含苯四甲酸的邻菲啰啉铜配合物的合成、晶体结构及电化学性质研究
2013-08-20黄文浩吴云龙谢吉民
朱 禹 黄文浩 朱 敏 吴云龙 谢吉民*,,2
(1 江苏大学化学化工学院,镇江 212013)
(2 南京大学配位化学重点实验室,南京 210093)
The construction by design of metal-organic frameworks (MOFs) using various secondary building units connected through coordination bonds,supramolecular contacts (hydrogen bonding, π-π stacking, etc.) has been an increasingly active research area[1-5]. In particular, multibenzenecarboxylate ligands have been shown to be good building blocks in the design of metal-organic materials with desired topologies owing to their rich coordination modes and abundant hydrogen-bonding interactions[6-7]. The 1,2,4,5-benzene-tetracarboxylic acid (H4BTEC) is a good choice for the construction of polymeric structures for the abundant carboxylic groups, which could act not only as hydrogen-bond acceptors but also donors.Furthermore, through careful control of the reaction conditions, partial or complete deprotonation occurs,leading interesting supra-molecular structures[8-12]with or without auxiliary ligands. Herein we report an interesting complex [Cu(phen)2Br](H3BTEC), obtained from the assembly reactions of the CuBr2and 1,10-phenanthroline in the presence of auxiliary ligand of H4BTEC.
1 Experimental
1.1 Materials and measurements
All chemicals used were of analytical grade.Solvents were purified by conventional methods. IR spectra were measured as KBr pellets on a Nicolet FT-170SX spectrometer in the range of 400 ~4 000 cm-1. Electronic absorption pectra were recorded on an Agilent 8453 spectrophotometer. Elemental analyses were performed with a Perkin-Elmer 240 instrument. TG measurements were performed on a NETZSCH STA 449C analyzer. Cyclic voltammetry was performed in a three-electrode cell using a Chi-730C electro-chemistry station.
1.2 Synthesis of [Cu(phen)2Br](H3BTEC)
1,10-Phenanthroline (24.0 mg, 0.12 mmol) was dissolved in hot acetonitrile (15 mL) and mixed with a solution of copper bromide (9.2 mg, 0.04 mmol) in methanol (2 mL) and stirred for 10 min. 30.4 mg H4BTEC (0.12 mmol) in methanol (2 mL) was added to the above solution and stirred for another 8 h at 40℃. The solution was filtered, evaporated in the air and prismatic green crystals of 1 were formed after 4 weeks. The ICP and elemental analysis results for C34H21BrCuN4O8Calcd.(%): C, 53.94; H, 2.80; N, 7.40;O, 16.91; Cu, 8.39; Found (%): C, 53.83; H, 2.71; N,7.22; O, 17.17; Cu, 8.15.
1.3 Crystal structure determination
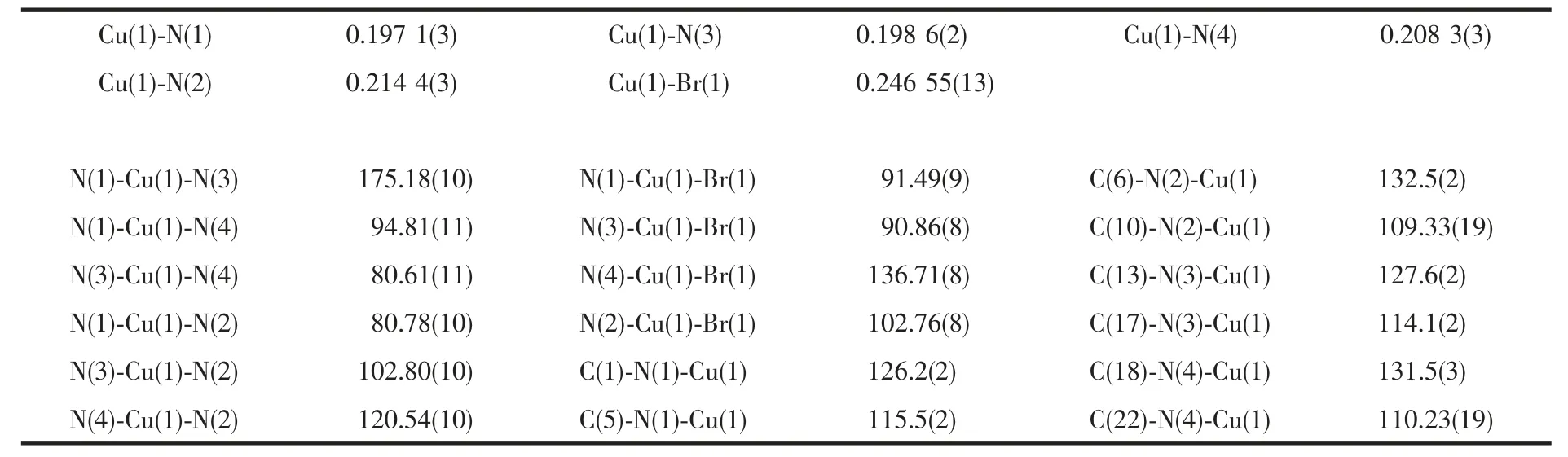
Table 1 Selected bond lengths (nm) and bond angles (°)
A crystal of the title compound with approximate dimensions of 0.28 mm ×0.24 mm ×0.22 mm was mounted on a glass fiber for data collection.Diffraction data was collected on a Rigaku saturn 724 CCD area detector diffracto-meter, equipped with a graphite-monochro-matized Mo Kα (λ=0.071 073 nm)radiation at 293(2)K.A total of 14 810 reflections were collected in the range of 2.78°≤θ≤26.02°, of which 5 827 were independent (Rint=0.032 1)and 4 657 were observed (I>2σ(I)). The crystal structure was solved by direct methods using difference Fourier synthesis with SHELXTL-97[13], and refined by full-matrix leastsquares method. The non-hydrogen atoms were refined anisotropi-cally and hydrogen atoms were added according to theoretical models. Intensity data were corrected for factors and empirical absorption. The final refinement converged at R=0.044 9, wR=0.086 4(w=1/[σ2(Fo2)+(0.034 9P)2+0.687P] where P=(Fo2+2Fc2)/3, S=1.059, (Δ/σ)max=0.001, (Δρ)max=300 e·nm-3and(Δρ)min=-438 e·nm-3. The selected bond lengths and bond angles are listed in Table 1.
CCDC: 858948.
2 Results and discussion
2.1 Crystal structure

Fig.1 Molecular structure of [Cu(phen)2Br](H3BTEC)

Fig.2 (a) View of the anion chains in complex 1, showing hydrogen bonds (dashed lines); (b) View of the anion chains showing hydrogen bonds and Br…π interactions (dashed lines)

Table 2 Hydrogen bond lengths and bond angles
The structure of the complex consists of coordination cation [Cu(phen)2Br]+and mono-deprotonated[H3BTEC]-anion. As illustrated in Fig.1, the Cu (Ⅱ)atom is five coordinated by four nitrogen atoms from two chelating bidentate 1,10-phenanthroline and one bromide anion (Cu1-N1 0.197 1(3) nm; Cu1-N2 0.214 4(3) nm; Cu1-N3 0.198 6(2) nm; Cu1-N4 0.208 4(3) nm; Cu-Br 0.246 54(13) nm) and exhibits a distorted square pyramidal geometry with the bromide atom occurs the axial position. All the bond lengths fall in normal range[14-15]. Interestingly, complexes with mono-deprotonated and uncoordinated H4BTEC as the title one are rare as far as we know[16]. The shortest distance between the approximately parallel H3BTECand phen is about 0.35 nm, which is within the common range for π-π interactions between two aryl rings[9]. There are considerable strong intramolecular hydrogen bonds, as well as large numbers of intermolecular hydrogen bonds. O-H…O hydrogenbonds (O3-H3B…O8i; O6-H6B…O2ii) form free H3BTEC-anions into an infinite chain (Fig.2a). As shown in Fig.2b, a kind of non conventional hydrogen bond (C12-H12A…Br1vi) which is seldom seen in previous reports[17]has been found in the structure; In addition, the distance between Br atom to ring Cgx(N4/C18-C22) is 0.346 87 nm, indicating that the Br…π interactions occurred in the structure (symmetry codes:x2-x,1-y,1-z)[18-20].Finally,the cation complexes are connected into a one-dimensional chain via C-H…Br hydrogen-bonding and Br…π interactions. The whole structure displays a three-dimensional supramolecular framework via these hydrogen bonds(Fig.3). Selected hydrogen bond lengths and bond angles are listed in Table 2.

Fig.3 Two-dimensional and three-dimensional abstract graphs of the cation and anion chains in 1
2.2 IR and UV analyses
According to the IR spectra, the wide band at 3 435 cm-1is attributed to hydroxyl group from the carboxyl groups. The C-H stretching mode for the phen ring is relatively weak and observed at about 3 043 cm-1. A protonated carboxylate group is present with the characteristic absorption at 1 716 cm-1[21].Bands centered about 1 650~1 500 cm-1are assigned to the symmetric mode of the carboxylate groups, and those at about 1 440~1 380 cm-1[22-23],to the asymmetric mode. Besides, the adsorption peaks of 1,10-phenanthroline shift from 1 420, 852 cm-1and 737 to 1 428 cm-1, 850 and 723 cm-1in the complex.
The UV spectra of 1 and the ligands in ethanol qualitatively were measured at room temperature from 200~400 nm. The spectra of phen and and H4BTEC both feature one main band located around 340 and 328 nm respectively, While the band of 1 locates around 334 nm. The shifts of absorption peaks in 1 attribute to electronic transition absorption of the ligands.
2.3 Thermogravimetric analysis
Thermal behaviors of the complex were investigated using thermogravimetry. As shown in Fig.4, the TG curve of 1 reveals that it is almost stable up to 202 ℃.The weight loss of 74.87%in the temperature range of 202~560 ℃corresponds to the partly decomposition of phen and H3BTEC-. The residual weight of the sample 1/2[Cu2Br2(CO3)] is ca. 23.28% (Calcd.22.99%).

Fig.4 TG curve of complex 1
2.4 Electrochemical properties
The electrochemical behavior of the complexe was investigated by cyclic voltam-metry (CV) in DMF containing 0.1 mol·L-1TBAP. Fig.5 displays the cyclic voltammogram for the complex 1 during scanning from -2.0 to 1.5 V in 0.1 V·s-1. It exhibits one reversible one-electron and irreversible oneelectron oxidations as well as one reversible oneelectron and two irreversible one-electron reductions.

Fig.5 Cyclic voltammogram of 1
[1] Wang J, Lin Z J, Ou Y C, et al. Inorg. Chem., 2008,47:4481-4489
[2] KONG Zhi-Guo(孔治国), WANG Ming(王明), WANG Qin-Wei( 王 庆 伟). Chinese J. Inorg. Chem.(Wuji Huaxue Xuebao), 2010,26(3):537-540
[3] Tao J, Shi J X, Tong M L, et al. Inorg. Chem., 2001,40:6328-6330
[4] Xie J M, Wu H, Kawakami D, et al. Inorg. Chem., 2008,47:1949-1952
[5] Stephenson M D, Hardie M J. Cryst. Growth Des., 2006,6:423-432
[6] Bai H Y, Ma J F, Yang J, et al. Cryst. Growth Des., 2010,10:1946-1959
[7] Wang T T, Xie J M, Xia C K, et al. Chinese J. Struct. Chem.,2010,29:1265-1269
[8] Xia C K, Lu C Z, Yuan D Q, et al. J. Mol. Struct., 2007,831:195-202
[9] Wang J, Lin Z J, Ou Y C, et al. Inorg. Chem., 2008,47:190-199
[10]Shi Q, Cao R, Sun D F, et al. Polyhedron, 2001,20:3287-3293
[11]Zhang L J, Xu J Q, Shi Z, et al. J. Solid State Chem., 2003,32:32-39
[12]Hu M L, Xiao H P, Wang S, et al. Acta Crystallogr. Sect.,2003,C59:m454-m455
[13]Sheldrick G M. SHELXL-97, Program for Crystal Structure Determination, University of Göttingen, Germany, 1997.
[14]Parker O J, Greiner G T, Brenemen G L. Polyhedron, 1994,13:267-271
[15]Murphy G, Sullivan C O, Murphy B, et al. Inorg. Chem.,1998,37:240-248
[16]Cho J H, Lough A T, Kim J C. Inorg. Chim. Acta, 2003,342:305-
[17]Steiner T. Acta Crystallogr. Sect., 1996,C52:2263-2266
[18]Schottel B L, Chifotides H T, Dunbar K R. Chem. Soc. Rev.,2008,37:68-83
[19]Reddy D S, Craig D C, Desiraju G R. J. Am. Chem. Soc.,1996,118:4090-4093
[20]WANG Yan(王彦), SHENG Yue-Wei(盛月溦), SUN Wei-Yin(孙为银).Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2009,25(7):1182-1186
[21]Cao R, Shi Q, Sun D F, et al. Inorg. Chem., 2002,41:6161-6168
[22]Majumder A, Gramlich V, Rosair G M, et al. Cryst. Growth Des., 2006:2355-2368
[23]Lei R, Zhang H H, Hu J, et al. Chinese J. Struct. Chem.,2010,29(4):655-659
