Ag+与Hg2+对G-四链体DNA结构的破坏及其对构筑DNA逻辑门的应用
2013-10-17朱莉娜王海仙孙润丰孔德明李孝增
朱莉娜 王海仙 孙润丰 李 平 孔德明 李孝增
(1天津大学化学系,天津 300072)
(2青海民族学院外语系,西宁 810007)
(3南开大学教育部功能高分子材料重点实验室,天津 300071)
It is widely accepted that genomic DNA is mainly presented asclassic Watson-Crick double helix.However,as for some DNAs with specific sequences,they may adopt some particular structures[1].For example,some G-rich DNA sequences can form a wide variety of inter-or intramolecular four-stranded structures by Hoogsteen-type base pairing,termed G-quadruplexes[2-5].G-quadruplexes are unique highordered nucleic acid structures attracting more and more attention[6-8],since G-rich sequences with the potential to form quadruplexes have been found in several biologically important regions such as telomeric DNA and many gene promoter regions[9].It has been reported thatthe formation ofG-quadruplex in telomere regions can inhibit telomerase function and the reagents with the ability to promote G-quadruplex formation can be used as potential anti-cancer drugs[10].Most studies on G-quadruplexes are focused on the promotion of G-quadruplex formation.Whereas,there have been rare report on the agents that can inhibit the formation of the G-quadruplex or disrupt G-quadruplex structures.However,the study on these agents should also be helpful for the treatment of some sort of diseases[11].For example,the promotion of telomerase activity by disrupting the G-quadruplex formation by telomere sequence may play an important role in the treatment of liver cirrhosis,etc[12].
Some cations,such as K+,Na+,NH4+,Mg2+,Pb2+,Sr2+and Tl+,can promote the G-quadruplex formation and impart G-quadruplex stabilization.The effects of these ions on G-quadruplex stability and polymorphism have been widely investigated[13-15].To the best of our knowledge,only two ions,Hg2+ion and Ag+ion,have beenreported to have the ability to disrupt G-quadruplex structures in the presence of high concentration of K+[16-19].Hg2+can destroy the G-quadruplex formed by the G-rich sequence containing T bases via forming THg2+-T base pair[16-17],and Ag+ion can chelate to N7 and C6O groups in G bases and can greatly interfere with the formation of G-quadruplexes(Fig.1)[18-19].Some G-quadruplex DNAzyme-based sensors for Hg2+and Ag+have been developed by utilizing the G-quadruplex-disrupting abilities of these two ions[16-19].It is necessary to investigate the abilities of the two ions to disrupt the G-quadruplex structures in detail.Such studies will provide valuable information for the investigation in the interactions between the metal ions and G-quadruplexes and the design of metal complex-based G-quadruplex disrupting reagents,and will also help to develop some important analytical applications of these interactions,for example,constructing DNA logic gates.
Recently,molecular switches and molecular logic gates have attracted considerable attention owing to the importance of the development of miniaturized devices[20-21].As a new generation of molecular logic gates,DNA molecular logic gates have attracted increasing interest in recent years due to the wellregulated structures of DNAs and their abilities to store genetic information[22].
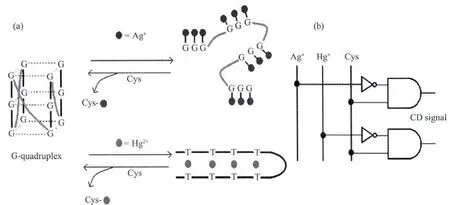
Fig.1 (a)Proposed mechanism of the disruption of G-quadruplexes by Ag+and Hg2+ions,and the reformation of G-quadruplexes in the presence of Cysteine(Cys).(b)The design of a DNA IMPLICATION logic gate
In the present study,the effects of Hg2+and Ag+ions on four representative G-quadruplexe structures were studied and compared by circular dichroism(CD) spectra and their G-quadruplex-disrupting mechanisms were also discussed.It is interesting that both of the two G-quadruplex-disrupting metal ions can tightly bind to the thiol group of cysteine(Cys).Thus,Cys may capture Ag+or Hg2+from DNA-Ag+or DNA-Hg2+complexes,promoting the reformation of G-quadruplexes.As a result,a DNA IMPLICATION logic gate was constructed by sequential addition of Ag+(or Hg2+)and Cys into G-quadruplex solutions.In this IMPLICATION logic gate,Ag+(or Hg2+)and Cys can be as the two inputs,and CD signal can be as the output.
1 Experimental
1.1 Materials and reagents
The oligonucleotides listed in Table 1 were purchased from Sangon Biotech.Co.,Ltd.(Shanghai,China).The concentrations of the oligonucleotides were represented as single-stranded concentration.Singlestranded concentration was determined by measuring the absorbance at 260 nm.Molar extinction coefficient was determined using a nearest neighbour approximation (http://www.idtdna.com/analyzer/Applications/OligoAnalyzer).AgNO3,Hg(Ac)2and L-cysteine(Cys)were obtained from Sigma.All chemical reagents were of analytical grade and used without further purification.Deionized and sterilized water (resistance >18 MΩ·cm-1)was used throughout the experiments.
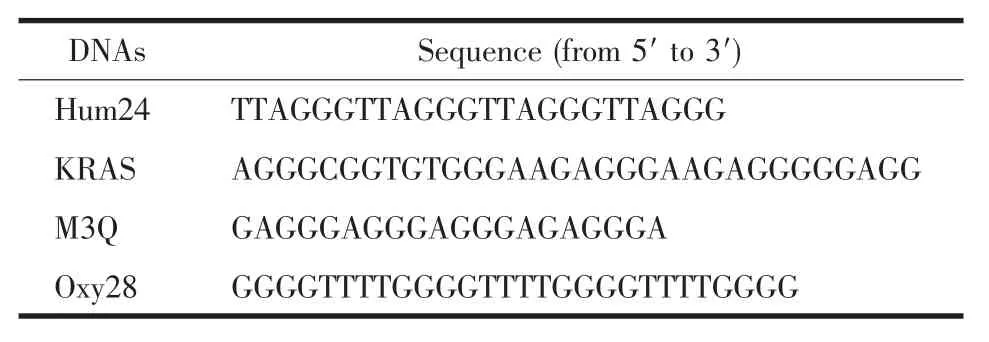
Table 1 Sequences of the oligonucleotides used in this work
1.2 Circular dichroism(CD)titration
3 mL reaction mixture was prepared in 10 mmol·L-1Tris-HAc buffer(pH=7.4)containing 1.5 μmol·L-1individual DNA oligonculeotide and 50 mmol·L-1KAc.In orderto ensure the formation ofG-quadruplex structures,the mixture was heated at 95℃for 5 min,cooled slowly to 25℃and then incubated at 25 ℃ overnight.To this mixture concentrated Ag+or Hg2+solution was added continuously,and the mixture was thoroughly mixed by repeated aspiration and injection.CD spectra of the mixtures were recorded between 200 and 320 nm in 1 mm path length cuvettes on a Jasco J-715 spectropolarimeter.Spectra were averaged from 3 scans recorded at 100 nm·min-1with a response time of 1 s and a bandwith of 1.0 nm.The CD signals and the concentrations of DNA oligonucleotides and metal ions were corrected for the amount of volume change during titration.
1.3 IMPLICATION logic operation
3 mL reaction mixture was prepared in 10 mmol·L-1Tris-HAc buffer(pH=7.4)containing 1.5 μmol·L-1individual DNA oligonculeotide and 50 mmol·L-1KAc.In orderto ensure the formation ofG-quadruplex structures,the mixture was heated at 95℃for 5 min,cooled slowly to 25℃and then incubated at25 ℃ overnight.To thismixture concentrated Ag+(or Hg2+)solution was added to reach a specified concentration,and CD spectrum of the mixture was recorded.Then,concentrated Cys solution was added to reach the same concentration as the metal ion,and CD spectrum of the mixture was recorded again.CD spectra were all recorded between 200 and 320 nm in 1 mm path length cuvettes on a Jasco J-715 spectropolarimeter.The spectra average was performed using the same procedure as that in section 1.2.
2 Results and discussion
2.1 Disrupting abilities of Ag+and Hg2+ions to the G-quadruplex formed by Hum24
The CD spectra of G-quadruplex-forming G-rich oligonucleotides in the presence of different concentrations of each ion were used to investigate the disruption of G-quadruplexes by the metal ions.It has been reported that CD spectra of a typical parallel G-quadruplex structure have a positive peak near 260 nm and a negative peak around 240 nm,whereas CD spectra of a typical antiparallel G-quadruplex structure have a positive peak at 295 nm and a negative peak close to 265 nm[23-24].Hum24,KRAS,M3Q and Oxy28(Table 1)were used as the four G-quadruplex-forming oligonucleotides.Hum24 is a G-rich oligonucleotide with the repeated subunit of the telomere from vertebrates.In the presence of 50 mmol·L-1K+,the CD spectrum of Hum24 displayed a positive peak at around 290 nm and a negative peak at 240 nm (Fig.2a),indicating that neither a typical antiparallel G-quadruplex nor a typical parallel G-quadruplex can be formed.It is very possible that Hum24 adopts a hybrid structure containing both syn-and anti-bonds[25-28].As shown in Fig.2a,the addition of Ag+ion leads to a gradual decrease in the positive CD signal intensity at 290 nm,reaching a plateau when the concentration of Ag+exceeded 6 μmol·L-1.According to the plot of CD signal density versus Ag+concentration,the IC50value,which represents the metal ion concentration required for 50% decrease of the signal intensity,can be calculated.The obtained IC50value is 2.2 μmol·L-1(Table 2).
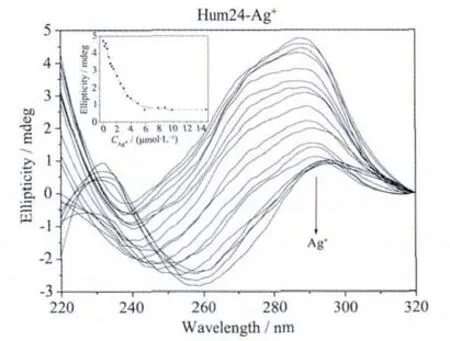
Fig.2a Ag+ion-mediated disruption of the G-quadruplex formed by Hum24.Ag+concentration-dependent change in CD spectra.The corrected concentrations of Ag+are (arrow direction):0,0.2,0.4,0.6,0.8,1.0,1.2,1.5,2.0,2.5,3.0,3.5,4.0,5.0,6.0,7.9,8.6,9.8 and 14.7 μmol·L-1.The inset represents the Ag+concentration-dependent change in CD signal at 290 nm.The solid line represents least square fit to the data
Under the same conditions,the addition of Hg2+could also lead to a gradual decrease in the positive CD signal intensity at 290 nm (Fig.2b),and the obtained IC50value is 7.5 μmol·L-1.(Table 2),which is higher than that of Ag+,indicating that the G-quadruplex-disrupting ability of Ag+is higher than that of Hg2+,at least for the G-quadruplex formed by Hum24.This can be easily interpreted by the reported G-quadruplex-disrupting mechanisms(Fig.1)[16-19].That is,Hg2+and Ag+ions can disrupt G-quadruplexes by forming T-Hg2+-T base pair and by chelating to G bases,repectively.As for Ag+ion,its binding sites in the G bases are just involved in the formation of G-quartets that are the basic structural motifs of G-quadruplexes.Therefore,when a Ag+ion binds with a G base in Hum24,the G base cannot participate in the formation of the corresponding G-quartet any more,and thenumberofG-quartetin theG-quadruplex decreases from 3 to 2.As a result,the stability of the G-quadruplex decreases greatly or stable quadruplex cannotbe formed any more.However,Hg2+ion interactes with T bases,which are located in the loops of the G-quadruplex.Thus,it is very possible that the interaction between Hg2+ion and T bases has less effect on G-quadruplex stability.
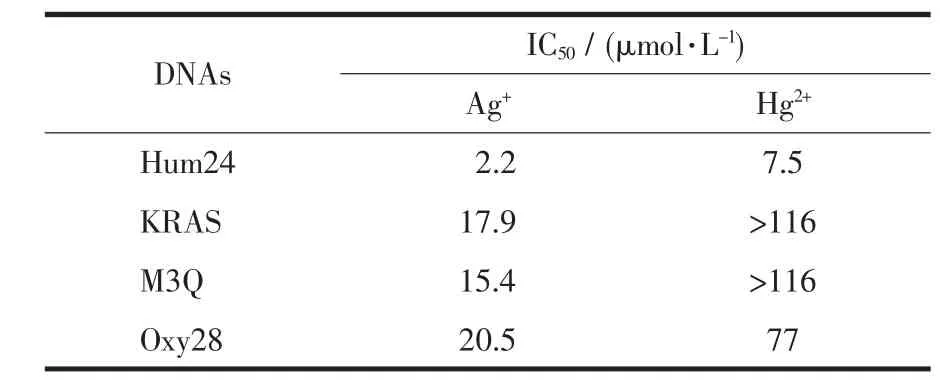
Table 2 Calculated IC50values in different G-quadruplex-metal ion systems
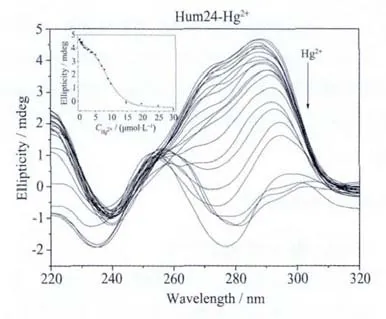
Fig.2b Hg2+ion-mediated disruption of the G-quadruplex formed by Hum24.Hg2+concentration-dependent change in CD spectra.The corrected concentrations of Hg2+are (arrow direction):0,0.2,0.4,0.6,0.8,1.0,1.2,1.5,2.0,2.5,3.0,3.5,4.0,5.0,6.0,7.0,7.9,8.9,9.8,14.7,19.6,24.8 and 29.7 μmol·L-1.The inset represents the Hg2+concentration-dependent change in CD signal at 290 nm.The solid line represents least square fit to the data
2.2 Disrupting abilities of Ag+and Hg2+ions to the G-quadruplex formed by KRAS
To further investigate the disrupting abilities of Hg2+and Ag+ions to G-quadruplexes,another oligonucleotide KRAS was used.KRAS is a 32-nucleotide G-rich sequence,which is located in the promoter of the human KRAS gene.The mammalian KRAS gene encodes for a guanine nucleotide binding protein of 21 kDa that is involved in an important cell-growth pathway[29-30].
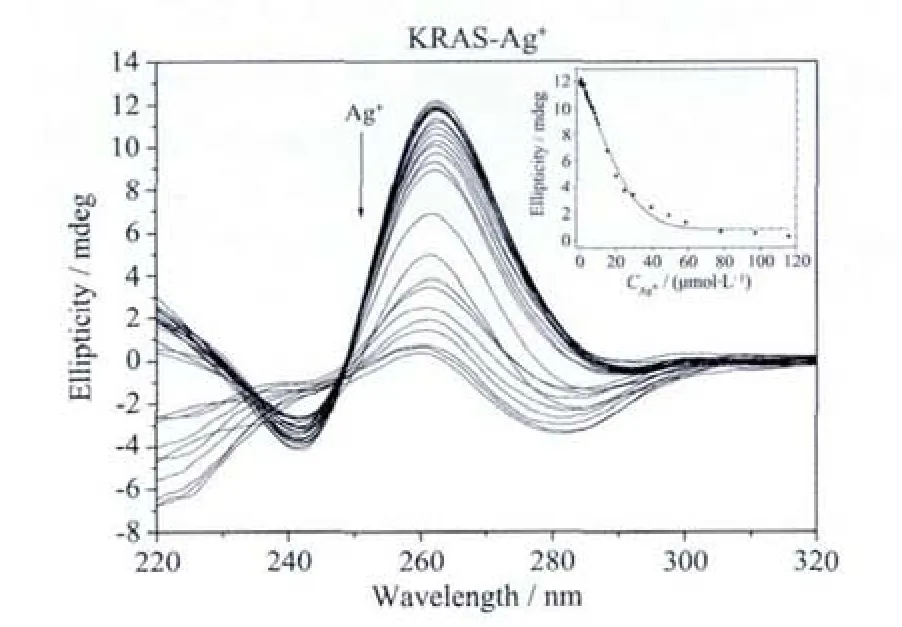
Fig.3a Ag+ion-mediated disruption of the G-quadruplex formed by KRAS.Ag+concentration-dependent change in CD spectra.The corrected concentrations of Ag+are (arrow direction):0,0.2,0.4,0.6,0.8,1.0,1.2,1.5,2.0,2.5,3.0,3.5,4.0,5.0,6.0,7.0,7.9,8.9,9.8,14.7,19.6,24.8,29.7,39.5,49.2,58.8,78.0,97.1 and 116.0 μmol·L-1.The inset represents the Ag+concentrationdependent change in CD signal at 262 nm.The solid line represents least square fit to the data
In the presence of 50 mmol·L-1K+,the CD spectrum of KRAS shows a positive peak at around 262 nm (Fig.3a),indicating that KRAS could form a parallel G-quadruplex[31-32].Increasing the concentration of the added Ag+ion causes a monotonic decrease of the positive CD signal,and the signal intensity nearly reaches zero when 116 μmol·L-1Ag+is added.The calculated IC50value is about 17.9 μmol·L-1(Table 2),which is much higher than that in Hum24-Ag+system.There are 12 G bases in Hum24,and all of them can participate in the formation of G-quartets.When any one of them interacts with Ag+ion,the conformation and stability of the G-quadruplex will be affected greatly.However,there are 21 G bases in KRAS sequence,and according to the previous reports,only 12 G bases in KRAS participate in the formation of G-quartets[31-32].That is to say,other 9 G bases are located in the loops of the G-quadruplex.When the G bases in the loops interact with Ag+ion,the G-quartets may not be destroyed,and the conformation and stability of the G-quadruplex may not be obviously affected.As a result,the KRAS-Ag+system has a higher IC50value than Hum24-Ag+system.
Under the same conditions,the addition of Hg2+could also lead to a gradual decrease of the positive CD signal intensity,but the decreasing rate was much lower than that in KRAS-Ag+system (Fig.3b).Even when 116 μmol·L-1Hg2+ion is added,the positive peak still remained a high signal intensity,indicating thatHg2+ion can notentirely destroy the G-quadruplex structure.The calculated IC50value was >116 μmol·L-1(Table 2),suggesting the weak ability of Hg2+ion to disrupt the G-quadruplex structure formed by KRAS.This can be easily interpreted.The weak disruption of Hg2+ion should be attributed to the only 2 T bases in KRAS sequence.
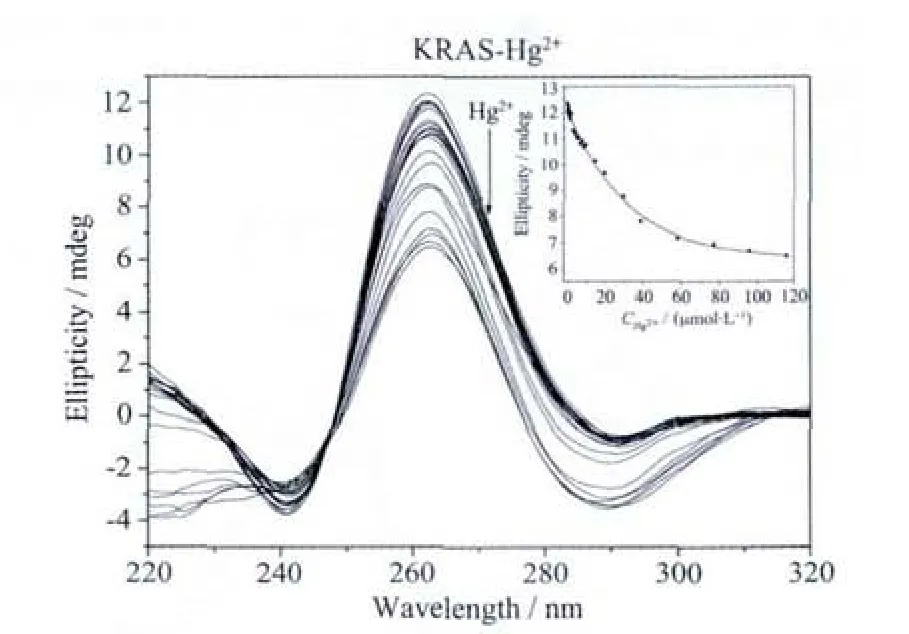
Fig.3b Hg2+ion-mediated disruption of the G-quadruplex formed by KRAS.Hg2+concentration-dependent change in CD spectra.The corrected concentrations of Hg2+are (arrow direction):0,0.2,0.4,0.6,0.8,1.0,1.2,1.5,2.0,3.0,4.0,5.0,6.0,7.0,7.9,8.9,9.8,14.7,19.6,24.8,29.7,39.5,49.2,58.8,78.0,97.1 and 116.0 μmol·L-1.The inset represents the Hg2+concentration-dependent change in CD signal at 262 nm.The solid line represents least square fit to the data
2.3 Disrupting abilities of Ag+and Hg2+ions to the G-quadruplex formed by M3Q
M3Q is a 20-nucleotide G-rich sequence located on the upstream of the initiation codon of MT3-MMP mRNA.Matrix metalloproteinases (MMPs)are zincdependent endopeptidases and coded by MT3-MMP mRNA.The upregulation of MMP protein expression level is associated with the invasiveness of many cancers[33-34].
In the presence of 50 mmol·L-1K+,M3Q also folded into a parallel G-quadruplex with a positive peak at around 262 nm in the CD spectrum (Fig.4a).With an increased concentration of Ag+ion,the CD signal intensity was decreased significantly,reaching zero at about 58 μmol·L-1Ag+.The calculated IC50value is 15.4 μmol·L-1(Table 2),which is much higher than that of the Hum24-Ag+system and a little lower than that of the KRAS-Ag+system.There are 14 G bases in M3Q,and 12 of them participate in the formation of G-quartets.The other two are located in the loops of the G-quadruplex.The number of the G bases in loops is just between those in the G-quadruplexes formed by KRAS and Hum24.This result further demonstrates the proposed G-quadruplex-disrupting mechanism of Ag+ion.
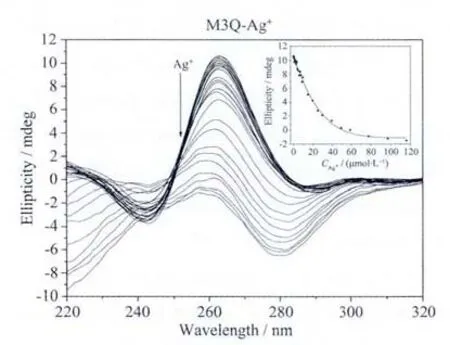
Fig.4a Ag+ion-mediated disruption of the G-quadruplex formed by M3Q.Ag+concentration-dependent change in CD spectra.The corrected concentrations of Ag+are (arrow direction):0,0.2,0.4,0.6,0.8,1.0,1.2,1.5,2.0,2.5,3.0,3.5,4.0,5.0,6.0,7.0,7.9,8.9,9.8,14.7,19.6,24.8,29.7,39.5,49.2,58.8,78.0,97.1 and 116.0 μmol·L-1.The inset represents the Ag+concentrationdependent change in CD signal at 262 nm.Thesolid line represents least square fit to the data
According to the reported G-quadruplexdisrupting mechanism of Hg2+ion[16-17],Hg2+ion should notdestroy the G-quadruplex formed by M3Q,because there is no T base in M3Q at all.To our surprise,however,the CD signal intensity increases with increasing the concentration of Hg2+ion in the low Hg2+concentration range (Fig.4b),suggesting that low concentrations of Hg2+can promote the folding of M3Q into G-quadruplex structure or increase the stability of the G-quadruplex.Further addition of Hg2+could also decrease the CD signal intensity though the signal intensity still remains at a very high level even when 116 μmol·L-1Hg2+is added.That is to say,besides the formation of T-Hg2+-T base pairs,Hg2+ion can also disrupt G-quadruplex structures through other way(s).
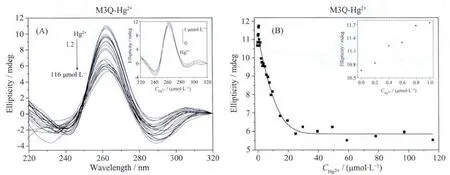
Fig.4b Hg2+ion-mediated disruption of the G-quadruplex formed by M3Q.(A)Hg2+concentration-dependent change in CD spectra.The corrected concentrations of Hg2+are (arrow direction):0,1.0,1.2,1.5,2.0,2.5,3.0,3.5,4.0,5.0,6.0,7.0,7.9,8.9,9.8,14.7,19.6,24.8,29.7,39.5,49.2,58.8,78.0,97.1 and 116.0 μmol·L-1.The inset represents the CD spectrum change in the Hg2+concentration range of 0~1.0 μmol·L-1.The corrected concentrations of Hg2+are(arrow direction):0,0.2,0.4,0.6,0.8 and 1.0 μmol·L-1. (B)Hg2+concentration-dependent change in CD signal at 262 nm.The solid line represents least square fit to the data.The inset represents the CD signal change in the Hg2+concentration range of 0~1.0 μmol·L-1
2.4 Disrupting abilities of Ag+and Hg2+ions to the G-quadruplex formed by Oxy28
Asforthe threeoligonucleotidesmentioned above,KRAS and M3Q adopt parallel G-quadruplex structure,Hum24 adoptsa hybrid G-quadruplex structure.To investigate the disrupting abilities of Hg2+and Ag+ions to G-quaduplexes with antiparallel structure,Oxy28 was used.Oxy28 is a 28-nucleotide G-rich sequence with the repeated subunit of the telomere from Oxytricha.In the presence of 50 mmol·L-1K+,the CD spectrum of Oxy28 displays a positive peak at around 293 nm and negative peak at around 260 nm,which is typical of antiparallel G-quadruplexes.As shown in Fig.5a,the CD signal intensity of Oxy28 also monotonically decreases with the increment of Ag+.The calculated IC50value is 20.5 μmol·L-1(Table 2),which is the highest one in the four G-quadruplexes studied in this work.This is not surprised.Because the G-quadruplex formed by Oxy28 contained four G-quartets,the stability of the G-quadruplex formed by Oxy28 is certainly higher than the G-quadruplexes containing three G-quartets.The higher stability might increase the difficulty of Ag+-mediated disruption of G-quadruplex structures.When one G-quartet is destroyed by Ag+and the number of G-quartet decreases from four to three,the stable G-quadruplex still exists.

Fig.5a Ag+ion-mediated disruption of the G-quadruplex formed by Oxy28.Ag+concentration-dependent change in CD spectra.The corrected concentrations of Ag+are (arrow direction):0,0.2,0.4,0.6,0.8,1.0,1.2,1.5,2.0,2.5,3.0,3.5,4.0,5.0,6.0,7.0,7.9,8.9,9.8,14.7,19.6,24.5,29.3,39.0,48.5,58.1,77.0,95.8 and 114.5 μmol·L-1.The inset represents the Ag+concentrationdependent change in CD signal at 293 nm.The solid line represents least square fit to the data
Oxy28 is a T-rich oligonucleotide.The addition of Hg2+should has significant effect on the G-quadruplex formed by Oxy28 through T-Hg2+-T base pair formation.As shown in Fig.5b,the addition of Hg2+indeed leads to the decrease of the CD signal intensity,but the decreasing rate is not so great as we expect.The calculated IC50value is 77 μmol·L-1,which is lower than those of KRAS and M3Q,but much higher than that of Hum24.The lower IC50value in comparison to KRAS and M3Q can be interpreted by the higher T-base number in Oxy28.The higher IC50value in comparison to Hum24 may be attributed to the higher stability of the G-quadruplex formed by Oxy28.
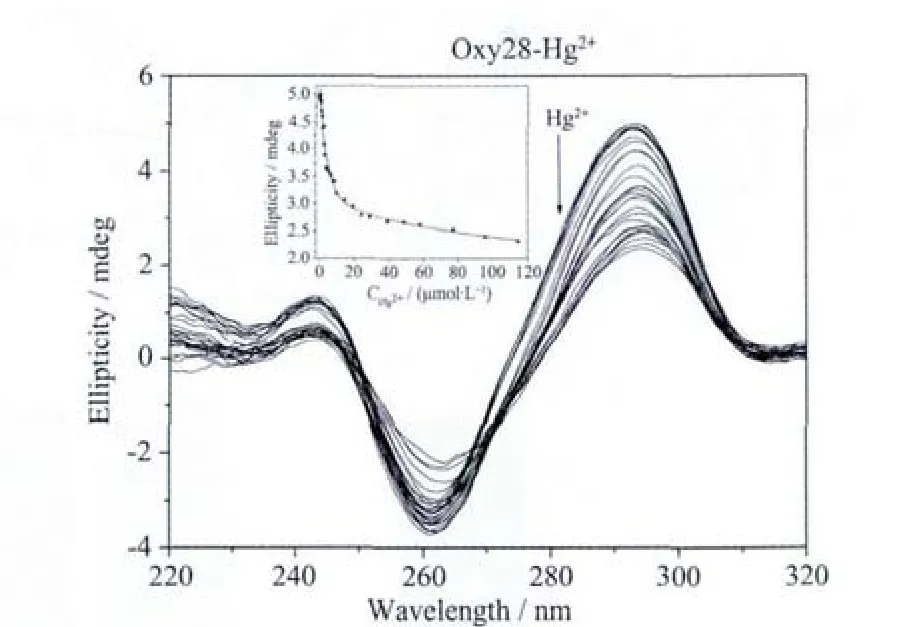
Fig.5b Hg2+ion-mediated disruption of the G-quadruplex formed by Oxy28.Hg2+concentration-dependent change in CD spectra.The corrected concentrations of Hg2+are (arrow direction):0,0.2,0.4,0.6,0.8,1.0,1.2,1.5,2.0,2.5,3.0,3.5,4.0,5.0,6.0,7.0,7.9,8.9,9.8,14.7,19.6,24.5,29.3,39.0,48.5,58.1,77.0,95.8 and 114.5 μmol·L-1.The inset represents the Hg2+concentrationdependent change in CD signal at 293 nm.The solid line represents least square fit to the data
Comparing with K+,Na+,NH4+,Mg2+,Pb2+,Sr2+and Tl+,Hg2+and Ag+show effective disruption abilities to G-quadruplex structures as described by above experiments.The ionic radius is a parameter that aptly describes how well guanine tetrads are stabilized by various cations,and K+and Sr2+with similar ionic radii of approximately 0.13 nm imparting G-quadruplex prominent stabilization are believed to fit exceptionally well in the cavities between guanine tetrads[13].The ionic radii of Hg2+and Ag+are similar with that of Na+(0.1 nm).According to the above fit model,they should show the similar effect on the stabilization of G-quadruplex structures with Na+.So we believe that the disruption abilities of Hg2+and Ag+to G-quadruplex structures may be due to the formation of the coordinate bonds between Hg2+or Ag+with the nitrogen and/or oxygen atoms in the bases of the strands,instead of the interaction with guanine tetrads.
2.5 Construction of DNA IMPLICATION logic gates
The experiments mentioned above show that both Ag+and Hg2+can disrupt G-quadruplex structures,though the disrupting abilities of them are highly dependent on the stability and base component of G-quadruplexes.It is reported that Cys,a thiol-containing amino acid,can tightly bind to Ag+or Hg2+[35-36].Thus the interactions between the two metal ions and DNA bases could be influenced by Cys.Based on this,many highly sensitive and specific Cys detection methods have been reported[37-40].Herein,using Ag+and Cys as the two inputs and the CD signal as output,a DNA IMPLICATION logic gate is designed.Fig.6 shows the operation of the IMPLICATION logic gate utilizing the influence of Ag+or/and Cys on the CD spectrum of Hum24.Similar spectrum variation can be observed in the operation to KRAS,M3Q and Oxy28.As shown in Fig.6,with no input or with Cys input alone,CD signal maintains at high levels with an output of 1.With Ag+input alone,the CD signal decreases sharply,giving an output of 0.With both Ag+and Cys inputs,the CD signal is recovered to almost its initial level with an output of 1,suggesting the disruption of the Ag+-DNA complex and the reformation of G-quadruplex structure.A bar graph of the output signals of the system is in Fig.6(B),and the truth table is in Fig.6(C).Using Hg2+and Cys as the two inputs,similar IMPLICATION logic gate can also be designed.

Fig.6 Operation of the IMPLICATION logic gate utilizing the influence of Ag+and/or Cys on the CD spectrum of Hum24.(A)CD spectra of Hum24 in the presence of different inputs.1:CAg+=0 μmol·L-1,CCys=0 μmol·L-1;2:CAg+=8 μmol·L-1,CCys=0 μmol·L-1;3:CAg+=0 μmol·L-1,CCys=8 μmol·L-1;4:CAg+=8 μmol·L-1,CCys=8 μmol·L-1.(B)The CD signal intensities at 290 nm in the presence of different inputs.The dashed line represents the defined threshold value.(C)Truth table for the DNA IMPLICATION logic gate
3 Conclusions
In conclusion, the G-quadruplex-disrupting abilities of Hg2+and Ag+ions were studied using four representative G-quadruplex-forming sequences.According to the experimental results,some primary conclusions can be drawn:(1)Hg2+and Ag+ions both exhibit the disrupting abilities to the representative G-quadruplex-forming sequences.Compared to Hg2+ion,Ag+ion can display higher G-quadruplex-disrupting ability. (2)Ag+ion can be used as a general G-quadruplex-disrupting reagent.Itcan disruptG-quadruplexes by interacting with G bases.Because G bases are necessary for G-quadruplex formation,the G-quadruplex-disrupting ability of Ag+ion can be applicable to any G-quadruplexes.(3)The G bases located in the loops of G-quadruplexes can also interact with Ag+ion.As a result,the presence of G bases in G-quadruplex loops can decrease the G-quadruplex-disrupting ability of Ag+ion.(4)The G-quadruplex-disrupting ability of Hg2+to T-rich G-quadruplexesishigherthan thatto T-poorG-quadruplexes,demonstrating that the formation of THg2+-T base pairs is one important way for Hg2+ion to disrupt T-rich G-quadruplexes.But because Hg2+ion can also disrupt G-quadruplexes without T bases(M3Q),Hg2+ion may also disrupt G-quadruplexes by other way(s).(5)The stability of the G-quadruplex has a great effect on the G-quadruplex-disrupting abilities of Ag+and Hg2+ions.These results may provide some important information for the development of highly efficient G-quadruplex-disrupting reagents, for example metal complexes-based G-quadruplexdisrupting reagents.
The G-quadruplex-disrupting abilities of Ag+and Hg2+,together with their strong interactions with Cys,provide the possibility to carry out an IMPLICATION logic opertation.Thus,using Ag+(or Hg2+)and Cys as the two inputs,CD signal as the output,a DNA IMPLICATION logic gate has been constructed.To the best of our knowledge,this logic gate is the third example of a molecular logic gate using CD signal as output[41-42],and the first example of a DNA IMPLICATION logic gate using CD signal as output.
[1]Gilbert D E,Feigon J.Curr.Opin.Struct.Biol.,1999,9:305-314
[2]Huppert J L.Chem.Soc.Rev.,2008,37:1375-1384
[3]Williamson J R.Annu.Rev.Biophys.Biomol.Struct.,1994,23:703-730
[4]Rhodes D,Giraldo R.Curr.Opin.Struct.Biol.,1995,5:311-322
[5]Zahler A M,Williamson J R,Cech T R,et al.Nature,1991,350:718-720
[6]Howell L A,Searcey M.ChemBioChem.,2009,10:2139-2143
[7]Shi S,Yao T M,Ji L N,et al.Dalton Trans.,2012,41:5789-5793
[8]Shi S,Yao T M,Ji L N,et al.J.Inorg.Biochem.,2013,121:19-27
[9]Lipps H J,Rhodes D.Trends Cell.Biol.,2009,19:414-422
[10]Neidle S.FEBS J.,2009,277:1118-1125
[11]Shalaby T,Hiyama E,Grotzer M A.Anticancer Agents Med.Chem.,2010,10:196-212
[12]Lechel A,Manns M P,Rudolph K L.J.Hepatol.,2004,41:491-497
[13]Simonsson T.Biol.Chem.,2001,382:621-628
[14]Gill M L,Strobel S L,Loria J P.Nucleic Acids Res.,2006,34:4506-4514
[15]Liu W,Liang H J,Fu Y.J.Phys.Chem.B,2011,115:13051-13056
[16]Li T,Dong S J,Wang E K.Anal.Chem.,2009,81:2144-2149
[17]Li T,Li B,Wang E K,et al.Chem.Commun.,2009,45:3551-3553
[18]Zhou X H,Kong D M,Shen H X.Anal.Chem.,2010,82:789-793
[19]Kong D M,Xu J,Shen H X.Anal.Chem.,2010,82:6148-6153
[20]Topal S Z,Gürek A G,Ertekin K,et al.Mater.Chem.Phys.,2010,121:425-531
[21]Chen X,Wang Y,Liu Q,et al.Angew.Chem.Int.Ed.Engl.,2006,45:1759-1762
[22]Xie W Y,Huang W T,Li N B,et al.Chem.Commun.,2012,48:82-84
[23]Paramasivan S,Rujan I,Bolton P H.Methods,2007,43:324-331
[24]Kong D M,Cai L L,Guo J H,et al.Biopolymers,2009,91:331-339
[25]Monchaud D,Yang P,Lacroix L,et al.Angew.Chem.Int.Ed.Engl.,2008,47:4858-4861
[26]Ambrus A,Chen D,Dai J,et al.Nucleic Acids Res.,2006,34:2723-2735
[27]Kong D M,Ma Y E,Guo J H,et al.Anal.Chem.,2009,81:2678-2684
[28]Luu K N,Phan A T,Kuryavyi V,et al.J.Am.Chem.Soc.,2006,128:9963-9970
[29]Malumbres M,Barbacid M.Nat.Rev.Cancer,2003,3:459-465
[30]Xodo L,Paramasivam M,Membrino A,et al.Nucleic Acids Symp.Ser.,2008,52:159-160
[31]Chen Z,Zheng K W,Hao Y H,et al.J.Am.Chem.Soc.,2009,131:10430-10438
[32]Paramasivam M,Membrino A,Cogoi S,et al.Nucleic Acids Res.,2009,37:2841-2853
[33]Hotary K,Li X Y,Allen E,et al.Genes Dev.,2006,20:2673-2686
[34]Morris M J,Basu S.Biochem.,2009,48:5313-5319
[35]Gruen L C.Biochim.Biophys.Acta,1975,386:270-274
[36]Burstein Y,Sperling R.Biochim.Biophys.Acta,1970,211:410-412
[37]Guo J H,Kong D M,Shen H X.Biosens.Bioelectron.,2010,26:327-332
[38]Li T,Shi L L,Wang E K,et al.Chem.Eur.J.,2009,15:3347-3350
[39]Ruan Y B,Li A F,Zhao J S,et al.Chem.Commun.,2010,46:4938-4940
[40]Jia S M,Liu X F,Li P,et al.Biosens.Bioelectron.,2011,27:148-152
[41]D′Urso A,Mammana A,Balaz M,et al.J.Am.Chem.Soc.,2009,131:2046-2047
[42]Zhou Y C,Zhang D Q,Zhang Y Z,et al.J.Org.Chem.,2005,70:6164-6170
