Synthesis and Fluorescence Properties of Terbium and Samarium Complexes with 2,4,6-Tri(2-pyridyl)-1,3,5-triazine
2013-10-21WANGAilingLIHaiyanZHANGHaixiaDUYanYUEBinCHUHaibinZHAOYongliang
WANG Ai-ling,LI Hai-yan,ZHANG Hai-xia,DU Yan,YUE Bin,CHU Hai-bin,ZHAO Yong-liang
(College of Chemistry and Chemical Engineering,Inner Mongolia University,Hohhot 010021,China)
1 Introduction
2 Experiments
2.1 Reagents and Instruments
Purities of rare earth oxide Tb4O7and Sm2O3are both 99.99%.TPTZ,p-hydroxybenzoic acid,terephthalic acid,nitric acid,anhydrous ethanol and other reagents are all of analytical grade.The mole concentrations of rare-earth ions were analyzed by the EDTA complexometric titration method with xylenol orange as indicator.Elemental analysis of C,H,N was performed with a Vario EL Cube elemental analyzer.The components of terbium and samarium elements in the complexes were determined by Optima 5300DV ICP emission spectrometer.The UV spectra (200~400 nm)of the ligands and complexes were recorded on Shimadzu UV-265 spectrophotometer.The IR spectra were measured with a Nicolet Nexus 670 FT-IR spectrometer by using the KBr pellet technique in the range of 4 000~400 cm-1.The phosphorescence spectrum of the complex Gd(C7H5O3)3and fluorescence excitation and emission spectra of all the complexes were measured by Edinburgh Analytical Instruments FLS-920 fluorescence spectra-photometer at 77 K and at room temperature,respectively.
2.2 Synthesis of Rare Earth Complexes
The ethanol solution of Tb(NO3)3was prepared by the reaction of Tb4O7with dilute solution of nitric acid and 10% H2O2,heating dissolved,evaporated,and then dissolved in anhydrous ethanol.
Preparation of samarium nitrate was similar to terbium nitrate except that Tb4O7was replaced by Sm2O3and not adding 10% H2O2solution.
TPTZ (0.6 mmol,0.187 2 g)was first dissolved in 40 mL of ethanol solution with stirring at 60 ℃.Then NH3·H2O (1∶4,volume ratio)was added into the solution with the pH value adjusted to 6.4~6.7.While being stirred,6 mL Tb(NO3)3(0.1 mol/L)ethanol solution was added into above TPTZ ethanol solution.The pH was readjusted to between 6 and 7 with NH3·H2O and the liquid was stirred at 60 ℃for 3 h,after which the mixture was separated by filtering and washing with ethanol and acetone.The product was dried at 50 ℃overnight,then the solid complex(1)Tb(TPTZ)(NO3)3(C2H5OH)was got.
The preparation of (2)Tb(TPTZ)2(NO3)3-(C2H5OH)and (3)Tb2(TPTZ)(NO3)6(C2H5OH)was similar to Tb(TPTZ)(NO3)3(C2H5OH)except that the molar ratios of TPTZ and Tb(NO3)3were 2∶1 and 1∶2,respectively.
The synthesis procedures for complexes (6)Sm-(TPTZ)(NO3)3(C2H5OH),(7)Sm(TPTZ)2-(NO3)3(C2H5OH)and (8)Sm2(TPTZ)(NO3)6-(C2H5OH)were similar to that of terbium complexes except that Tb(NO3)3was replaced by Sm(NO3)3.
Anionic ligand p-hydroxybenzoic acid (0.165 7 g,1.2 mmol)was dissolved in ethanol,and 0.124 8 g(0.4 mmol)TPTZ was added into the solution.The solution was heated to 60 ℃with stirring,then 4 mL 0.1 mol/L Tb(NO3)3was added.The pH value of the solution was adjusted to 6.4~6.7,after which the solution was stirred at 60 ℃for 2 h and then stood overnight.The mixture was filtered and washed three times by ethanol.After dried at 50 ℃for 24 h,the complex (4)Tb(TPTZ)(C7H5O3)3-(C2H5OH)was obtained.
The preparation of (5)Tb2(TPTZ)2(C8H4O4)-(NO3)4(C2H5OH)was similar to that of Tb(TPTZ)-(C7H5O3)3(C2H5OH)except that the molar ratio of Tb(NO3)3∶TPTZ∶C8H4O4was 2∶2∶1.
The synthesis procedures for (9)Sm(TPTZ)-(C7H5O3)3(C2H5OH)and (10)Sm2(TPTZ)2-(C8H4O4)(NO3)4(C2H5OH)were similar to that of terbium complexes (4)and (5)except that Tb(NO3)3was replaced by Sm(NO3)3。
3 Results and Discussion
3.1 Composition Analysis and Properties of the Complexes
The elementary analysis results and mole conductivity values of the complexes are listed in Table 1.From the elementary analysis data,it can be calculated that their compositions agree with the above complexes described.The products are all white powder solid which are stable in the air and are soluble in dimethylformamide (DMF)and dimethyl sulfoxide (DMSO)but insoluble in water,ethanol,acetone and ethyl ether.The small values of mole conductivity (13.6~19.5 S·cm2·mol-1,Table 1)of the complexes in DMF reveal that only a small section of the complexes ionizes in DMF and thesecomplexes are all non-electrolytes[5-6].
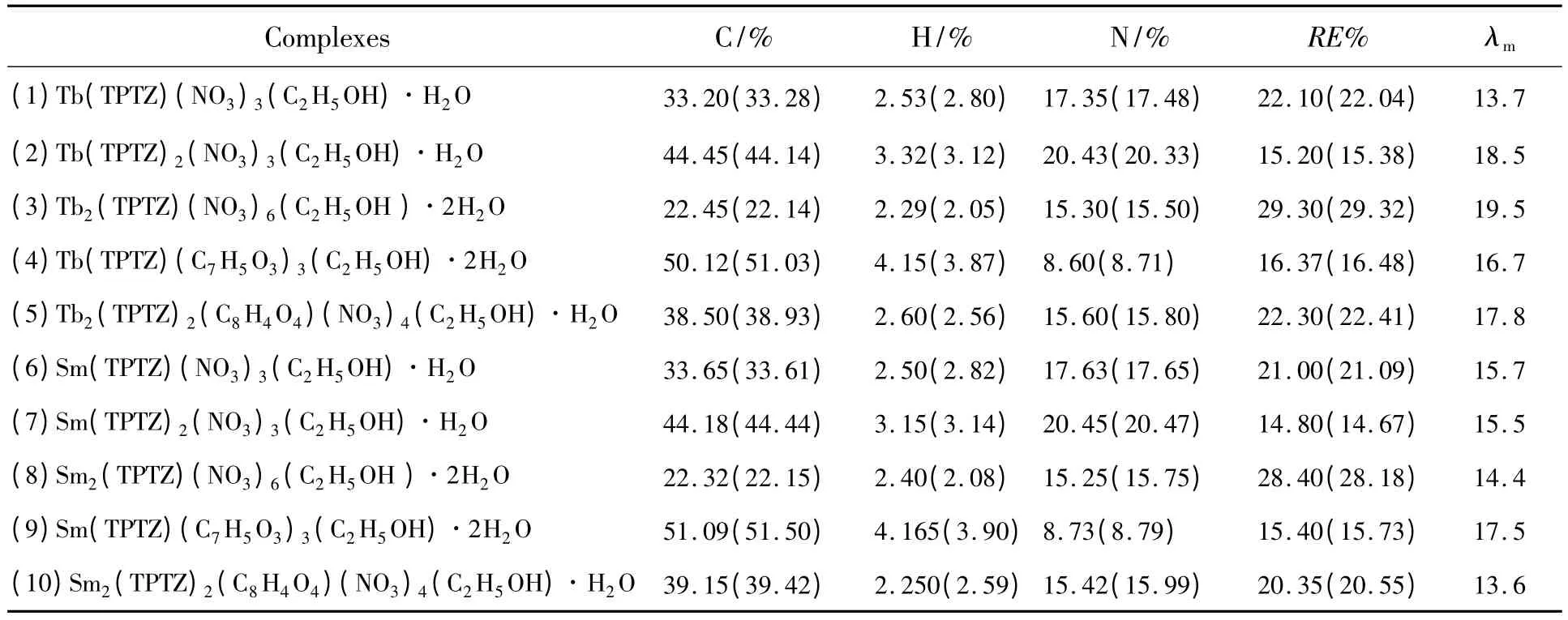
Table 1 Elemental analyses and mole conductivity values (S·cm2·mol -1)of the complexes
3.2 UV-Vis Absorption Spectra
The UV-Vis spectra for the dissociative ligands and corresponding complexes were shown in Fig.1.DMF was used as a reference and solvent.The concentrations of the ligands and complexes were all 1 ×10-5mol/L.The spectra of complexes containing nitrate were similar,and only the spectrum of the complex Tb(TPTZ)(NO3)3(C2H5OH)·H2O was presented.
As seen from the spectra,the ligands TPTZ,phydroxybenzoic acid and terephthalic acid show strong absorption peaks at 282,285 and 272 nm,respectively.After coordinating to Tb3+,there is an obvious shift to 278 nm and an increase of absorption intensity,which indicate that these ligands are all coordinated to Tb3+.There is no difference in the UV-Vis spectra of the complexes with the same ligands but different cation ions.
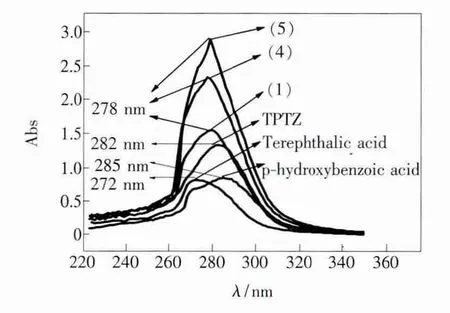
Fig.1 UV-Vis absorption spectra of ligands and complexes
3.3 Infrared Spectra
Within the scope of 4 000~400 cm-1,IR spectra of the complexes and the ligands were determined and shown in Fig.2.The peak at 1 371 cm-1that ascribed to the ring stretching of both the pyridine and triazine rings of ν(C ═N)of TPTZ[7]moved 6~15 cm-1toward high wavenumber in the complexes.This indicated that the nitrogen atoms of the rings was coordinated to rare earth ions.The strong pyridine ring characteristic absorption band(1 527 cm-1)which was ascribed to ν(C ═N)and ν(C ═C)moved 3~4 cm-1toward low wavenumber after coordination,which also demonstrated TPTZ coordinates to the rare earth ion[8].
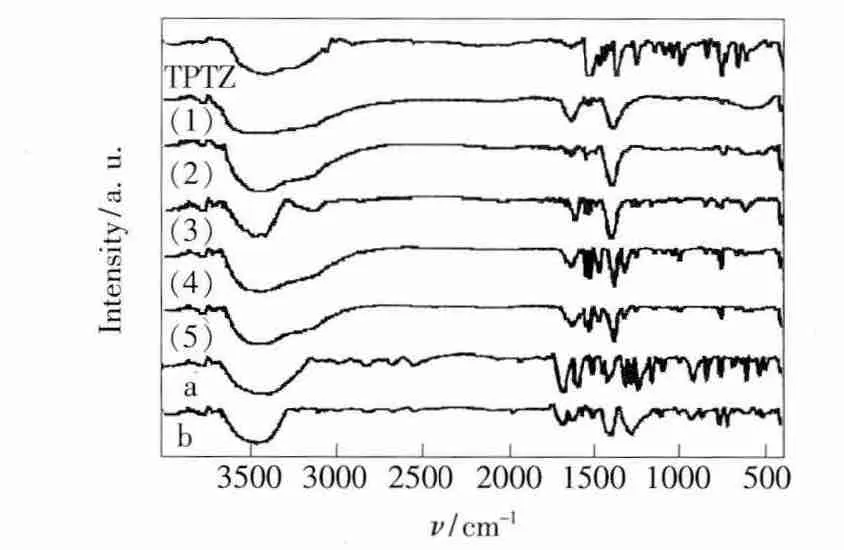
Fig.2 IR spectra of ligands and complexes.(a)p-hydroxybenzoic acid.(b)Terephthalic acid.(1)~ (5)TPTZ and complexes.
As shown in Fig.2,the coordinated nitrate ions show ν1(out-of-plane deformation)at 789 cm-1,ν2(doubly degenerate stretching)at 1 234 and 1 451 cm-1,and ν3(doubly degenerate inplane bending)at 691 and 742 cm-1,which means that the nitrate ions coordinate Tb3+and Sm3+ions with bidentate structure[9].Comparing the IR data of p-hydroxybenzoic acid and terephthalic acid before and after coordination with rare earth ions,it can be seen that the free carboxylic ion changes significantly after coordinated.The ν(C ═O)peak of p-hydroxybenzoic acid at 1 676 cm-1disappears and two peaks at 1 612 cm-1(νas(COO-))and 1 493 cm-1(νs(COO-))appear in the complex.Also,the peak of terephthalic acid at 1 684 cm-1disappears and two peaks at 1 608 cm-1(νas(COO-))and 1 487 cm-1(νs(COO-))appear in the complexes.These changes suggest that the ligands coordinate with rare earth ions by bidentate carboxylate groups[10].
3.4 Fluorescence Spectra
The phosphorescence spectra of the Gd(C7H5O3)3complex was measured with an excitation wavelength of 355 nm in methylene dichloride.The concentration of the gadolinium complex was 1 ×10-4mol/L.As shown in Fig.3,the emission peak of the complex appears at 580 nm,from which the triplet state energy level of p-hydroxybenzoic acid is calculated to be approximately 17 241 cm-1.
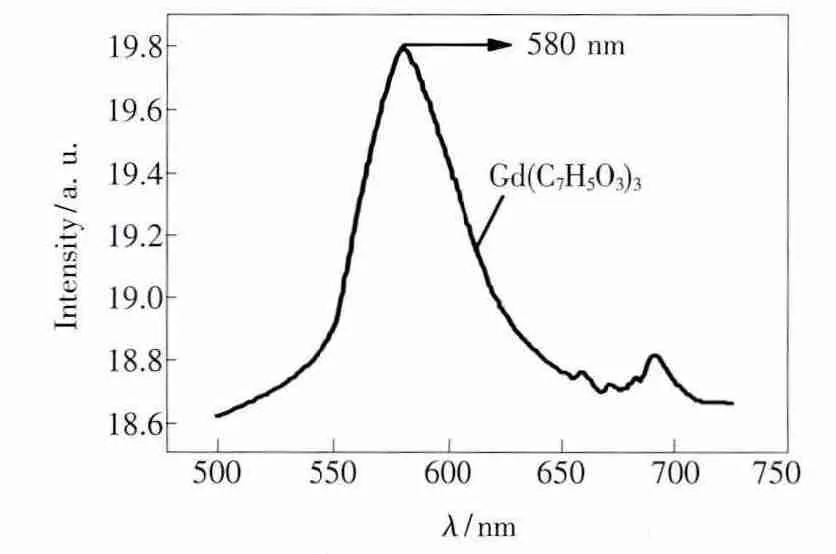
Fig.3 The phosphorescence spectrum of Gd(C7H5O3)3in methylene dichloride
The excitation spectra of the complexes were obtained by monitoring the emission wavelength of the terbium complexes at 545 nm and samarium complexes at 595 nm within the scope of 220~450 nm.The emission spectra of all complexes were determined at the excitation slit width of 3 nm and the emission slit width of 1 nm.The most efficacious excitation wavelength was at 355 nm for terbium complexes and at 370 nm for samarium complexes.The emission spectra of these terbium complexes show four typical emission bands at 490,542,583 and 620 nm,corresponding to the5D4→7F6,5D4→7F5,5D4→7F4,and5D0→7F3transitions of Tb3+ion,respectively (Fig.4).The main transition from5D4to7F5at 542 nm is responsible for the green colored emission of Tb3+ion[11].
The fluorescence emission spectra data of the complexes are listed in Table 2.All these complexes exhibit strong luminescence emission,which indicate that TPTZ ligand with a triplet energy level of 21 277 cm-1[12]is a good organic ligand and it is beneficial to absorb light energy and effectively transfer energy to Tb3+and Sm3+ions,and emit the characteristic fluorescence of the two ions.
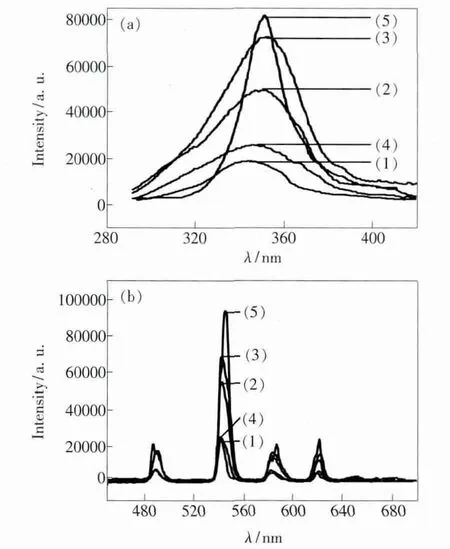
Fig.4 Fluorescence exitation spectra (a)and emission spectra (b)of terbium complexes
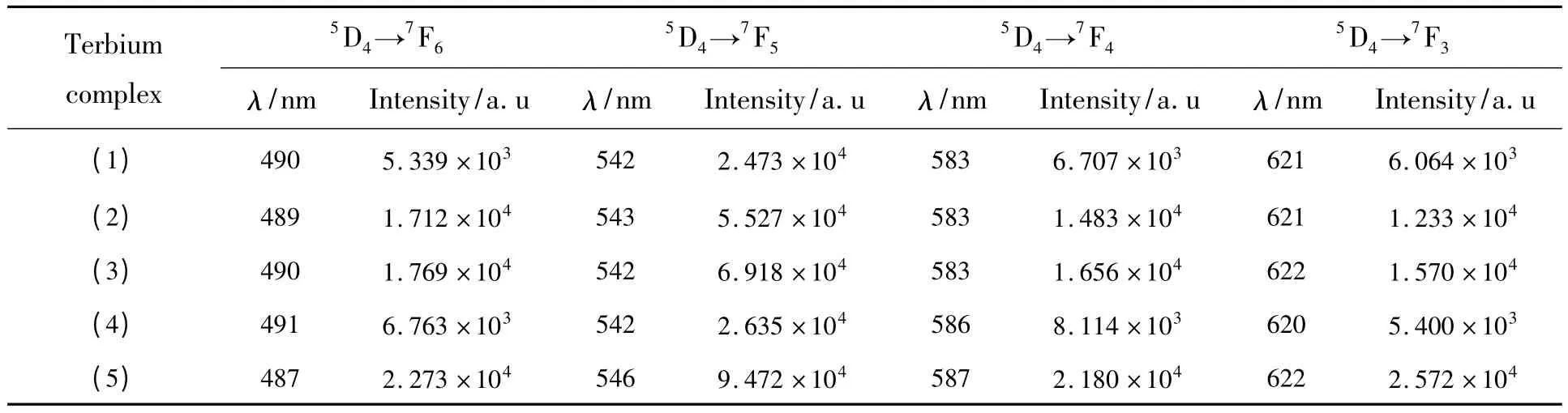
Table 2 (a)Fluorescent emission spectra data of the complexes (1)to (5)
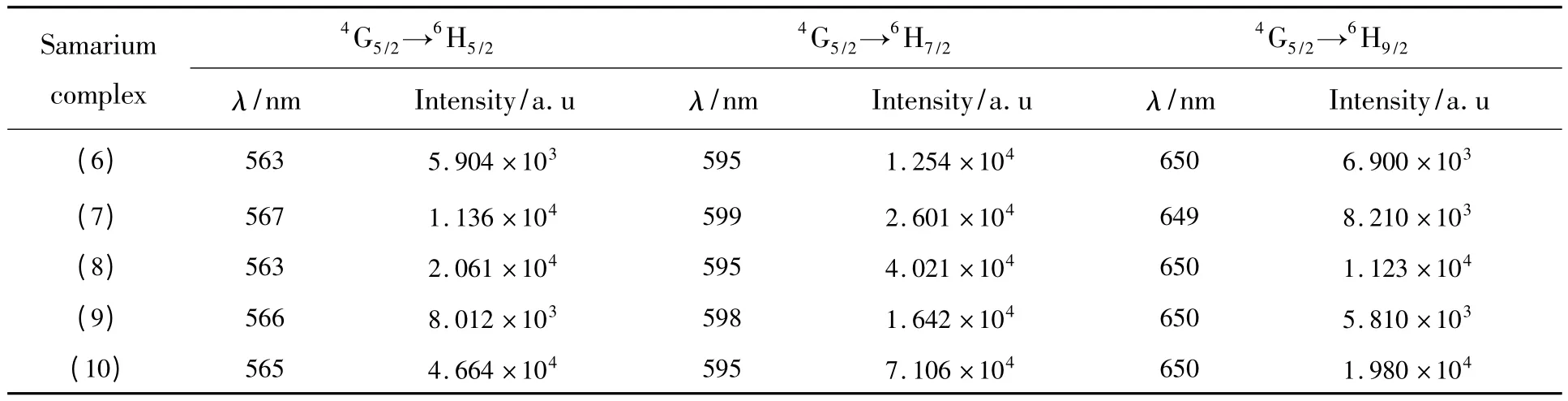
Table 2 (b)Fluorescent emission spectra data of complexes (6)to (10)
4 Conclusion
In summary,ten solid complexes REn(TPTZ)xLy(RE=Tb and Sm,L=NO-3,C7H5O-3and C8H4O2-4)were synthesized and characterized.The oxygen atoms of nitrate and carboxyl group and nitrogen atoms of TPTZ coordinated to the rare earth ions.All complexes show outstanding fluorescence.As the concentration of TPTZ or luminous center ion increases,the fluorescent intensity has been enhanced for the complexes with the same ligands.And there is no phenomenon of concentration-quenching effect to some extent.Based on the triplet energy level of phydroxybenzoic acid and fluorescence intensity of the corresponding complexes,it can be inferred that the aromatic ligands of such low triplet energy level can couple with other appropriate aromatic ligands to form wonderful luminous lanthanide complexes.It is of significance to use such material to design complexes with strong fluorescent intensity and optimize the luminescent properties of lanthanide complexes.
[1]Ji X L,Li B,Jiang S C,et al.Luminescent properties of organic-inorganic hybrid monoliths containing rare earth complexes[J].J.Non-Cryst.Solids,2000,275(1-2):52-58.
[2]Latva M,Takalo H,Simberg K,et al.Enhanced EuⅢion luminescence and efficient energy transfer between lanthanide chelates within the polymeric structure in aqueous solutions[J].J.Chem.Soc.Perkin Trans.Ⅱ,1995,1995(5):995-999.
[3]Silva C R,Wang J F,Carducci M D,et al.Synthesis,structural characterization and luminescence studies of a novel europium (Ⅲ)complex[Eu(DBM)3(TPTZ)](DBM:dibenzoylmethanate;TPTZ:2,4,6-tri(2-pyrid-yl)-1,3,5-triazine)[J].Inorg.Chim.Acta,2004,357(2):630-634.
[4]Sabbatini N,Guardigli M.Luminescence lanthanide complexes as photochemical supramolecular devices [J].Coord.Chem.Rev.,1993,123(1-2):201-228.
[5]Tayor M D,Carter C P,Wynter C L.The infrared spectra and structure of the rare-earth benzoates[J].J.Inorg.Nucl.Chem.,1968,30(6):1503-1511.
[6]Ouchi A,Suzuki Y,Ohki Y,et al.Structure of rare earth carboxylates in dimeric and polymeric forms [J].Coord.Chem.Rev.,1988,2(1):29-43.
[7]Hadadzadeh H,Maghami M,Simpson J,et al.Nickel(Ⅱ)polypyridyl complexes of 2,4,6-tris(2-pyridyl)-1,3,5-triazine[J].J.Chem.Crystallogr.,2012,42(7):656-667.
[8]Das A,Marschner C,Cano J,et al.Synthesis,crystal structures and magnetic behaviors of two dicyanamidebridged di-and polynuclear complexes of cobalt(Ⅱ)derived from 2,4,6-tris(2-pyridyl)1,3,5-triazine and imidazole[J].Polyhedron,2009,28(12):2436-2442.
[9]Curtis N F,Curtis Y M.Some nitrato-amine nickel(Ⅱ)compounds with monodentate and bidentate nitrate ions[J].Inorg.Chem.,1965,4(6):804-809.
[10]Liu L,Xu Z,Lou Z,et al.Luminescent properties of a novel terbium complex Tb(o-BBA)3(phen)[J].J.Rare Earth,2006,24(2):253-256.
[11]Zhang A Q,Pan Q L,Jia H S,et al.Synthesis,characteristic and intramolecular energy transfer mechanism of reactive terbium complex in white light emitting diode[J].J.Rare Earth,2012,30(1):10-16.
[12]Zhao Y F,Zhao Y L,Bai F,et al.Fluorescent property of the Gd3+-doped terbium complexes and crystal structure of[Tb(TP TZ)(H2O)6]Cl3·3H2O[J].J.Fluoresc.,2010,20(3):763-770.
[13]Wen X C,Zhao Y L,Wang L M,et al.Synthesis and fluorescence properties of europium,terbium doped Zn2+,Cd2+and Cr3+complexes[J].J.Rare Earth,2007,25(6):679-683.
[14]Gerisler H F,Hellwege K H.Zeitsch riftfur physic a hadrons and nuclei[J].Physik,1953,136(3):293-302.
[15]Yan B,Zhang H J,Wang S B,et al.Spectroscopic study of luminescence and energy transfer of binary and ternary complexes of rare earth with aromatic carboxylic acids and 1,10-phenanthroline[J].Spectrosc.Lett.,1998,31(3):603-613.
[16]Yan B,Zhang H J,Wang S B,et al.Intramolecular energy transfer mechanism between ligands in ternary rare earth complexes with aromatic carboxylic acids and 1,10-phenanthroline [J].J.Photochem.Photobiol:A,1998,116(3):209-214.
