基于2-丙基-4,5-二甲酸咪唑和4,4-联吡啶的镉(Ⅱ)配位聚合物的合成、晶体结构及荧光性质
2013-08-20邓记华钟地长梅光泉
邓记华 钟地长 梅光泉
(1 宜春学院,江西省高等学校应用化学与化学生物学重点实验室,宜春 336000)
(2 赣南师范学院化学与化学工程学院,赣州 341000)
During the past decade, coordination polymers have been attracted considerable attention due to their intriguing structures[1]and potential applications in fields as luminescence[2], magnetism[3], catalysis[4], as well as gas storage and separation[5]. The diverse metal ions and/or metal-containing clusters as the nodes and a variety of organic ligands by coordination bonds can lead to the formation of thousands of coordination polymers. Imidazole-4,5-dicarboxylate (H3IDC), a nitrogen heterocycle carboxylate ditopic ligand with multi-coordination sites, has been extensively used as building block to construct functional complexes[6-9].However, to the best of our knowledge, the coordination chemistry of 2-propyl-imidazole-4,5-dicarboxylate (H3PIDC) that is the derivative of H3IDC, has been studied very limitedly[10-11]. In this article, we report the synthesis, crystal structure and luminescent property of a novel Cd (Ⅱ)coordination polymer based on H3PIDC and 4,4-bipyridine (bpy),[Cd2(HPIDC)2(bpy)(H2O)2] (1), which is a onedimensional (1D) zigzag ribbon containing rare square tetranuclear cadmium(Ⅱ)structural units.
1 Experimental
1.1 Syntheses
A mixture of Cd(NO3)2·4H2O (0.5 mmol, 0.154 g), 2-propyl-imidazole-4,5-dicarboxylate (0.5 mmol,0.099 g), 4,4-bipyridine (bpy, 0.5 mmol, 0.078 g), 0.5 mL ammonia and 8.5 mL distilled water were mixed and stirred for ten minutes. The resulting solution was transferred to a 20 mL Teflon-lined stainless steel vessel and heated at 160 ℃for 48 h. After the oven was cooled to room temperature at a rate of 10 ℃·h-1,colorless block-shaped crystals of 1 were isolated by filtration, washed with water, and dried in air. Yield:0.093 g, 46% base on H3PIDC. Anal. Found(%): C,38.51;H,3.55;N,10.46.Calcd.for C26H28Cd2N6O10(%):C, 38.58; H, 3.49; N, 10.38.
1.2 X-ray structure determination
Single-crystal data for 1 were collected on a Bruker Smart 1000 CCD diffractometer with graphitemonochromated Mo Kα radiation (λ=0.071 073 nm) at 173(2) K. All empirical absorption corrections were applied using the SADABS program[12]. The structure was solved using direct method, which yielded the positions of all non-hydrogen atoms. These were refined first isotropically and then anisotropically. The disorder of the propyl group of HPIDC2-anion was treated by performing half occupancies initially, which were refined as 0.53 finally. The C6 and C6′ were fixed at the same coordinates and were constrained with the same displacement parameters by using EXYZ and EADP instructions respectively. The displacement parameters of C7, C7′, C8, C8′ atoms was also restricted by EADP instruction. In addition, to obtain a reasonable structure, the C-C bond distances of the disorder part were constrained with 0.154 nm by using DFIX instruction. Positions of the hydrogen atoms attached to C atoms were geometrically placed and isotropically refined as a riding mode, while these attached to O atoms and N atoms were positioned from the difference Fourier synthesis and constrainedly refined with the N-H 0.091 nm, O-H 0.084 nm,and Uiso(H)=1.2Ueq(N), Uiso(H)=1.5Ueq(O). All calculations were performed using the SHELXTL system of computer programs[13].
CCDC: 860811.
2 Results and discussion
2.1 Crystal structure

Table 1 Crystal data and structure refinements for 1
The result of single X-ray diffraction analysis indicates that there are three crystallographically independent Cd (Ⅱ) in 1. As shown in Fig.1, Cd1 coordinates to four N atoms from two individual HPIDC2-anions and two bpy molecules, and two O atoms from two individual HPIDC2-anions, resulting in distorted N4O2octahedral geometry. Cd2 shows N3O3octahedral geometry. The three N atoms are from three individual HPIDC2-anions, and the three O atoms come from two individual HPIDC2-anions and one coordinated H2O molecule. Cd3 exhibits distorted N2O4octahedral geometry. The two N atoms come from two individual HPIDC2-anions, and the four O atoms are from two individual HPIDC2-anions and two coordinated H2O molecules. It is interesting to find that the H3PIDC ligand only loses two H atoms,including the H atoms of the imidazole group and one carboxylate group, while the other caroboxylate group has not been deprotonated, which is indicated by the marked difference between C10-O5 (0.122 4 nm) and C10-O6 (0.128 1 nm). The resulted HPIDC2-anion,employing a bridging chelating coordination mode,coordinates with the Cd(Ⅱ)ions. Through the bridging of four μ2-HPIDC2-anions, one Cd1, two Cd2 and one Cd3 are connected together to form a rare square tetranuclear Cd (Ⅱ) structural units (Fig.1b). Each tetranuclear Cd(Ⅱ)unit is further linked with another two equivalent Cd(Ⅱ)units through four bpy molecules,resulting in an one-dimensional zigzag ribbon (Fig.1c).To date, only a few polynuclear cadmium (Ⅱ)units existing in coordination polymers have been reported in the literatures[14-16], and such a perfect square tetranuclear cadmium(Ⅱ)has not been observed before this work.

Fig.1 (a) Coordination evironment of three crystallographically independent Cd(Ⅱ)ions and the coordination modes of HPIDC2-anion and bpy molecule; (b) Rare square tetranuclear Cd(Ⅱ)bridged by four μ2-HPIDC2-anions;(c) 1D zigzag ribbon in 1
There are rich intra/intermolecular hydrogen bonds observed in 1 (Fig.2, Table 2). The terminal ligand H2O (O9) donates H atom to the carboxylate oxygen O6 to form strong O-H…O hydrogen bond (RO9…O6=0.269 1(6) nm), which makes the 1D zigzag ribbons connected together to form a 2D supramolecular layers (Fig.2a). The adjacent 2D supramolecular layers are further linked together through four types of OH…O hydrogen bonds (RO9…O1=0.296 9(6) nm; RO9…O2=0.301 7(7)nm;RO10…O3=0.283 3(6)nm;RO10…O4=0.294 1(6)nm) to form a 3D supramolecular network (Fig.2b). It is interesting to find that under the similar reaction conditions, using ligand imidazole-4,5-dicarboxylate(H3IDC) instead of H3PIDC ligand led to the formation of a 3D cage-based coordination polymer[7]. This different result indicates that the substituent of propyl group plays an important role in tuning the structures of coordination polymers, which may be ascribed to the steric hindrance effect of the propyl groups[17].
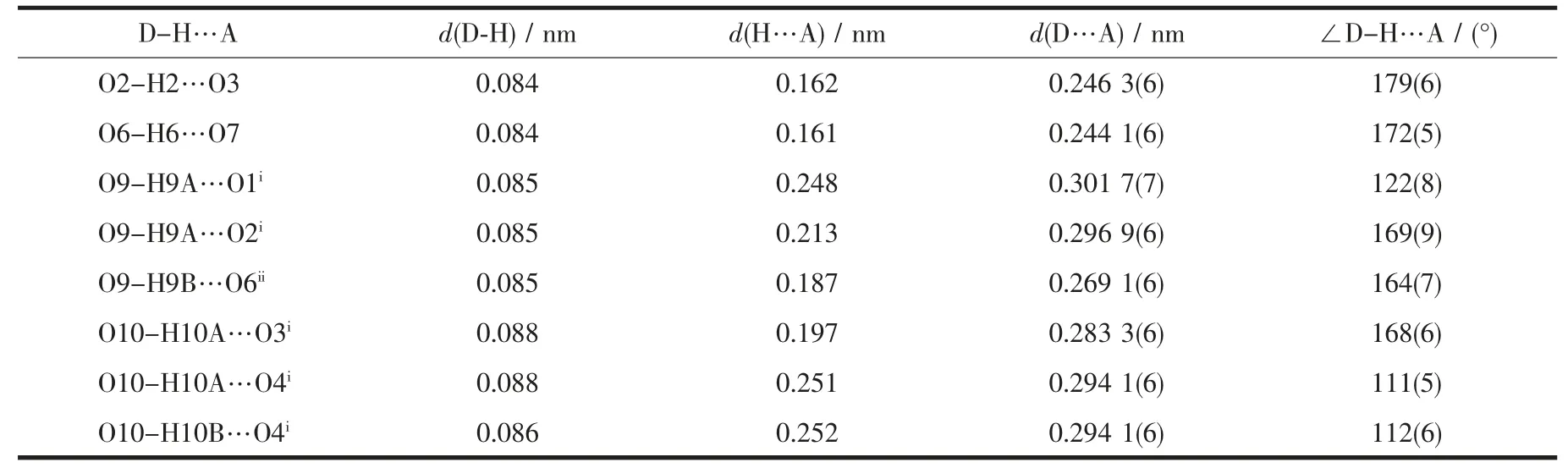
Table 2 Hydrogen bonds observed in 1
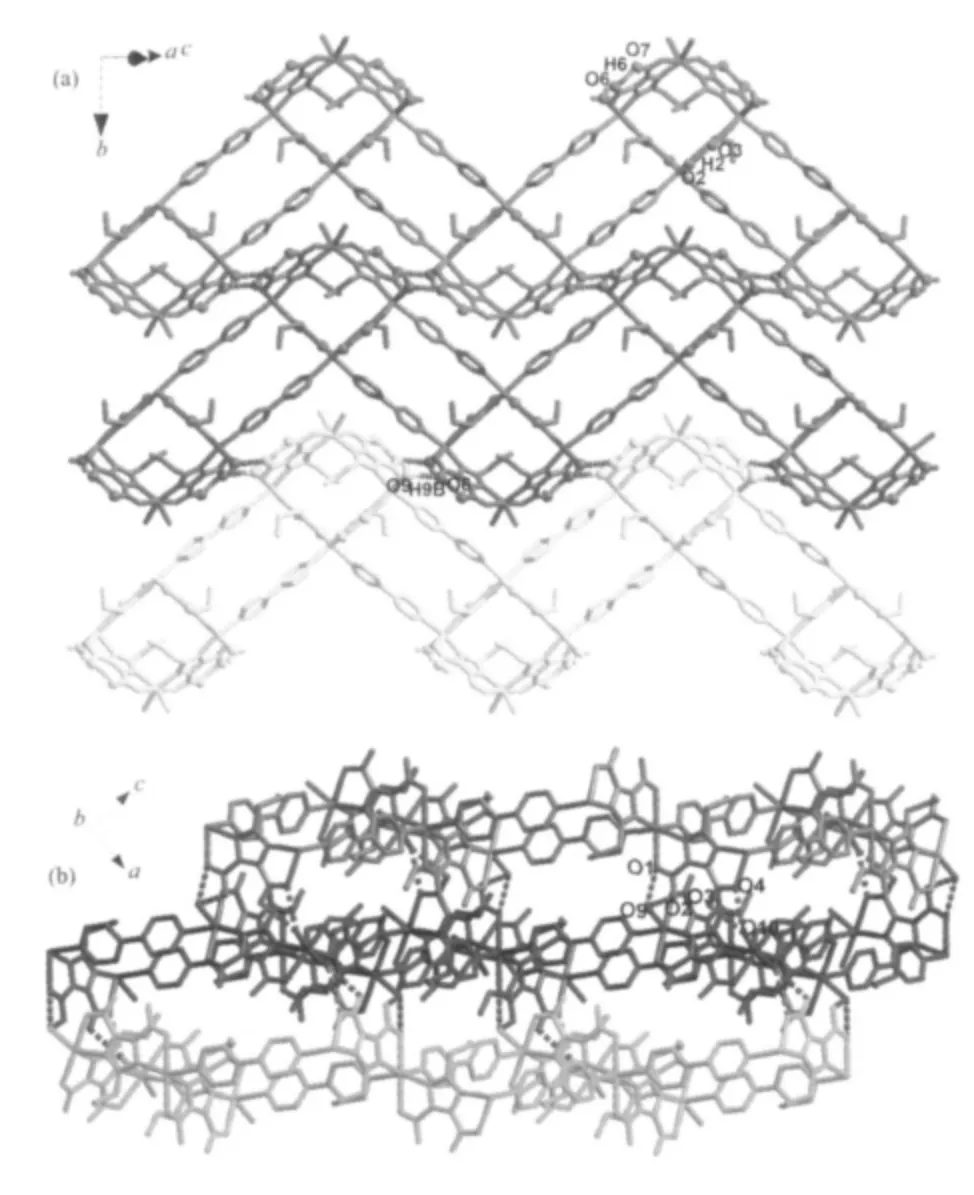
Fig.2 (a) 2D supramolecular sheet stabilized by intra/intermolecular hydrogen bonds; (b) 3D supramolecular network of 1
2.2 Thermal stability
The thermogravimetric analyses (TGA) was carried out on a Netzsch TG-209 Thermogravimetry Analyzer in air atmosphere. As shown in Fig.3, the TG curve of 1 exhibits an initial weight loss of 4.6%from the room temperature to 198 ℃, corresponding to the removal of two coordinated H2O molecules per formula unit (calcd. 4.4%). After that temperature, the framework began to decompose through another three steps of weight losses upon further heating.
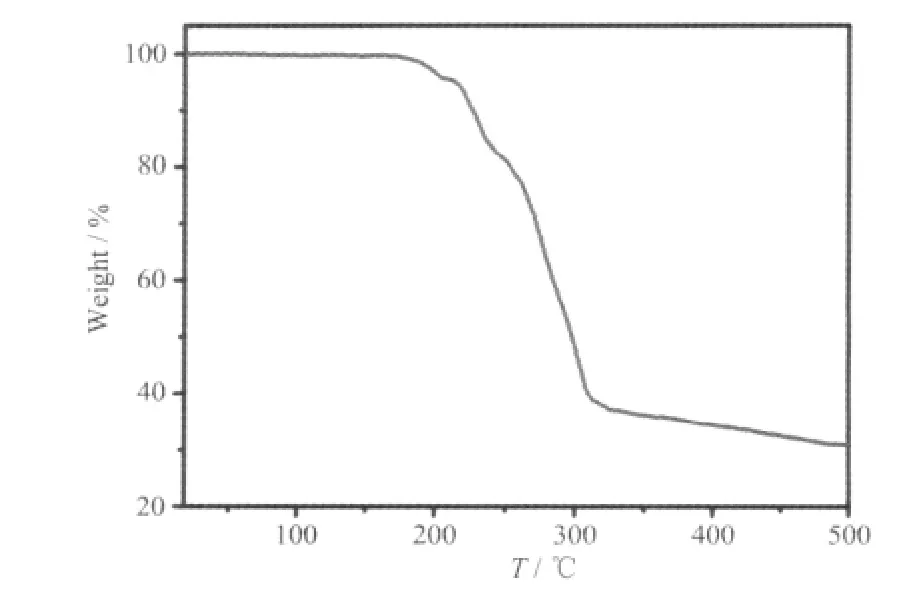
Fig.3 TG curve of 1
2.3 Luminescence
Cd(Ⅱ)is an ideal metal center used for building luminescent coordination polymers, as it has d10electronic configuration. Built from Cd (Ⅱ) and conjugated organic ligand, 1 may exhibit excellent luminescent behavior. Therefore, the photoluminescent property of 1 was investigated on a RF-5301PC spectrometer in the solid state at room temperature.As depicted in Fig.4, it can be observed that 1 exhibits fluorescence upon excitation at 252 nm. The strong emission maximum at 427 nm can be tentatively assigned to ligand-to-metal charge transfer(LMCT)and/or metal-to-ligand charge transfer (MLCT),which has been observed in other Cd (Ⅱ)coordination polymers[18].
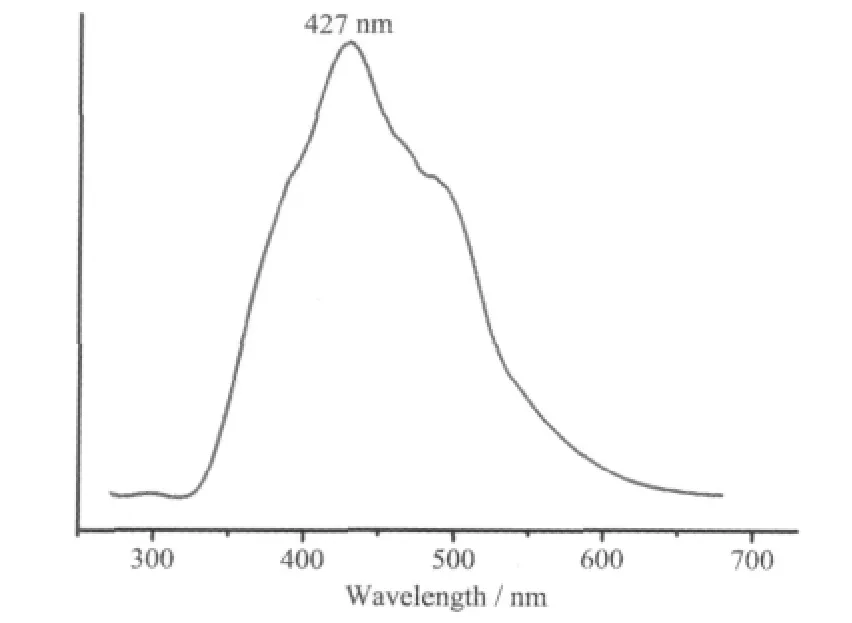
Fig.4 Fluorescent emission spectra for 1 in the solid state at ambient temperature
[1] Zhong D C, Meng M, Zhu J, et al. Chem. Commun., 2010,46:4354-4356
[2] Deng J H, Yuan X L, Mei G Q. Inorg. Chem. Commun.,2010,13:1585-1589
[3] Kurmoo M. Chem. Soc. Rev., 2009,38:1353-1379
[4] Ma L Q, Abney C, Lin W B. Chem. Soc. Rev., 2009,38:1248-1256
[5] Pan L, Olson D H, Ciemnolonski L R, et al. Angew. Chem.,Int. Ed., 2006,46:616-619
[6] Sun Y Q, Zhang J, Chen Y M, et al. Angew. Chem. Int. Ed.,2005,44:5814-5817
[7] Lu W G, Su C Y, Lu T B, et al. J. Am. Chem. Soc., 2006,128:34-35
[8] Alkordi M H, Liu Y, Larsen R W, et al. J. Am. Chem. Soc.,2008,130:12639-12641
[9] Nouar F, Eckert J, Eubank J F, et al. J. Am. Chem. Soc.,2009,131:2864-2870
[10]Feng X, Zhao J, Liu B, et al. Cryst. Growth Des., 2010,10:1399-1408
[11]Meng C X, Li D S, Zhao J, et al. Inorg. Chem. Commun.,2009,12:793-795
[12]BRUKER. SMART, SAINT, and SADABS, Bruker AXS Inc., Madison, Wisconsin, USA, 1998.
[13]Sheldrick G M. Acta Cryst., A64, 2008:112-122
[14]Deng H, Qiu Y C, Li Y H, et al. Chem. Commun., 2008:2239-2241
[15]Zhang J, Liu R, Feng P, et al. Angew. Chem. Int. Ed., 2007,46:8388-8391
[16]Zheng S L, Yang J H, Yu X L, et al. Inorg. Chem., 2004,43:830-838
[17]Huang X C, Lin Y Y, Zhang J P, et al. Angew. Chem. Int.Ed., 2006,45:1557-1559
[18]NIE Xue(聂雪), QU Jing-Nian(屈景年), CHEN Man-Sheng(陈 满 生). Chinese J. Inorg. Chem.(Wuji Huaxue Xuebao),2011,27:2267-2270
