension at 72 °C for 7 min. After the 1/10 amplification of the PCR reaction mixture, it was analyzed by electrophoresis in 1.5% agarose and 0.5 µg/mL ethidium bromide and visualized with an ultraviolet transilluminator.
We began the study upon approval of the Ethics and Research Committee of the Hospital de Infectología and with the patients’ signed consent.
3. Results
The mean age of the patients included in this study was 52.7 years (ranging from 28 to 77 years) (Table 1).
The clinical diagnosis was based on the presence of rachialgia (pain in the spine) following an inflammatory pattern, limitation of movement and imaging findings (MRI/ CT/X-ray).
As described in Table 2, of 7 clinical TB specimens, 14% were AFB smear positive and 86% were smear negative. In smear positive specimen, the number of bacilli per highpower field was less than 10, confirming the paucibacillary nature of these samples. Histopathology was suggestive of TB in 86% specimens and all cases had histological features suggestive of OTB, confirmed by the presence of caseation necrosis, epithelioid cell granulomas and Langerhans giant cells. In 39% of patients of whom OTB was highly suspected clinically, imaging studies (MRI/CT/X-ray) were suggestive of TB.
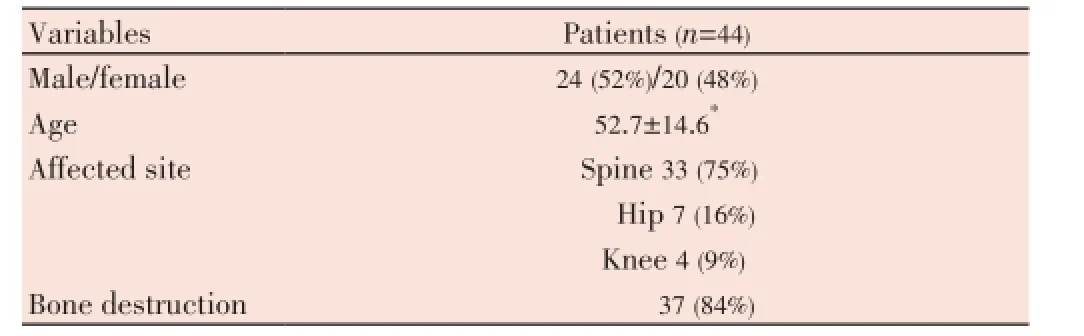
Table 1 Characteristics of osteoarticular tuberculosis patients.

Table 2 Results obtained by 4 different methods.
Thirty nine negative controls were negative by inhouse nested PCR. In-house nested PCR validated 91% of all specimens as positive. Considering clinical diagnosis as the diagnostic gold standard, overall sensitivity and specificity of our in-house nested PCR was 90.9% and 97.4%, respectively; positive predictive value was 97.5% and negative predictive value was 90.4% (Table 3).
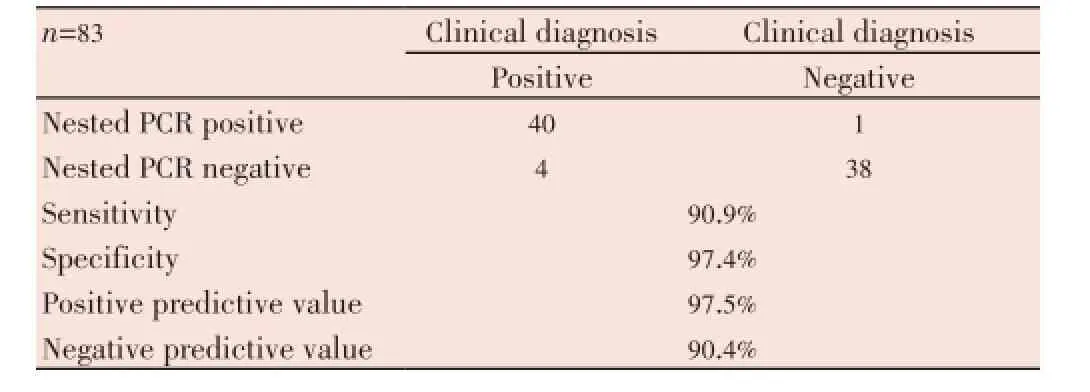
Table 3 Comparison clinical diagnosis confirmation with nested PCR.
An ethidium bromide stained gel ofM. tuberculosisDNA amplified by the designed multiprimer system is shown in Figure 1A, lane 2. As expected, three bands, corresponding to a 396-bp fragment resulting from the amplification of the species-specific MTP40 gene, to a 984-bp amplification fragment from the IS6110 insertion sequence present in all mycobacteria from the complex, and to a 506-bp amplification fragment from the alpha antigen gene common to all described mycobacteria, were observed. In Figure 1B, lane 2, 3 and 4, are shown as a band, corresponding to a 223-bp fragment resulting from the amplification in nested PCR, amplifying an internal segment of the specific gene MTP40.
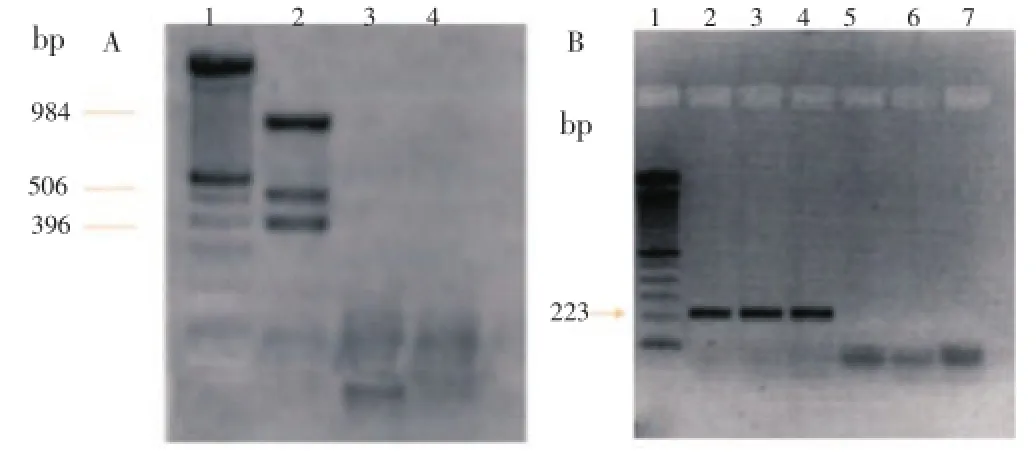
Figure 1. PCR electrophoresis. A. Amplification with multiprimer PCR. Lane 1: 100 bp (Promega) marker; Lane 2: M. tuberculosis DNA (positive control); Lane 3: Negative sample; Lane 4: Water (negative control). B. Amplification with nested PCR. Lane 1: 100 bp marker (Promega); Lane 2: M. tuberculosis DNA (positive control); Lanes 3 and 4: Positive samples; Lanes 5 and 6: Negative samples; Lane 7: Water (negative control).
In our series, therapy was administered following the usual standardized guidelines for 12 months, plus ethambutol during the first 2 months of treatment; the patients’ clinical course was good and no complications developed in 39 (89%) of cases.
4. Discussion
The mean age of the patients included in this study was 52.7 years, coinciding with that reported in other studies where the reported average age was 55[24,25].
Our study group had a higher percentage of spondylodiscitis than that reported in the literature[3,4,26]. We did, however, concur with previously reported data on the incidence of extra spinal TB[9-11].
Among the patients in whom we obtained biopsy material/ pus, 6/7 samples were positive by imaging studies, histopathology and PCR. AFB smear was also positive in this group. Our results, in terms of AFB staining and histology results, also concur with previous reports in the literature[27].
In-house nested PCR was positive in 91% of clinically suspected OTB but 4 suspicious cases had a negative PCR. There was no PCR inhibition, as internal control was amplified in each specimen. False PCR negativity could be attributed therefore, to a low bacterial load or sampling error.
Unfortunately, extra spinal OTB has no pathognomonic imaging features and in advanced stages, it may mimic other disease processes. Therefore, appropriate laboratory tests are mandatory in order to confirm the diagnosis. Infections with low numbers of bacilli are frequently seen in OTB, a clinical situation where an early and sensitive diagnosis is of utmost importance. Nested PCR has great potential in improving the clinicians’ ability to clinically diagnose suspected TB in a timely manner. This, in turn, ensures early treatment.
The advantages of PCR include: (1) It is a highly efficient and rapid method for disease diagnosis (48 h). (2) A PCR result is of great value in early diagnosis, particularly in infections of certain body systems where disease progression is rapid and detection by culture method is time-consuming. (3) Since PCR is a very sensitive technique and can detect as few as one to two mycobacteria in the specimen, treatment may be initiated based on this result, if there are associated clinical signs of the disease. (4) It can differentiate typical and atypical mycobacteria. (5) PCR requires a very small specimen, even microliters obtained by fine needle aspirate can be tested. PCR can also detect very low levels AFB in clinical specimens and results are available within two days. However, misleading results can occur, such as the smallest amount of contaminating DNA can be amplified. A PCR-positive result does not always correlate with culture results and PCR is not a substitute for culture; it is an ancillary study complementing the routine battery of laboratory tests conducted for the rapid and definitive diagnosis of TB.
Its disadvantages include an inability to differentiate live from dead organisms, since it does not depend on bacterial replication and it provides no information on disease activity. Hence, a rational approach favors a combination of the patients’ clinical presentation and PCR in order to solve the diagnostic dilemma of OTB.
In our study, although PCR increased the number of definite diagnoses and provided highly specific and sensitive results within 48 h, it is expensive and not routinely available in TB endemic countries. Minimally, if possible, AFB smear and histopathology of tissue specimens can be carried out at a much lower cost and can increase the number of definite diagnoses. Nested PCR has great potential in improving the clinicians’ ability to rapidly diagnose clinically suspected TB, which will ensure early patient treatment[28].
In conclusion, PCR is a quick, highly sensitive and specific modality for OTB diagnosis. In cases associated with severe bone destruction, as in our patient population, its performance in serum samples is most useful.
Conflict of interest statement
The authors declare that there no conflict of interest.
Acknowledgements
This study was supported by FIS, IMSS, Grant No. 2009-785-011.
Comments
Background
This is a good work and very informative for evaluation diagnosis of OTB patients using serum samples by nested PCR. Authors have developed a technique for diagnosis of OTB in serum samples and correlates nested PCR to clinical, radiological and molecular diagnosis, which is good for diagnosis and treatment of OTB patients.
Research frontiers
Several techniques have been used for diagnosis of OTB patients, and in this study, serum samples are used to determine the patients suffering with OTB disease. This study has been focused on developing new early stage diagnosis technique for TB disease.
Related reports
There are very limited reports on this type of diagnosis studyfor TB disease and it is very sensitive technique for diagnosis and start treatment in early stage.
这类形容词还有好好儿的、慢慢儿的、薄薄儿的、细细儿的、高高儿的、暗暗儿的、悄悄儿的等,但总得来说,这类形容词不是很多。
Innovations and breakthroughs
In the present study, authors have evaluated a sensitive and less time consuming technique for diagnosis of TB disease using serum samples.
Applications
There are many time-consuming diagnosis techniques used to diagnose TB patients. This technique is very useful for detection of early stage TB and provides good treatment for patients.
Peer review
This is a very useful study, in which authors have evaluated the diagnosis techniques for detection of TB patients. The results obtained in this work clearly suggested that diagnosis technique has been designed to detect early stage TB patients.
[1] Talbot JC, Bismil Q, Saralaya D, Newton DA, Frizzel RM, Shaw DL. Musculoskeletal tuberculosis in bradford-a 6-year review. Ann R Coll Surg Engl 2007; 89: 405-409.
[2] Kumar M, Kumar R, Srivastva AK, Naq VL, Krishnani N, Maurya AK, et al. The efficacy of diagnostic battery in pott’s disease: a prospective study. Indian J Orthop 2014; 48(1): 60-66.
[3] Koptan W, ElMiliqui Y, Elsharkawi M. Single stage anterior reconstruction using titanium mesh cages in neglected kyphotic tuberculous spondylodiscitis of the cervical spine. Eur Spine J 2011; 20: 308-313.
[5] Merino P, Candel FJ, Gestoso I, Baos E, Picazo J. Microbiological diagnosis of spinal tuberculosis. Int Orthop 2012; 36: 233-238.
[6] Lotfinia I, Vahedi P. Late-onset post-diskectomy tuberculosis at the same operated lumbar level: case report and review of literature. Eur Spine J 2010; 19(Suppl 2): 226-232.
[7] Umredkar A, Mohindra S, Chhabra R, Gupta R. Vertebral body hyperostosis as a presentation of Pott’s disease: a report of two cases and literature review. Neurol India 2010; 58: 125-127.
[8] Fuentes Ferrer M, Gutiérrez Torres L, Ayala Ramírez O, Rumayor Zarzuelo M, del Prado González N. Tuberculosis of the spine. A systematic review of case series. Int Orthop 2012; 36: 221-231.
[9] De Backer AI, Vanhoenacker FM, Sanghvi DA. Imaging features of extraaxial musculoskeletal tuberculosis. Indian J Radiol Imaging 2009; 19(3): 176-186.
[10] D’Souza MM, Sharma R, Tripathi M, Mondal A. F-18 fluorodeoxyglucose positron emission tomography/computed tomography in tuberculosis of the hip: a case report and brief review of literature. Indian J Nucl Med 2011; 26(1): 31-33.
[11] Lidder S, Lang K, Haroon M, Shahidi M, El-Guindi M. Tuberculosis of the knee. Orthop Rev (Pavia) 2009; 1(2): e24.
[12] Tuli SM. General principles of osteoarticular tuberculosis. Clin Orthop Relat Res 2002; 398: 11-19.
[13] Corti M, Villafañe MF, Yampolsky C, Ambroggi M, Palmieri O. Spondylodiscitis with spinal epidural abscess and psoas Mycobacterium tuberculosis. Rev Panam Infectol 2007; 9: 50-53. Spanish.
[14] Chomczynski P. A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. Biotechniques 1993; 15: 532-534, 536-537.
[15] Borremans M, de Wit L, Volckaert G, Ooms J, de Bruyn J, Huygen K, et al. Cloning, sequence determination, and expression of a 32-kilodalton-protein gene of Mycobacterium tuberculosis. Infect Immun 1989; 57: 3123-3130.
[16] Parra CA, Londoño LP, Del Portillo P, Patarroyo ME. Isolation, characterization, and molecular cloning of a specific Mycobacterium tuberculosis antigen gene: identification of a species-specific sequence. Infect Immun 1991; 59: 3411-3417.
[17] Thierry D, Brisson-Noel A, Vincent-Levy-Frebault V, Nguyen S, Guesdon JL, Gicquel B. Characterization of a Mycobacterium tuberculosis insertion sequence, IS6110, and its application in diagnosis. J Clin Microbiol 1990; 28: 2668-2673.
[18] Del Portillo P, Murillo LA, Patarroyo ME. Amplification of a species-specific DNA fragment of Mycobacterium tuberculosis and its possible use in diagnosis. J Clin Microbiol 1991; 29: 2163-2168.
[19] Kitaura H, Ohara N, Matsuo T, Tasaka H, Kobayashi K, Yamada T. Cloning, sequencing and expression of the gene for alpha antigen from Mycobacterium intracellulare and use of PCR for the rapid identification of Mycobacterium intracellulare. Biochem Biophys Res Commun 1993; 196: 1466-1473.
[20] Matsuo K, Yamaguchi R, Yamazaki A, Tasaka H, Terasaka K, Yamada T. Cloning and expression of the gene for the crossreactive alpha antigen of Mycobacterium kansasii. Infect Immun 1990; 58: 550-556.
[21] Matsuo K, Yamaguchi R, Yamazaki A, Tasaka H, Yamada T. Cloning and expression of the Mycobacterium bovis BCG gene for extracellular alpha antigen. J Bacteriol 1988; 170: 3847-3854.
[22] Ohara N, Matsuo K, Yamaguchi R, Yamazaki A, Tasaka H, Yamada T. Cloning and sequencing of the gene for alpha antigen from Mycobacterium avium and mapping of B-cell epitopes. Infect Immun 1993; 61: 1173-1179.
[23] Gori A, Franzetti F, Marchetti G, Catozzi L, Corbellino M. Specific detection of Mycobacterium tuberculosis by mtp40 nested PCR. J Clin Microbiol 1996; 34: 2866-2867.
[24] Ruiz G, Garcia Rodrigues J, Guerri ML, Gonzalez A. Osteoarticular tuberculosis in a general hospital during the last decade. Clin Microbiol Infect 2003; 9: 919-923.
[25] Houshian S, Poulsen S, Riegels-Nielsen P. Bone and joint tuberculosis in Denmark: increase due to immigration. Acta Orthop Scand 2000; 71: 312-315.
[26] Arathi N, Ahmad F, Huda N. Osteoarticular tuberculosis-a three years’ retrospective study. J Clin Diagn Res 2013; 7(10): 2189-2192.
[27] Jain AK, Jena SK, Singh MP, Dhammi IK, Ramachadran VG, Dev G. Evaluation of clinico-radiological, bacteriological, serological, molecular and histological diagnosis of osteoarticular tuberculosis. Indian J Orthop 2008; 42: 173-177.
[28] Kamara E, Mehta S, Brust JC, Jain AK. Effect of delayed diagnosis on severity of Pott’s disease. Int Orthop 2012; 36: 245-254.
10.12980/APJTB.4.201414B112
*Corresponding author:Unidad de Investigación Médica en Inmunología e Infectología, Hospital de Infectología, CMNR, IMSS, Av. Jacarandas y Seris, Col. La Raza, PC: 02990. México, D.F., México.
Tel: (+52) (55) 5724-5900, ext, 24321;
Fax: (+52) (55) 5353-0989;
E-mail: gelorriaga@webtelmex.net.mx
Foundation Project: This study was supported by FIS, IMSS (Grant No. 2009-785-011).
Article history:
Received 4 May 2014
Received in revised form 9 May, 2nd revised form 16 May, 3rd revised form 23 May, 2014
Accepted 3 Jul 2014
Available online 28 Jul 2014
Methods:A total of 44 consecutive serum specimens were collected from clinically suspected OTB patients, based on clinical and radiological [X-ray or magnetic resonance imaging/computed tomography] features. They were screened by in-house nested PCR. In addition, a few specimens were examined by Gram stain, acid-fast bacilli stain, histopathology and routine bacterial culture. A total of 39 specimens were collected from patients suffering from other bone diseases of nontuberculous origin and included as negative controls.
Results:Of the 44 clinically suspected OTB patients, in-house nested PCR was positive in 40 (91%) cases; PCR was negative in 38 (97%) negative controls. Sensitivity and specificity of our inhouse nested PCR was 90.9% and 97.4%, respectively. The PCR report was available within 48 h. It was possible to standardize serum PCR technique and in positive cases, a good correlation was observed in terms of an adequate treatment response.
Conclusions:Nested PCR in serum samples is a rapid, highly sensitive and specific modality for OTB detection. PCR should be performed in addition to clinical evaluation, imaging studies, acidfast bacilli staining, culture and histopathology diagnosis, if possible.
猜你喜欢
杂志排行
Asian Pacific Journal of Tropical Biomedicine的其它文章
- Antimicrobial activity against periodontopathogenic bacteria, antioxidant and cytotoxic effects of various extracts from endemic Thermopsis turcica
- Proteomics analysis of antimalarial targets of Garcinia mangostana Linn.
- The presence of eucalyptol in Artemisia australis validates its use in traditional Hawaiian medicine
- Jeju seaweeds suppress lipopolysaccharide-stimulated proinflammatory response in RAW 264.7 murine macrophages
- Antioxidant potential of Rumex vesicarius L.: in vitro approach
- Cytotoxicity screening of Melastoma malabathricum extracts on human breast cancer cell lines in vitro
