一维配位聚合物{[CO(DPA)(H 2O)4]·(DPDO)·(H 2O)}N(H 2DPA=2,2′-联苯二酸,DPDO=N,N′-二氧化-4,4′-联吡啶)的晶体结构和光谱性质
2013-09-15党东宾
潘 慧 柏 龚 安 冰 党东宾
(河南大学化学化工学院,河南省多酸化学重点实验室,开封 475004)
The construction of coordination polymers via self-assembly of predesigned ligands with appropriate metal ions has attracted increasing interests,due to the intriguing structure motifs and their potential applications in catalysis, molecular adsorption,magnetism,optical devices and molecular sensors[1-4].The polycarboxylates as one important subfamily of ligands are often employed to construct interesting framework structures because of not only their abundant coordination modes (such as monodentate,bidentate and chelate)but also their abilities to act as hydrogen-bond acceptors and donors[5-7].Compare with rigid organic polycarboxylic ligands,the flexible V-shaped 2,2′-biphenyldicarboxylate (H2dpa)are often used as bridging ligands to assemble different coordination polymers with metal ions via the cooperative effect of various possible coordination modes and the flexibility of the ligand around the C-C bond[8-11].On the other hand,the introduction of the second organic component affords interestingly extended structures and functional properties.This strategy is currently one of the powerful methods in the design of coordination polymers[12-13].
As a part of our continuing investigations on coordination polymers[14-20],herein,we reported the synthesis and crystal structure of a one-dimensional Co(Ⅱ) coordination polymer{[Co(dpa)(H2O)4]·(dpdo)·(H2O)}n(1)containing the bridging ligand 2,2′-biphenyldicarboxylate (H2dpa) and free organic component 4,4′-bipyridine-N,N′-dioxide(dpdo).
1 Experimental
1.1 Materials and methods
All chemicals were of reagent grade quality obtained from commercial sources and used without further purification.Ligand dpdo was synthesized and characterized by a previously reported procedure[21].Elemental analyses(C,H and N)were carried out on a Perkin-Elmer 240C analytical instrument.IR spectra were recorded from KBr pellets with a Nicolet 170 SXFT-IR spectrophotometer in the 4 000~400 cm-1region.The UV-Vis spectra were obtained on a Shimazu UV-250 spectrometer in the range of 800~190 nm in the solid state.X-ray powder diffraction patterns were recorded on a D/max-γA rotating anode X-ray diffractometer with Cu sealed tube(λ=0.154 178 nm).The thermogravimetric analysis was carried out on a Perkin-Elmer-7 thermal analyzer at a heating rate of 10℃·min-1from 25 to 800℃,and the luminescent spectra were performed on a Hitachi F-7000 fluorescence spectrophotometer.
1.2 Synthesis of the polymer
{[Co(dpa)(H2O)4](dpdo)(H2O)}n(1): A mixture containing Co(Ac)2·4H2O(0.125 g,0.5 mmol),H2dpa(0.121 g,0.5 mmol),dpdo (0.094 g,0.5 mmol)and NaOH (0.020 g,0.5 mmol)in 8 mL deionized water was sealed in a 25 mL Teflon lined stainless steel container and heated at 120 ℃ for 3 d.Red block crystals of 1 were collected in a 62%yield.Anal.for C24H26CoN2O11:calcd.(%):C 49.92,H 4.54,N 4.85;found(%):C 50.15,H 4.48,N 4.95.IR (cm-1,KBr pellet):v=3 392(m),3 107(m),1 601(w),1 574(m),1 548(s),1 475(m),1 449(w),1 406(s),1 232(s),1 190(s),1 024(w),839(s),760(m),547(m).
1.3 Crystal structure determination
A suitable sample of size 0.40 mm ×0.31 mm ×0.18 mm was chosen for the crystallographic study and then mounted on a Bruker Smart APEXⅡCCD diffractometer withωandφscan mode in the range of 1.77°<θ<25.00°.All diffraction measurements were performed at room temperature using graphite monochromatized Mo Kα radiation(λ=0.071 073 nm).A total of 12 354 (4 299 independent,Rint=0.034 6)reflections were measured.The structure was solved by direct methods and refined on F2by using fullmatrix least-squares methods with SHELXL-97 program[22-23].All non-hydrogen atoms were refined anisotropically by full-matrix least-squares techniques.All hydrogen atoms associated with carbon atoms were placed in the calculated positions and refined as a riding mode.The hydrogen atoms of water molecules were located from the successive difference Fourier syntheses and refined with the isotropic displacement parameters setting to 1.2 times those of their carrier atoms.Structure solution and refinement based on 3473independentreflectionswith I>2σ(I).Spacegroup,lattice parameters and other relevant information are listed in Table 1,and selected bond lengths and angles are given in Table 2.
CCDC:922034.
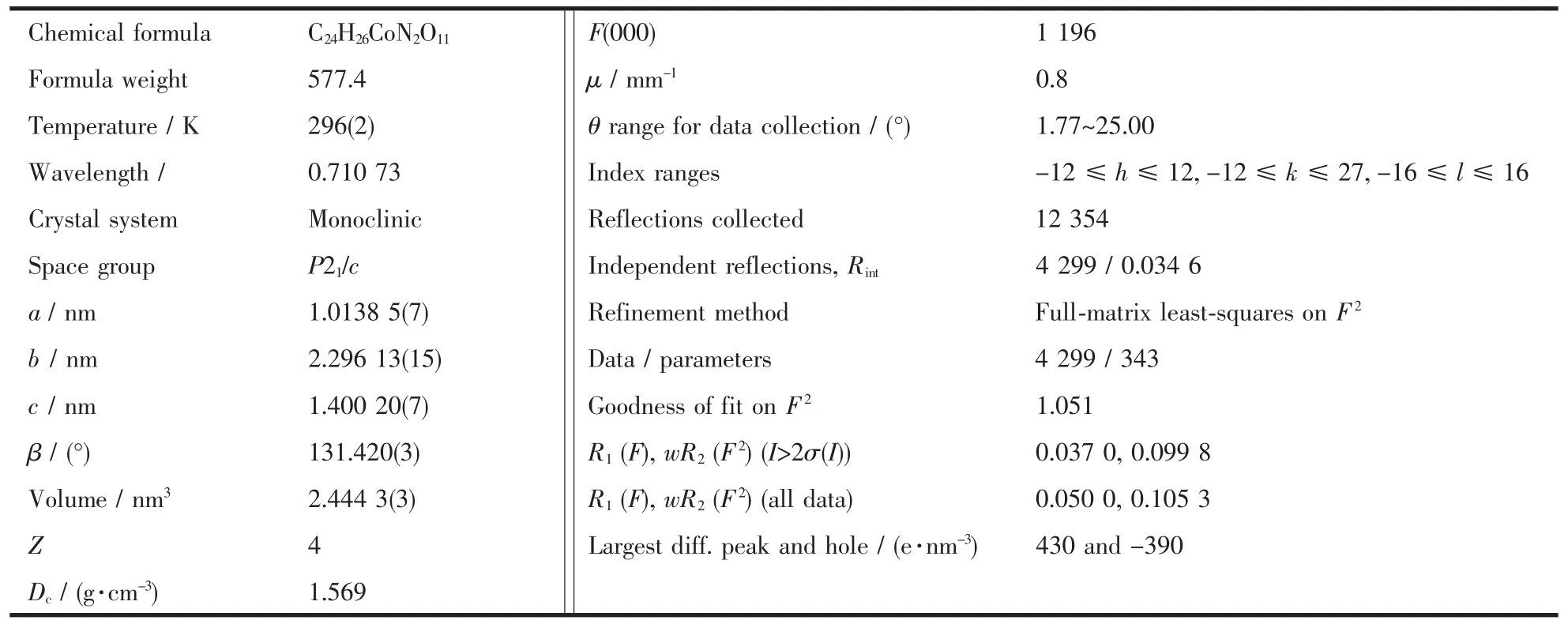
Table 1 Summary of crystal data and refinement results for the polymer 1

Table 2 Selected bond lengths(nm)and angles(°)of the polymer 1
2 Results and discussion
2.1 IR and UV-Vis spectra
In the IR spectrum of polymer 1,the moderate broad band at 3 392 cm-1is assigned to theν(OH)stretching frequency indicating the presence of water molecule.The characteristic bands of the carboxyl groups are shown at 1 548 cm-1for antisymmetric stretching and 1 406 cm-1for symmetric stretching.The separation value of 142 cm-1indicates that the carboxyl groups of H2dpa function in the unidentate coordination mode[24].Moreover,two strong bands at 1 232 and 1 190 cm-1indicate the existence of dpdo[25].These assignments were finally confirmed by X-ray crystallography(see below).
As depicted in Fig.1,the electronic spectrum of the polymer 1 in the solid state shows three absorption bands in the region between 340 and 800 nm.The intense band around 355 nm is due to the ππ* transition of the aromatic units.The bands observed at 495 and 672 nm can be attributed to a parity forbidden d-d type transition of Co(Ⅱ)[26].
2.2 Crystal and molecular structure
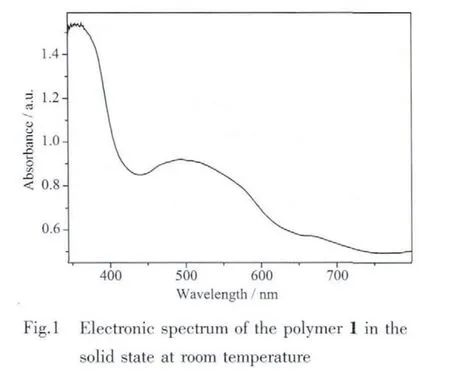
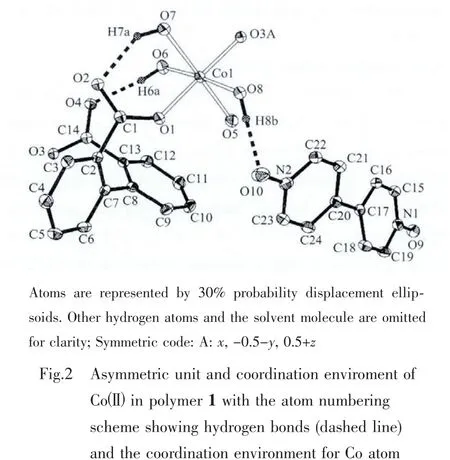
Single-crystal X-ray structural analysis revealed that the structure of 1 exhibits a three-dimensional supramolecular framework containing free organic units 4,4′-bipyridine-N,N′-dioxide (dpdo)based on one-dimensional polymeric chains,which formed through the coordination interaction of Co(Ⅱ)and the bridging organic ligands 2,2′-biphenyldicarboxylate(dpa2-).Polymer 1 crystallizes in a monoclinic space group P21/c.The asymmetric unit consists of one Co(Ⅱ)ion,one dpa2-,one independent dpdo,four coordinated water molecules and one crystal water molecule.As shown in Fig.2,Co(1)atom is coordinated by two monodentate carboxylate oxygen atoms O(1)and O(3A)from two dpa2-ligands(O(1)trans to O(3A),Co(1)-O(1),0.202 3(2)nm;Co(1)-O(3A),0.203 7(2)nm,O(1)-Co(1)-O(3A),177.48(7)°),four oxygen atoms O(5),O(6),O(7)and O(8)from four coordinated water molecules with the average Co(1)-O distant of 0.215 nm to attain a distorted octahedral geometry.Each dpa2-ligand is bound to two Co(Ⅱ)centers through two monodentatecarboxylategroupswith the Co(1)…Co(1B)(symmetric code B:x,-0.5-y,-0.5+z)separation of 0.701 nm and each Co(Ⅱ)center is coordinated by two dpa2-ligands thereby generating a one-dimension helical chain architecture(Fig.3).The helical pitch is 1.400 2(7)nm(the unit cell length along the crystallographic c axis),which is given by one full rotation around the 21helical axis.Similar monohelical chain structure has also been observed in the related polymers[27-28].Consequently,the 2,2′-biphenyldicarboxylate adopts a twist conformation with the torsion angle of-63.1(3)°around C(2)-C(7)-C(8)-C(13)bonds.The angle between two phenyl rings is 63.5°.In addition,four types of O(5)-H(5a)…O(4A),O(6)-H(6a)…O(4),O(7)-H(7a)…O(2)and O(8)-H(8a)…O(2A)(symmetry code:A:x,-1/2-y,1/2+z)hydrogen bonds are found in the 1D helical chain and also play an important role in stabilizing network(Table 3).It is interesting to note that the existence of O(1W)-H(1Wa)…O(7),O(1W)-H(1Wb)…O(8),O(5)-H(5b)…O(1WC)and O(6)-H(6b)…O(1WC)(symmetry code:C:-1+x,-1/2-y;-1/2+z)hydrogen bonds between the 1D chains and solvent water molecules gives further rise to a 2D supramolecular network,in which each solvent water molecule acts as a 4-connected node(Fig.4).
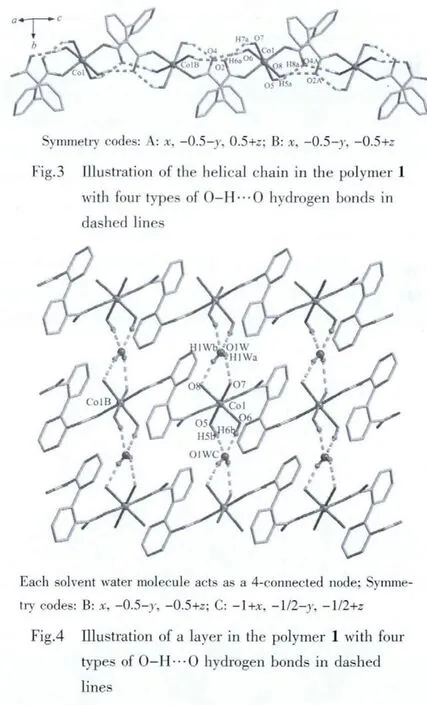
Detailed structural analysis revealed that in the solid state the adjacent layers are separated by dpdo units.The O(7)-H(7b)…O(9D),O(8)-H(8b)…O(10)and two types of C(19)-H(19a)…O(3F),C(22)-H(22a)…O(2G)(symmetry codes:D:1-x,-1/2+y,1/2-z;F:1-x,-y,-z;G:x,-1/2-y,1/2+z)hydrogen bonds are observed,which lead to a 3D supramolecular structure of 1(Fig.5).The O-H…O are between O(7),O(8)of the coordinated water molecules as hydrogen-bond donors and the oxygen atoms O(9),O(10)of dpdo as acceptor.Two types of C-H…O hydrogen bonds are found between the carbon atoms of dpdo and the oxygen atoms of the two coordinated water molecules.At the same time,the adjacent dpdo molecules are connected together through C(15)-H(15a)…O(10E)and C(23)-H(23a)…O(9H)(symmetry codes:E:-1+x,y,z;H:1+x,y,z)hydrogen bonds to form a dimer structure.Herein,each dpdo exhibits slightly twist conformation due to the existence of multiform hydrogen bonds with the dihedral angle of two pyridyl rings of 21.0°.Although these hydrogen bonds interactions are weak compared to the metal-oxygen coordination bonds,it is suggested that these interactions are important in the packing of the molecules.
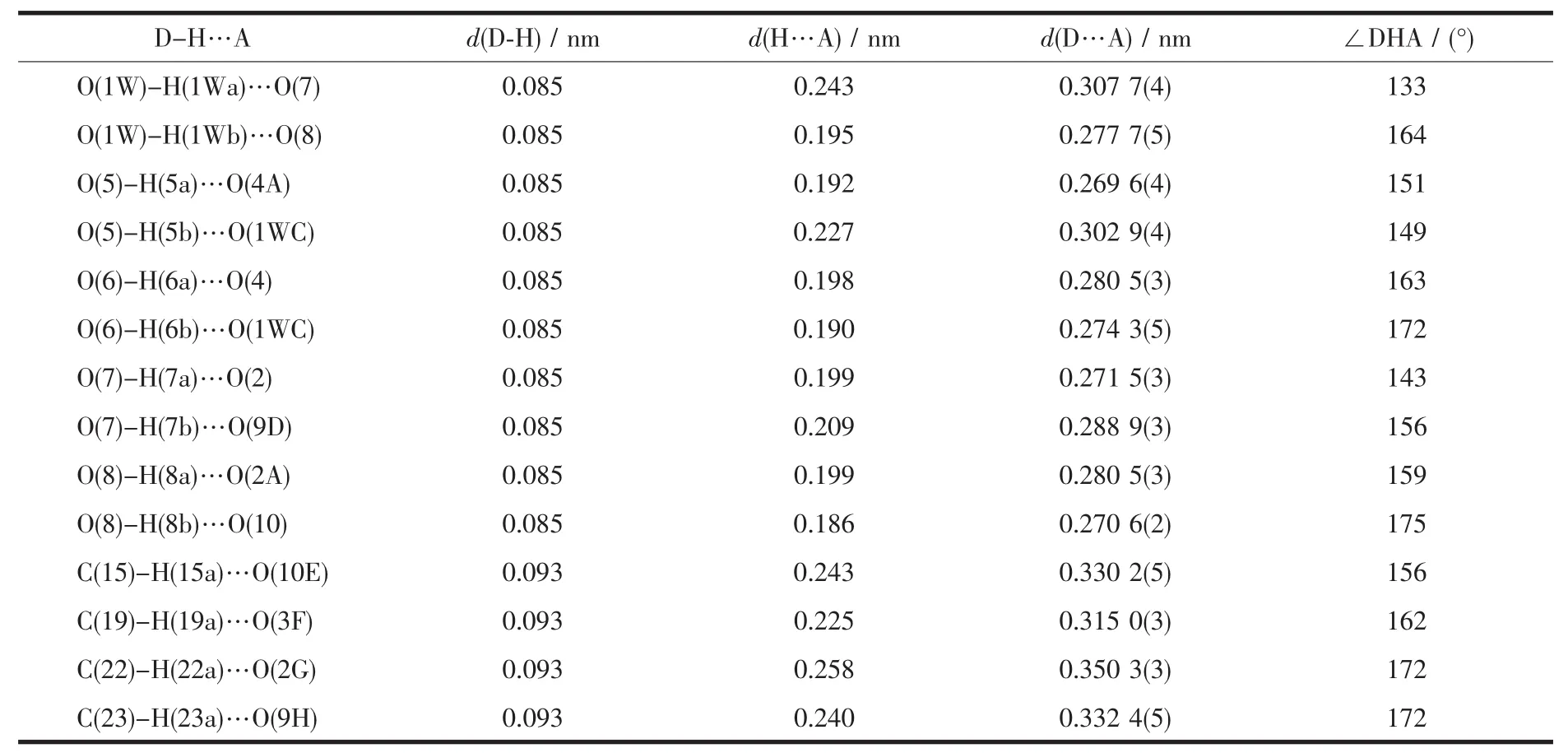
Table 3 Hydrogen bonding interactions of the polymer 1

2.3 Luminescence properties
The solid-state photoluminescent spectra of the polymer 1 and dpdo have been measured at room temperature.As shown in Fig.6,upon the excitation at 275 nm,the polymer 1 shows one emission peak at 430 nm,while the emission maximum of dpdo is at 460 nm upon excitation at 275 nm.Moreover,the results of the reported literatures reveal that 2,2′-biphenyldicarboxylate shows the main emission peak at 459 nm which is attributed to the π*-π orπ*-n transitions[29-30].Compared to that of ligands,the nature of the emissive behavior of the polymer 1 may be assigned mainly to the intraligand π*-π or π*-n electronic transitions[31-33].Similar emissions have been observed in the related complexes[34-35].

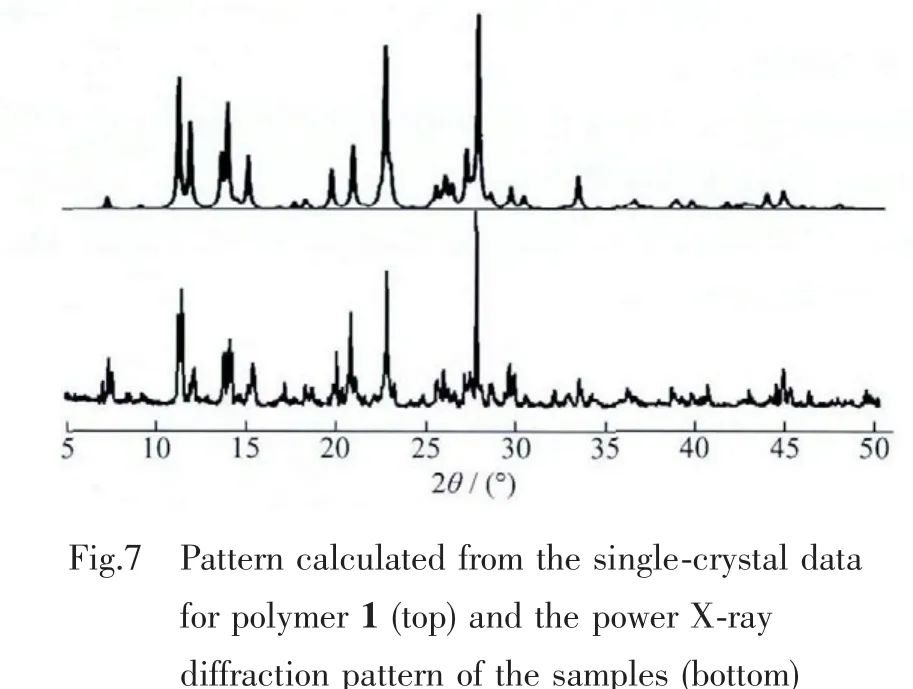
2.4 X-ray powder diffraction(XRPD)
As shown in Fig.7,the experimental XRPD pattern of the bulk product of 1 is in good agreement with the simulated XRPD pattern from single-crystal X-ray diffraction,indicating the phase purity of the sample.The intensity difference between experimental and simulated XRPD patterns is due to the variation in preferred orientation of the powder sample during collection of the experimental XRPD.
2.5 Thermal analysis

The thermogravimetric analysis (TGA)of 1 is carried out under nitrogen condition at a heating rate of 10 ℃·min-1from 25 to 800 ℃ (Fig.8).The TGA curve shows three-step weight loss processes.The first loss of 9.37%in the range of 105~145 ℃,corresponds tothe release of threewater molecules(Calcd.9.35%),and one endothermal peak at 105℃is observed in the DTA curve.Upon further raising the temperature till 265℃,another two water molecules are lost with the weight lose of 6.18% (Calcd.6.24%),and one exothermal peak at 265℃in the corresponding DTA curve.The last step gives a loss of 71.80%(Calcd.71.42%)in the range of 265~405 ℃,corresponding to the loss of all the organic ligands,and the final residue should be CoO.Moreover,a strong exothermal peak is observed at 405℃in DTA which suggests the occurrence of a phase transitions.
3 Conclusions
In this paper,a 1D polymer{[Co(dpa)(H2O)4](dpdo)(H2O)}n1 containing two organic components,dpdo(4,4′-bipyridine-N,N′-dioxide)and H2dpa(2,2′-biphenyldicarboxylate),has been synthesized and characterized by IR spectroscopy,electronic spectroscopy.1 exhibits a three-dimensional supramolecular framework containing free dpdo components based on one-dimensional polymeric chains,which formed through the coordination interaction of Co(Ⅱ)and dpa2-.The photoluminescence measurement of 1 shows the main emission peak at 430 nm in the solid state.
Refenrence:
[1]Zhang Z J,Zhao Y G,Gong Q H,et al.Chem.Commun.,2013,49:653-661
[2]Carson F,Agrawal S,Gustafsson M,et al.Chem.Eur.J.,2012,18:15337-15344
[3]Wang K B,Geng Z R,Zheng M B,et al.Cryst.Growth Des.,2012,12:5606-5614
[4]Bai Z S,Qi Z P,Lu Y,et al.Cryst.Growth Des.,2008,8:1924-1931
[5]Liu G X,Zhu K,Xu H M,et al.CrystEngComm,2009,11:2784-2796
[6]Selvakumar P M,Suresh E,Subramanian P S.Inorg.Chim.Acta,2008,361:1503-1509
[7]Liu G X,Xu Y Y,Wang Y,et al.Inorg.Chim.Acta,2010,363:3932-3938
[8]Wang X L,Guo Z C,Liu G C,et al.CrystEngComm,2013,15:551-559
[9]Ding C X,Li X,Ding Y B,et al.Cryst.Growth Des.,2012,12:3465-3473
[10]Stylianou K C,Rabone J,Chong S Y,et al.J.Am.Chem.Soc.,2012,134:20466-20478
[11]Zhou X Y,Guo Y L,Shi Z H,et al.Dalton Trans.,2012,41:1765-1775
[12]Platero-Prats A E,Bernini M C,Medina M E,et al.CrystEngComm,2011,13:4965-4972
[13]Wang R H,Hong M C,Luo J H,et al.Chem.Commun.,2003:1018-1019
[14]LI Zheng(李正),ZHAO Yue(赵越),WANG Peng(王鹏),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2012,28(7):1469-1476
[15]Zhao Y,Luo L,Liu C,et al.Inorg.Chem.Commun.,2011,14:1145-1148
[16]Dang D B,Gao H,Bai Y,et al.J.Chem.Crystallogr.,2010,40:332-336
[17]Dang D B,Li M M,Bai Y,et al.Spectrochim.Acta,Part A,Mol.Biomol.Spectrosc.,2013,103:101-107
[18]Dang D B,Li M M,Bai Y,et al.Synth.Met.,2012,162:2075-2080
[19]Bai Y,Wang J L,Dang D B,et al.Spectrochim.Acta,Part A,Mol.Biomol.Spectrosc.,2012,97:105-110
[20]ZHANG Zhong(张众),LIU Ji-Zhong(刘积中),GAO Pe(高鹏),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2012,28(1):195-200
[21]Wolfle I,Lodaya J,Sauerwein B,et al.J.Am.Chem.Soc.,1992,114:9304-9309
[22]Sheldrick G M.Acta Crystallogr.,1990,A46:467-473
[23]Sheldrick G M.Acta Crystallogr.,2008,A64:112-122
[24]Rueff J M,Pillet S,Claiser N,et al.Eur.J.Inorg.Chem.,2002:895-900
[25]Yin P X,Zhang J,Li Z J,et al.Inorg.Chim.Acta,2007,360:3525-3532
[26]Aizawa S,Funahashi S.Inorg.Chem.,2002,41:4555-4559
[27]Chen Z Y,Luo D B,Kang M P,et al.Inorg.Chem.,2011,50:4674-4676
[28]Dong D P,Sun Z G,Tong F,et al.CrystEngComm,2011,13:3317-3320
[29]Yin PX,Zhang J,Li Z J,et al.Cryst.Growth Des.,2009,9:4884-4896
[30]Wang R H,Yuan D Q,Jiang F L,et al.Cryst.Growth Des.,2006,6:1351-1360
[31]Wu H,Dong Z W,Liu H Y,et al.Dalton Trans.,2008:5331-5341
[32]Ashiry K O,Zhao Y H,Shao K Z,et al.Solid State Sci.,2007,9:1006-1011
[33]Xiong K C,Jiang F L,Yang M,et al.Dalton Trans.,2012,41:540-545
[34]Farnum G A,Lucas J S,Wang C Y,et al.Inorg.Chim.Acta,2011,368:84-95
[35]Gai Y L,Jiang F L,Xiong K C,et al.Cryst.Growth Des.,2012,12:2079-2088
