Preparation and characterization of TiO2-SiO2-Fe3O4 core-shell powders in nano scale
2013-12-20XUHongyan徐宏妍DIWUJiangtao第五江涛LIZhiyong李智勇
XU Hong-yan (徐宏妍), DIWU Jiang-tao (第五江涛), LI Zhi-yong (李智勇)
(School of Materials Science and Engineering, North University of China, Taiyuan 030051, China)
Preparation and characterization of TiO2-SiO2-Fe3O4core-shell powders in nano scale
XU Hong-yan (徐宏妍), DIWU Jiang-tao (第五江涛), LI Zhi-yong (李智勇)
(School of Materials Science and Engineering, North University of China, Taiyuan 030051, China)
TiO2/SiO2/Fe3O4nanoparticles have bigger specific area which can greatly increase the efficiency of photo-catalysis. The TiO2/SiO2/Fe3O4particles in nano scale were prepared with reduction method at high temperature in this paper,and their morphology, particle size and magnetic property were characterized by transmission electron microscope (TEM), X-ray diffraction (XRD) and magnetometer. The results show that the grain sizes of Fe3O4, SiO2-Fe3O4and TiO2-SiO2-Fe3O4particles were 50 nm, 70 nm and 120 nm, respectively. With the modification of SiO2, Fe3O4magnetic cores are protected from oxidation. Moreover, by the addition of TiO2function layer, TiO2-SiO2-Fe3O4functional nanoparticles, with the saturation magnetization density of 34.1 emu/g, is magnetically recoverable. The processes of this method are so simple that the nanoparticles can be produced in large quantity.
core-shell structure; nanoparticles; magnetic separation
CLD number: O643 Document code: A
Recently, environmental problems are urgent[1-6], especially, the water sources have been polluted during the economic development. Therefore, many researches have engaged in solving this problem[7-10]. TiO2plays an important role in treating polluted water, thus the method to prepare recoverable TiO2[11-16]is absolutely demanded.
This paper focuses on the research on preparation method of TiO2-SiO2-Fe3O4nanoparticles. To introduce Fe3O4as magnetic cores, the nanoparticles TiO2-Fe3O4can be recovered after treating polluted water. However, the interface between the two layers makes the efficiency of photocatalysis decrease deeply[17]. Therefore, a middle layer, SiO2, is introduced. The recoverable TiO2-SiO2-Fe3O4nanoparticles, with core-shell structure, are obtained in this work.
Mass production of the TiO2-SiO2-Fe3O4nanoparticles is a key problem for application. The advantages of these methods lie in simple process, none lead-in pollution agent and none controlling of PH value. Owing to these conveniences, the methods proposed in this work allow the TiO2-SiO2-Fe3O4nanoparticles can be produced in large quantity. It is the beginning of industrial production of the TiO2/SiO2/Fe3O4nanoparticles.
1 Experiments
Ferric chloride (AR) and carbon were used as reactants in a ratio of 1∶2 to obtain the Fe3O4magnetic particles at 430 ℃ for 3 h under hypoxemia condition. After reaction, the products were milled adequately, in order to get uniform Fe3O4particles. Tetraethoxysilane (TEOS), hydrochloric acid (AR), alcohol and deionized water (18.25 MΩ) were mixed in definite ratio of 9.2∶1∶24.6∶2 to obtain SiO2solation. Then deionized water was purified through ultra-pure (UPR) system.
The Fe3O4particles were put into a baker with SiO2solation and let them sit for three hours, then the wet particles were heated at 500 ℃ for 2 h to obtain SiO2-Fe3O4particles. The SiO2-Fe3O4particles were then put into another baker with TBOT, nitric acid, alcohol and deionized water whose ratio was 60.6∶1.2∶25∶2 for 30 min with ultrasonic agitation to encapsulate with TiO2. Stewed for another three hours, the wet particles were heated at 500 ℃ for 2 h and TiO2-SiO2-Fe3O4particles were produced finally.
The compositions of the particles were measured by Buker D8 Advance X-ray diffraction (XRD), the particles patterns and sizes were characterized by JEOL JEM-1200EX transmission electron microscopy (TEM), and the magnetic properties were characterized by a lake shore 7307 vibrating sample magnetometer.
2 Results and discussion
Fig.1 shows the XRD spectra of the Fe3O4, SiO2-Fe3O4and TiO2-SiO2-Fe3O4particles. The spectrum of Fe3O4particles (line a) shows that Fe3O4and α-Fe2O3coexist in the sample, which demonstrates that Fe3O4nanoparticles are obtained by high temperature reduction method. Fe3O4particles are oxidized partially to Fe2O3. While in the spectrum of SiO2-Fe3O4particles (line b), the characteristic peaks of α-Fe2O3disappear, which illustrates that the Fe3O4particles are prevented from being oxidized by the modification of SiO2. The XRD spectrum of TiO2-SiO2-Fe3O4particles (line c) detects the existence of the anatase TiO2. This performance certifies that the SiO2-Fe3O4particles are encapsulated perfectly with TiO2.
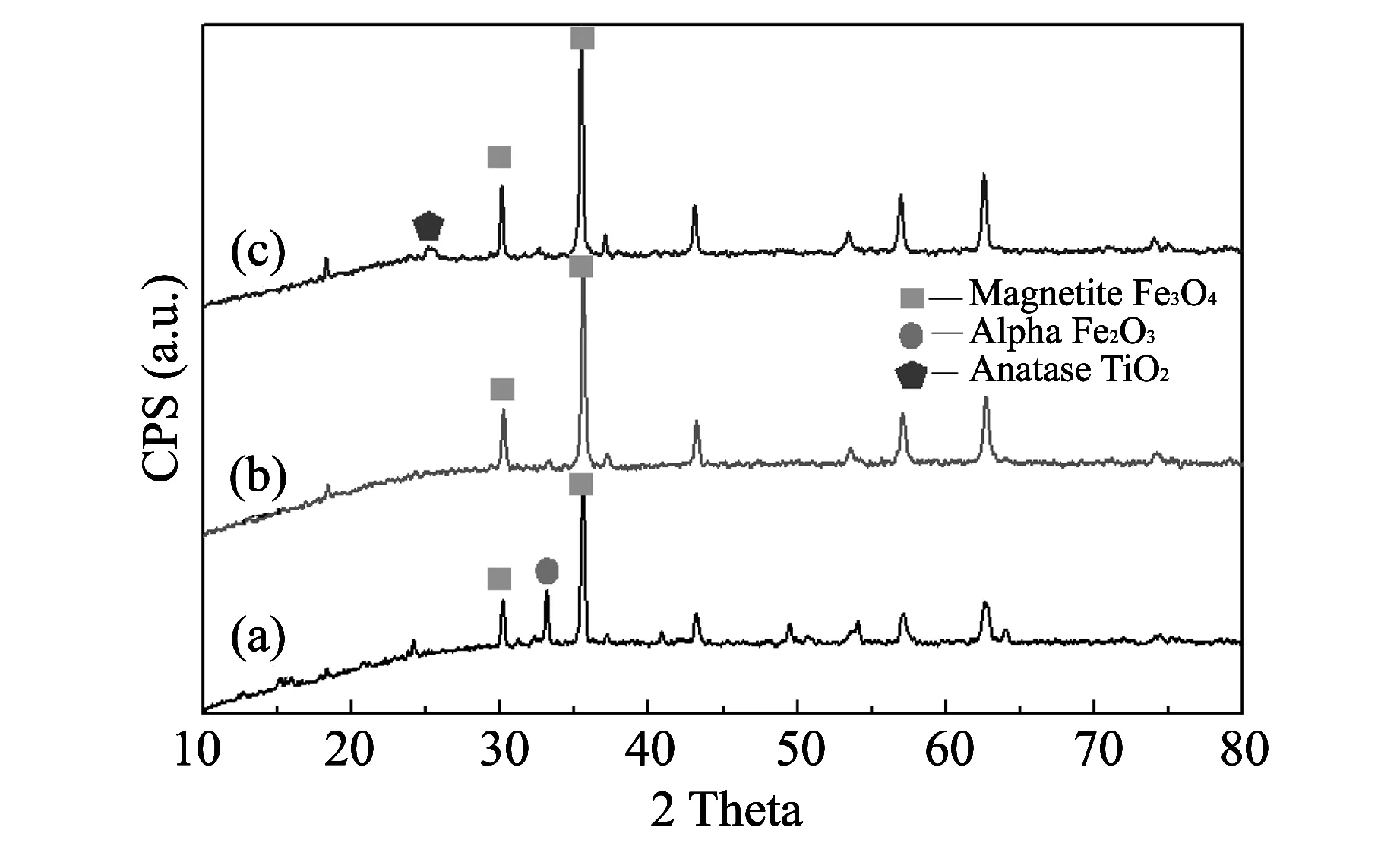
Fig.1 XRD spectra of (a) Fe3O4, (b) SiO2-Fe3O4 and (c) TiO2-SiO2-Fe3O4 particles
Fig.2 shows the TEM images of Fe3O4, SiO2-Fe3O4and TiO2-SiO2-Fe3O4particles. This result shows that Fe3O4nanoparticles are triangle with the edge size of about 50 nm. Fig.2(b) shows that SiO2is coated uniformly on magnetite nucleolus Fe3O4particles, and the rims of Fe3O4particles are not sharp and black any more. The size of SiO2-Fe3O4nanoparticles is of about 70 nm. It is clear in Fig.2(c) that SiO2-Fe3O4particles are encapsulated with many little spherical TiO2particles, and the size of TiO2-SiO2-Fe3O4particles is of about 120 nm.
The hysteresis cycles (see Fig.3) show that the saturation intensities of the Fe3O4, SiO2-Fe3O4and TiO2-SiO2-Fe3O4particles are 34.9,36.3 and 34.1 emu/g, respectively. It is clear that SiO2-Fe3O4particles have intermediate saturation intensity, which hints that the Fe3O4particles are prevented from oxidation by SiO2modification, and SiO2-Fe3O4particles are well encapsulated by anatase TiO2. Moreover, the saturation intensity of the TiO2-SiO2-Fe3O4particles is large enough for recovery.

Fig.2 TEM images of nanoparticles
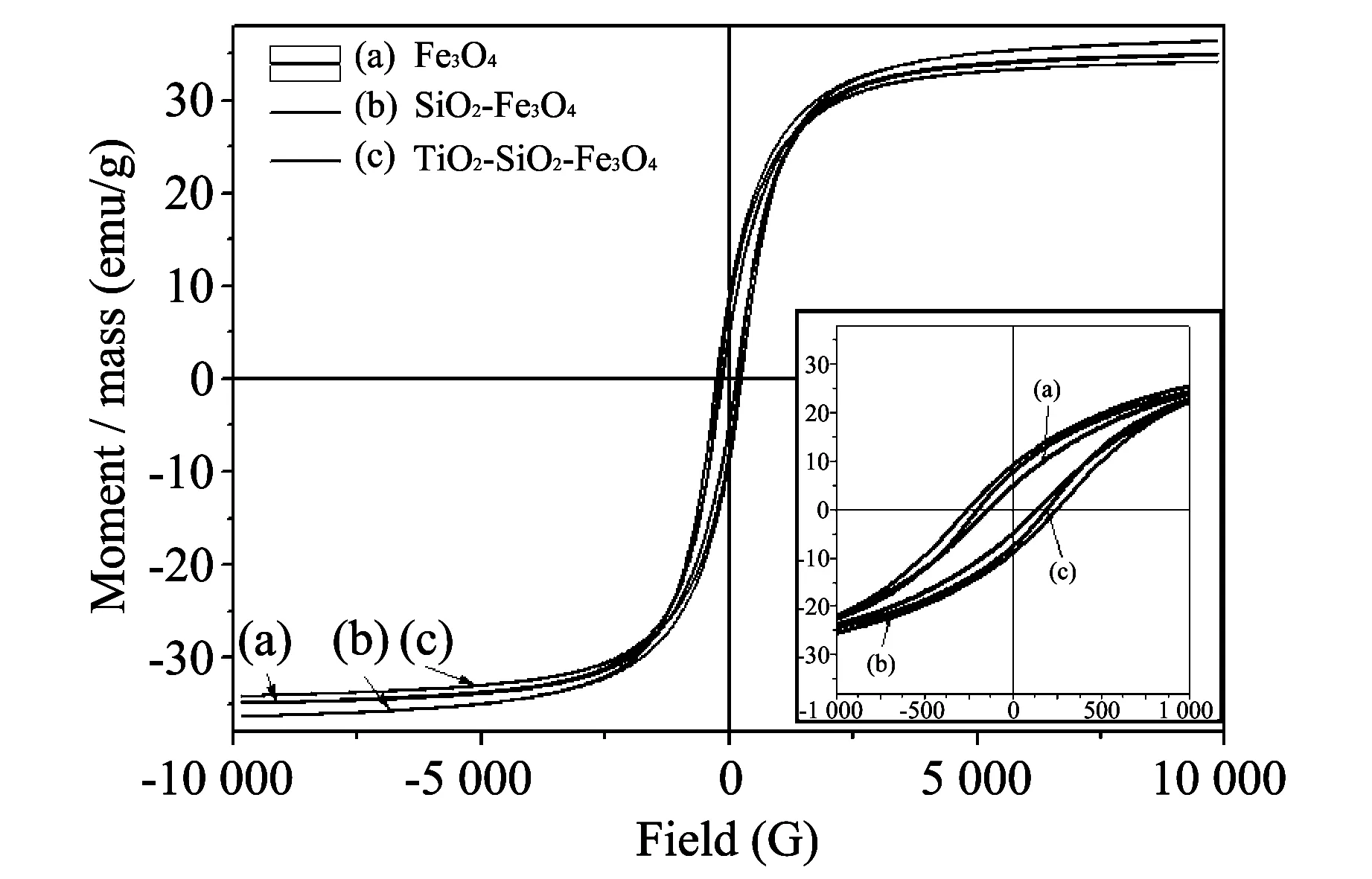
Fig.3 Hysteresis cycles of (a) Fe3O4, (b) SiO2-Fe3O4 and (c) TiO2-SiO2-Fe3O4 particles
The retentive magnetism of the Fe3O4, SiO2-Fe3O4and TiO2-SiO2-Fe3O4nanoparticles are 4.8 emu/g, 9.2 emu/g and 7.8 emu/g, respectively. These small values show that the particles have good dispersity.
3 Conclusion
The new approach used in the present work to synthesize Fe3O4magnetic nanoparticles is convenient and triumphant. With the modification of SiO2, Fe3O4magnetic nanoparticles are prevented from oxidation. Moreover, core-shell structure of TiO2-SiO2-Fe3O4functional nanoparticles with the size of 120 nm was also obtained in this work. The synthesized TiO2-SiO2-Fe3O4functional particles have saturation intensity of 34.1 emu/g and retentive magnetism of 7.8 emu/g. All these properties of the nanoparticles demonstrate that the methods researched in this paper is simple and the nanoparticles are easy for recovery. Solving the problem of mass production is the next goal of our research.
[1] Galvis H M T, Bitter J H, Khare C B, et al. Supported iron nanoparticles as catalysts for sustainable production of lower olefins. Science, 2012, 335(6070): 835-838.
[2] Fujishima A, Honda K. Electrochemical photolysis of water at a semiconductor electrode. Nature, 1972, 238(5338): 38-45.
[3] Carey J H, Lawrence J, Tosine H M. Photodechlorination of PCB's in the presence of titanium dioxide in aqueous suspension. Bulletin of Environmental Contamination and Toxicology, 1976, 16(5): 697-701.
[4] ZHANG Qiang, XUE Chen-yang, YUAN Yan-ling, et al. Fiber surface modification technology for fiber-optic localized surface plasmon resonance biosensors. Sensors, 2012, 12(3): 2729-2741. [doi:10.3390/s120302729]
[5] Ranjan M. Doubly localized surface plasmon resonance in bimodally distributed silver nanoparticles. Journal of Nanoscience and Nanotechnology, 2012, 12(6): 4540-4545.
[6] YANG Yang, WANG Hong, LI Jian-xin, et al. Novel functionalized nano-TiO2loading electrocatalytic membrane for oily wastewater treatment. Environmental Science & Technology, 2012, 46(12): 6815-6821. [doi: 10.1021/es3000504]
[7] ZHENG Xiong, CHEN Yin-guang, WU Rui. Long-term effects of titanium dioxide nanoparticles on nitrogen and phosphorus removal from wastewater and bacterial community shift in activated sludge. Environmental Science & Technology, 2011, 45(17): 7284-7290. [doi: 10.1021/es2008598]
[8] WANG Jian-ting, Moore J, Laulhe S, et al. Fluorophore-gold nanoparticle complex for sensitive optical biosensing and imaging. Nanotechnology, 2012, 23: 095501-13. [doi: 10.1088/0957-4484/23/9/095501]
[9] Pruden A L, Ollis D F. Photoassisted heterogeneous catalysis, the degradation of tricilloroethylene in water. Catalysis, 1983, 82(2): 404-417.
[10] Verwey J W M, Vander D V D, Dirksen G J, et al. Luminescence processes in the crystaline and glass modifications of LnBGeO5-type compositions. Journal of Solid State Chemistry, 1990, 89(1): 106-117.
[11] Massatr R. Preparation of magnetite nanoparticles. IEEE Transactions on Magnetics, 1981, Mag-17: 11247-11250.
[12] Molday R S. Magnetic iron-dextran microspheres. US Patent: 4452773.1984-06-05. http://www.google.com/patents/US4452773.
[13] BAO Shu-juan, ZHANG Xiao-gang, LIU Xian-ming, et al. Preparation and photocatalytic activity of TiO2/SiO2/Fe3O4. Chinese Journal of Applied Chemistry, 2004, 21(3): 261-265.
[14] SHEN Li-fen, Laibinis P E, Hatton T A. Bilayer surfactant stabilized magnetic fluids: synthesis and interactions at interfaces. Langmuir, 1999, 15(2): 447-453.
[15] Fang L M, Zu X T, Li Z J, et al., Synthesis and characteristics of Fe3+-doped SiO2nanoparticles via sol-gel-calcination or sol-gel-hydrothermal route. Journal of Alloys and Compounds, 2008, 454: 261-267.
[16] Avasaralaa B K, Tirukkovalluria S R, Bojjab S. Photocatalytic degradation of monocrotophos pesticide-an endocrine disruptor by magnesium doped titania. Journal of Hazardous Materials, 2011, 186(2/3): 1234-1240.
[17] GAO Jun-ning, HUANG Ying, HUANG Fei, et al. Progress in study on core-shell magnetic TiO2photocatalyst. Materials Guide, 2007, 21: 6.
date: 2013-10-12
Ministry of Human and Social Security of Shanxi Province, Natural Science Foundation for Young Scientists of Shanxi province (No.2011011020-2); Shanxi Province Foundation for Returness (No.2008062)
XU Hong-yan(xuhongyan@foxmail.com)
1674-8042(2013)04-0402-03
10.3969/j.issn.1674-8042.2013.04.021
猜你喜欢
杂志排行
Journal of Measurement Science and Instrumentation的其它文章
- A 4-layer method of developing integrated sensor systems with LabVIEW
- Calibration and compensation methods of installation error of electronic compass
- Design of shared bus DSP board in vector network analyzer
- Error modeling and analysis of inclinometer based on digital accelerometer
- Analysis of condition monitoring methods for electromotor on oil platform
- U disk recorder based on CH376 and ATmega 128
