Purification of Antimicrobial Peptide from Antarctic Krill (Euphausia superba) and its Function Mechanism
2013-07-29ZHAOLingYINBangzhongLIUQiandCAORong
ZHAO Ling, YIN Bangzhong, LIU Qi*, and CAO Rong
Purification of Antimicrobial Peptide from Antarctic Krill () and its Function Mechanism
ZHAO Ling, YIN Bangzhong, LIU Qi, and CAO Rong
,,,266071,..
The preliminary purification and antimicrobial mechanism of antimicrobial peptide from Antarctic Krill were studied in this paper. The results showed that the molecular weight range of antimicrobial polypeptide (CMCC-1) obtained by cation exchange chromatography was between 245-709D as detected by molecular sieve chromatography, and the minimum inhibition concentration (MIC) of CMCC-1 againstwas 5.0mgmL. The antimicrobial mechanism of CMCC-1 was studied withas indicator bacterium. Compared with control group, the results of the experimental group in whichwas treated with CMCC-1 were as follows: 1) CMCC-1 could inhibit cell division at logarithmic phase.2) The protein and reducing sugar content, and the conductivity of culture medium increased, and the activity of alkaline phosphatase and β-galactosidase could be detected in theculture medium.3) Observation under scanning electron microscope revealed that somatic morphology became irregular, and then somatic surface became coarse. The cell became much smaller, and most somatic cells gathered. The boundary between cells became dim and finally fused as a whole. 4) Observation under transmission electron microscope showed that the surface ofbecame rough and the reproducing ability was restrained. The cell wall became thin and the cytoplasm shrunk. Substances inside cell leaked out, which caused cellsdeath.5)SDS-PAGE analysis showed that some bands disappeared, and the residual bands became vague. 6) The genomic DNAelectrophoresisresults showed that the genomicDNA bandsofwere not degradedbut the brightness significantly reduced. Thus, it is supposed that CMCC-1 could destroy the cell wall and membrane of, increase the cell membrane permeability and the leaking-out of intracellular substances, and thus cause the death of.
Antarctic Krill; antimicrobial peptide; purification; antimicrobial mechanism
1 Introduction
Antarctic Krill is the key species of ecosystem in the Antarctic Ocean. It is not only an important resource but also rich in nutrients with high protein and low lipid (Shen., 2008). Researches of many domestic and overseas scientists have concentrated more on Antarctic krill processing and less on the active substances in it (Wu., 2008). Antarctic Krill is the primary diet of many whales, fish and squid, so their survival could be affected by a reduction in krill abundance due to fishing (Inigo., 1990)
Studies demonstrate that Antarctic krill contains many active substances, such as anti-hypertensive peptide, UV shielding material, polar lipids, astaxanthin, chitin and so on (Hatanaka., 2009; Sun., 2001; Liu., 2010). However, reports about the purification and antibacterial mechanism of the antibacterial peptide have not been found.
In recent years, antibacterial peptides have played a crucial role in resisting bacterial infection, and currently been isolated from ascidians, crustaceans, amphibians, mammals, plants and bacteria (Hara., 1996; Alejandro., 2011). Most of them can be defined as low molecular weight protein and do not produce nonspecific antibody (Hara., 1996; Schnapp., 1996). Hence, many scientists have been devoting themselves to developing antibacterial peptides from other species. Thus, the research and development of antimicrobial peptide from Antarctic Krill is of great significance.
2 Material and Methods
2.1 Preparation of Antimicrobial Peptide from Antarctic Krill
The Antarctic Krill was captured from the Scotia waters in the west of the Atlantic in 2010.
Protamex was used for the enzymolysis of Antarctic Krill homogenate. The enzymatic hydrolysate was degreased and centrifuged until the end of enzymolysis, then the sediment was removed. The antimicrobial peptide from Antarctic Krill obtained by ultrafiltrationwas freeze-dried and stored at-20℃.
2.2 CM-52 Carboxymethyl Cellulose Cation Ex- change Chromatography
The antimicrobial peptide from Antarctic krill was isolated by CM-52 carboxymethyl cellulose ion exchange column. The column was balanced with 10mmolLsodium acetate buffer (pH 4.0) for at least two hours , then gradient elution was carried out with 0-0.4molLNaCl buffer with elution rate of 0.4mLmin(5mL per tube) (Gregory., 1996). According to the elution curve, solution in related tube was merged. Paper disc diffusion method was used to select the fraction with the strongest antimicrobial activityagainst. The component was freeze-dried and stored at-20℃.
2.3 Determination of the Minimum Inhibitory Concentration
The fraction with the strongestactivity againstwas dissolved in distilled water to a concentration of 40mgmL. Double broth dilution method was used to dilute the solution. Paper disc diffusion method was used for the determination of inhibitory effect, and the process was repeated twice. After the medium was incubated at 37℃ for 24h, the inhibition zones were then measured. Peptide concentration corresponding to the minimum inhibition zone is the minimum inhibitory concentration (MIC).
2.4 The Relative Molecular Weight Distribution
A certain amount of the component with the strongestactivity towas dissolved in mobile phase, then filtered through 0.45μm membrane and packed into the column. Peak time was recorded and the relative mol- ecular weight was calculated.
Chromatographic conditions: column (TSK gel 2500 PWXL 300mm × 7.8mm);column temperature ( 40℃); mobile phase (acetonitrile/water/TFA, 50/50/0.1 (V/V/ V) ); flow rate (0.5 mLmin); wavelength ( 220nm).
2.5 Antibacterial Mechanism Analysis
was cultured for 12h and inoculated in LB culture solution with bacterial counts of 10CFUmL. The antibacterial peptide from Antarctic krill was added into the culture solution with minimum inhibitory concentration. The solution was cultivated at 37℃ in the mixed oscillation. The following experiments were performed subsequently.
2.5.1 The growth curve of.treated by anti- microbial peptide from Antarctic Krill
The OD values of culture solution were determined every one hour by spectrophotometer under 550nm (Lu., 2005; Wang., 2006). With ODvalue during theincubation time, the growth curve ofwas drawn. The OD value of culture solution without peptide was used as the control.
2.5.2 Determination of alkaline phosphatase activity
Measuring its OD value each hour at 405nm as mentioned in literature (Sun., 2005), with OD values of normal culture as the control.
2.5.3 Determination of conductivity of culture medium
The conductivity of supernatant was measured by DDS- 11A digital conductivity meter every one hour (Lee., 1998), with the conductivity of normal culture as the control.
2.5.4 Determination of activity of β-galactosidase enzyme
The absorbance was measured every one hour at 405 nm (Zhou., 2009; Jing., 1006), with absorbance of normal culture as the control.
2.5.5 Samples preparation for scanning electron microscopy
The culture medium at the 3rd, 5th, 7th, and 9th hour was centrifuged (5000rmin, 10min) respectively. The precipitate was washed with 0.1molLphosphate buffer three times, fixed with 3% glutaraldehyde at room temperature for four hours, and then washed with phosphate buffer (pH 6.8) again, fixed with 1% osmium acid for 90min, then rinsed with phosphate buffer, gradient dehydration by30, 50, 70, 80, 90, and 100% (V/V) ethanol, and percutaneous ethanol washed twice for 10min. After that 50% isoamyl acetate replaced once, 100% twice, each for 30min, and thendried before adhering samples, coated film and observed (Wang., 2004), while the normal culturedwas chosen as control.
2.5.6 Samples preparation for transmission elec- tron microscope
The medium at the 3rd, 5th, and 7th, and 9th hour was centrifuged (5000rmin, 10min) respectively.The sediment was washed by phosphate buffer for 3 times, and then fixed 4h by 2.5% glutaraldehyde at 4℃, gradient dehydration by 30, 50, 70, 90, and 100% (V/V) ethanol, and percutaneous ethanol washed two times for 10 min. The following steps were embedding,solidifying, sectioning, dyeing, and observing (Wang., 2004), while the normal cultivation was chosen as the control.
2.5.7 Effect of antimicrobial peptide from Antarctic Krill on the expression protein of.
Adding 5mgmLCMCC-1, then cultivation with shaking at 37℃ in the mixed oscillation. Sampling at the 3rd, 5th, and 7th, and 9th hour respectively, solution was, centrifuged (5000rmin, 10min) and the precipitate was kept, which was hydrolyzed by lysozyme at 37℃ for 30 min with the adding of suitable double distilled water. Joining 2x Loading buffer, boiling water bath for 10 min, shaking fiercely for fracturing DNA, and thencentrifuging (10000rmin, 5min).At the last, the supernatant was used for SDS-PAGE (15% separation gel; 5% concentrated gel), while the normal cultivation ofwas chosen as the control.
2.5.8 Effect of antimicrobial peptide from Antarctic Krill on genomic DNA of.
Bacterial genomic DNA was extracted by Kit from 2 mL solution and saved in TE buffer. DNA solution was kept at 37℃for 60min after adding 5mgmLCMCC-1. Expression ofGenomic DNA was observed by agarose electrophoresis, and the normal cultivation ofwas used as control group.
3 Results and Discussion
3.1 The Ingredient and Ratio of CMCC-1
The mixture of antimicrobial peptide from Antarctic krill contained 6 fractions of different molecular weights (Fig.1(a)). Peptides in aqueous solution were merged according to the elution peak, then freeze-dried and stored at-20 ℃.
Peak-1 showed the strongest inhibition against(Fig.1(b)), so the following experiments were based on peak-1(named CMCC-1) as the research object.
Known by calculation, CMCC-1 contained three dif-ferent components: the components with molecular wei- ghts of 709 D, 429 D and 245 D accounted for 5.1%, 22.7%, 5.1% and 72.2%, respectively.

Fig.1 The components of antimicrobial peptide from Antarctic Krill and their bacteriostatic spectrum. a. CM-52 carboxymethyl cellulose cation exchange chromatography; b. The peak of the bacteriostatic spectrum.
3.2 The MIC of CMCC-1
It can be seen from Fig.2 that the minimum inhibit- tory concentration of CMCC-1 was 5mgmL.

Fig.2 Minimum inhibitory concentration of CMCC-1 (the concentration decreasing in turn from 1 to 8).
3.3 The Antibacterial Mechanism of CMCC-1
3.3.1 Effects of CMCC-1 on growth curve of.
CMCC-1 inhibited the growth ofobviously. The growth of bacteria was at a lower level and no logarithmic growth period was observed in the experiment group (Fig.3(a)). The reason may be that CMCC-1 inhibited cell division in exponential phase of.
3.3.2 Effects of CMCC-1 on cell wall permeability of.
Alkaline phosphatase is located in cell plasma membrane. Its activity can not be detected outside the cell under normal circumstances. Alkaline phosphatase is releasedwhen the permeability of cell wall is changed. Thus, it can help to judge if the cell permeability changes or not through detecting the content of alkaline phosphatase in culture media (Sun., 2005).
The content of alkaline phosphatase in the control group was very low, while content of alkaline phosphatase in experiment group was significantly higher, and the content increasedwith the extension of incubation time. The content of alkaline phosphatase becamegraduallystable after 7 hours (Fig.3(b)). This may show that CMCC-1 changed the permeability of the cell wall and resulted in alkaline phosphatase permeating into the nutrient solution.
3.3.3 Effects of CMCC-1 on cell membrane permeability of.
The assay results of conductivity rate
The conductivity rate of culture solution with CMCC-1 was significantly higher than that of the control group (Fig.3(c)). With the extension of training time, the con- ductivity rate of experiment group continuously increased. However, the conductivity rate of control group remained at a lower level. This phenomenon showed that a lot of electrolyte had permeated in the medium. Hence, CMCC- 1 could inhibit the growth of bacteria by changing the permeability of cell membrane and causing constant electrolyte extravasation (such as K).
The measurement results of β-galactosidase activity
β-galactosidase is usually within the plasma membrane in microbial cells. Only when cell plasma membrane permeability is broken, it will be released outside.
The content of β-Galactosidase maintained a lower level of content in the media of control group (Fig.3(d)). The values were obviously lower than that of the group with peptide added. It is supposed that CMCC-1 further destroyed the permeability of cell membrane, then β-galactosidase was released outside the cells.

Fig.3 Effects of CMCC-1 on cell wall and cytoplasmic membrane permeability of Staphylococcus aureus. a. Effects of CMCC-1 on growth curve of S. aureus; b. Effects of CMCC-1 on cell wall permeability of S. aureus; c. The assay results of conductivity rate; d. Effects of CMCC-1 on cell membrane permeability of S. aureus.
3.3.4 Results of scanning electron microscopy observation for.treated by CMCC-1
Figs.4(a)-(e) were respectively morphological structu- res ofnormally cultured or treated by CMCC-1.
Under the scanning electron microscope, it can be seen that normalcell is a long ball, and the surface is smooth and glossy. There are distinct boundaries between cells, and the arrangement of cells is in pairs or short chains, or distinct grape-like gathering (Fig.4(a)). Three hours after being treated by CMCC-1, the shape of cell became irregular, and its surface rough. Cells arranged loosely and in disorder (partially single distribution, partially showing knot phenomenon) (Fig.4(b)). Five hours later, the shape became more irregular, shriveled, with cells being adhesive to each other, and the boundaries between cells becamevague. Thus, the results showed that CMCC-1 could damage cell wall or restrain its synthesis, a large number of cells adhered together, which might be due to the producing of polysaccharides after the cell wall was broken (Fig.4(c)). After treated 9h, cell surface was damaged more seriously, and part of cell membrane had ruptured. There was obviousleakage of cytoplasmic because boundaries between cells were almost lost. A few cell remained spherical, and cells almost integrated as a whole. The results indicated that the cell wall ofmight be destroyed by CMCC-1, cell membrane had broken to some extent, and the leakage of intracellular substances resulted in adhesion among cells of(Figs.4(d)-(e)).
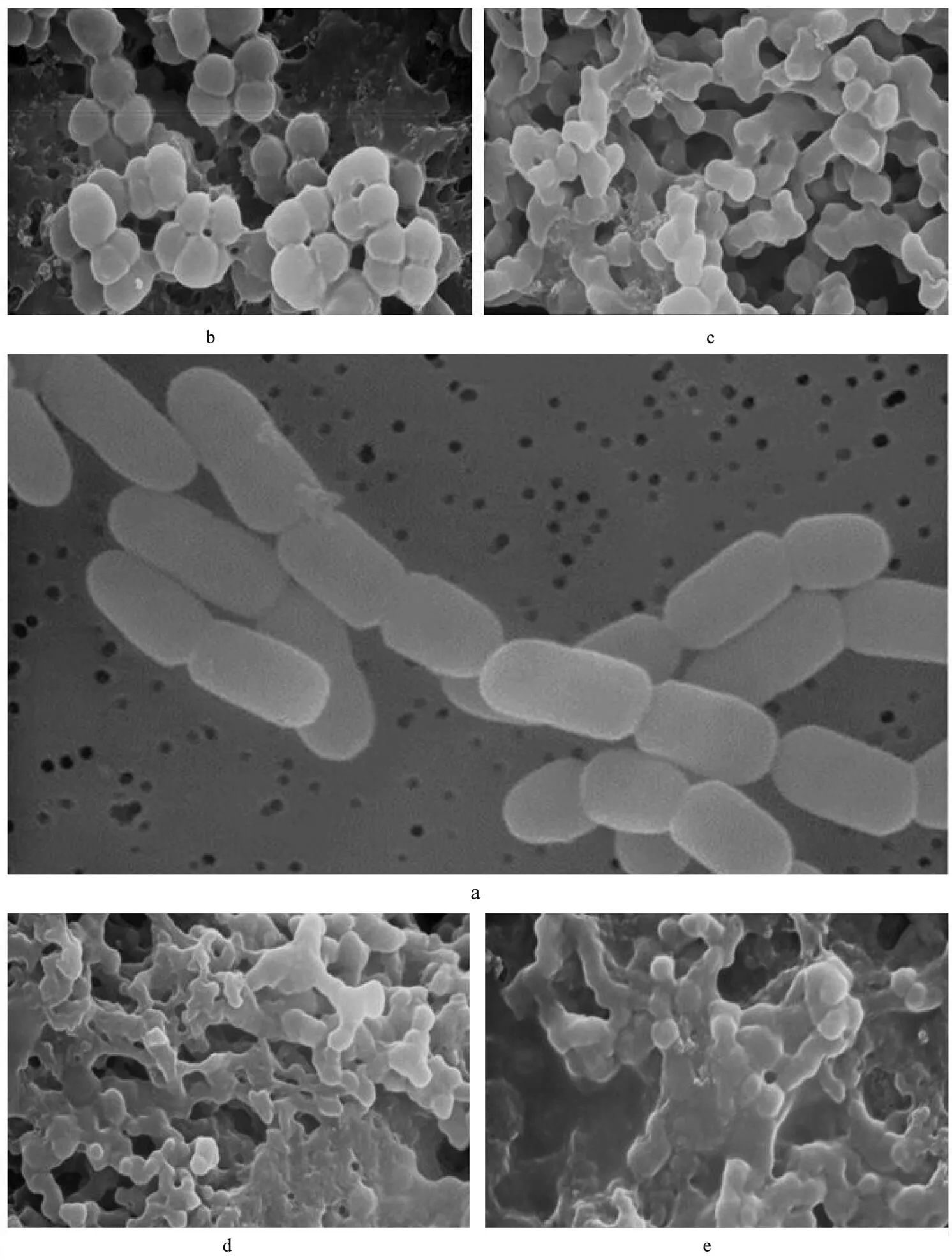
Fig.4 The graphs of scanning electron microscopy. a. Morphological structure of S. aureus; b. Morphological structure of S. aureus aftertreated by CMCC-1 3h; c. Morphological structure of S. aureus aftertreated by CMCC-1 5h; d. Morphological structure of S. aureus aftertreated by CMCC-1 7h; e. Morphological structure of S. aureus aftertreated by CMCC-1 9 h.
3.3.5 Results of transmission electron microscope observation on.treated by CMCC-1
Figs.5(a)-(e) were the ultrastructure ofin control and experiment group.
The cell wall and plasma membranemorphology of control group was unbroken and smooth, the combination among them was close, and cytoplasm had uniform distribution (Fig.5(a)). The proliferation ability of normalis especially strong. However, it was observed from the transmission electron microscope photograph oftreated byCMCC-1 three and five hours later that cell surface became rough, cell wall fractured, and propagation capacity was inhibited (Fig. 5(b)-(c)). Seven or nine hours later, cell wall became thin and shrank. Biggerinterior cavity, leakage of essential molecules and final death of cell were observed (Fig.5(d)-(e)).
3.3.6 Effect of CMCC-1 on expressed protein of.
SDS-PAGE experiments showed that the number of protein bands of control group was larger than that of experiment group and the band color was deeper (Fig. 6(a)). In experiment group, the protein bands with molecular weight between 40 to 70kD gradually disap-peared along with the increase of treating time, and the other protein bands became dim gradually. After treated by CMCC-1 for 9h, most protein bands became indistinct. Therefore, it was proposed that CMCC-1 entered into the cells of, made some functional proteins degrad- ed or inhibited the synthesis of certain proteins directly.

Fig.5 The graphs of transmission electron microscope. a. Ultrastructure of S. aureus; b. Ultrastructure of S. aureus aftertreated by CMCC-1 3 h; c. Ultrastructure of S. aureus aftertreated by CMCC-1 5 h; d. Ultrastructure of S. aureus aftertreated by CMCC-1 7 h; e. Ultrastructure of S. aureus aftertreated by CMCC-1 9 h.
3.3.7 Effect of CMCC-1 on genomic DNA of.
Compared with the electrophoretic graphs of genomic DNA of normal, the brightness of bandsof experiment group obviously reduced, and the shape did not diffuse (Fig.6(b)). However, there was no significant difference between them in migration rate. The results showed that CMCC-1 did not cause fracture of the genomic DNA, but led to the decline of DNA synthesis, resulting in the brightness reduction of DNA bands.
4 Conclusions
The molecular weight range of antimicrobial poly- peptide (CMCC-1) was between 245-709D. Though the exact mode of action of antimicrobial peptide on bacteria was still not very clear, it was proposed that CMCC-1 de- royed the cell wall and cytoplasmic membrane of, damaged the stability of intracellular environment and thus led to the leakage of essential molecules. It de- graded some functional proteins or directly inhibited the synthesis of certain protein,which could cause bacteria to be unable to develop resistance and cause cell death. At present, the structure of more than 70 kinds of antibacterial peptides has been identified. Many domestic and overseas scientists have been making their efforts to find new structure for its important applications (Schnapp., 1996). Thus, the research and development of antimicrobial peptide from Antarctic Krill is valuable.

Fig.6 Electrophoretic graphs. a. SDS-PAGE of whole proteins of S. aureus; b. Electrophoretic graphs of ge- nomic DNA (M represents l DNA/Hind III maker; 1 represents genomic DNA map of noraml S. aureus; 2 represents genomic DNA map of S. aureus treated by CMCC-1 for 30 min).
Acknowledgements
Financial support by the National Science and Tech- nology Pillar Program (2013BAD13B03), National Na- tural Science Foundation of China (Grant 31201311) and Special Scientific Research Funds for Central Non-profit Institutes, Yellow Sea Fisheries Research Institute (2060 3022012001) are greatly acknowledged.
Alejandro, M. S. M., Abimael, D. R., Roberto, G. S. B., and Nobuhiro, F., 2011.Marine pharmacology in 2007–8: Marine compounds with antibacterial, anticoagulant, antifungal, anti- inflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities; affecting the immune and nervous system, and other miscellaneous mechanisms of action.,, 153: 191- 222.
Gregory, M., Neumann, R. C., Gideon, M., and Polya, M., 1996. Purification and sequencing of yellow mustard seed napin small and large chains that are phosporylated by plant calcium-depended protein kinase and are calmodulin antagonist., 119: 49-66.
Gu, S. Q., Wang, X. C., and Lu, D. X., 2004. Study on the antibacterial mechanism of carp protamine.(), 26 (6): 100-104.
Hara, S., and Yamakawa, M., 1996.Production inof Moricin, a novel type antibacterial peptide from the Silkworm,, 220: 664-669.
Hatanaka, A., Miyahara, H., Suzuki, K. I., and Sato, S., 2009. Isolation and identification of antihypertensive peptides from Antarctic Krill., 74 (4): 116-120.
Inigo, E., Jonathan, L. W., Douglas, G. B., and Kenneth, G. F., 1990. Implications of a new acoustic target strength for abundance estimates of Antarctic krill, 345: 338-340.
Lee, H. J., Choi, G. J., and Cho, K. Y., 1998. Correlation of lipid peroxidation in Botrytis cinerea caused by dicarbo- ximide fungicides with their fungicidal activity., 46: 737-741.
Liu, L., Liu, C. C., Zhao, Y., Wang, X. C., and Li, J. L., 2010. Recent advances in the healthcare function and food safety of Antarctic Krill., 31 (17): 443-447.
Lu, W., Ren, G. P., and Song, J. M., 2005.Determination of content of peptides in protein hydrolysates., 26 (1): 7169-7171.
Pan, Y. J., Hao, Y. J., Qu, H., Shan, Y., Li, D. S., and Du, R. Q., 2006. Preliminary studies on antibacterial mechanism and analysis of antibacterial activity of chitosans., 31 (6): 361-365.
Schnapp, D., Kemp, G. D., and Smith, V. J., 1996. Purification and characterization of a proline-rich antibacterial peptide, with sequence similarity to bactenecin-7, from the haemocytes of the shore crab.., 240: 532-539.
Shen, X., Sun, S., Wang, H. Q., Wang, M. X., Ren, J. F., Zhang G. T., and Liu, B., 2008. Mitochondrial genomic characteris-tics of Euphausia Superba and its application as molecular markers., 39 (5): 446-454.
Sun, S., and Yan, X. J., 2001. Active substances in the Antarctic Krill., 13 (3): 213-216.
Sun, X., Wang, J. C., Li, H. T., Du, G. C., and Guo, D. S., 2005. A study on the antibacterial mechanism of rosmarinic acid.(), 18 (4): 41- 45.
Wang, G. L., Jiang, D., and Fang, H. J., 2004a. Study on bacteriostatic action and mechanism of arisaema consanguineum schott., 35 (3): 280-285.
Wang, G. L., Jiang, D., Fang, H. J., and Li, X., 2004b.Study on mutation breeding of high bacteriocin-producing strain WJK84-1 and antimicrobial mechanism against crown gall disease of plant.,44 (1): 23-28.
Wang, G. L., Tang, J. H., Jiang, D., Fang, H. J., and Liu, Z. B., 2006.Bacteriostatic action and mechanism of Sophora flavescens ait on01 C84010., 39 (5): 1018-1024.
Wu, W. P., and Xie, Y. L., 2010. Antarctic Krill and Krill fishery., 25 (1): 10-13.
Zhao, L., Cao, R., Liu, Q., and Wei, Y. X., 2011. Antimicrobial activity of polypeptides from Antarctic Krill., 32: 112-116.
Zhou, X. H., Zhang, X. X., Zhu, X. L., Wei, W., and Zhu, Z. J., 2009.Preliminary study on antibacterial activity and mechanism of propolis., 34 (5): 233- 236.
(Edited by Ji Dechun)
10.1007/s11802-013-2180-2
ISSN 1672-5182, 2013 12 (3): 484-490
. Tel: 0086-532-85830760 E-mail: liuqi@ysfri.ac.cn
(October 22, 2012; revised March 8, 2013; accepted March 21, 2013)
© Ocean University of China, Science Press and Springer-Verlag Berlin Heidelberg 2013
杂志排行
Journal of Ocean University of China的其它文章
- Evaluation of Antitumor, Immunomodulatory and Free Radical Scavenging Effects of A New Herbal Prescription Seaweed Complex Preparation
- Shallow Water Body Data Processing Based on the Seismic Oceanography
- Prediction of China’s Submerged Coastal Areas by Sea Level Rise due to Climate Change
- A Homogeneous Linear Estimation Method for System Error in Data Assimilation
- The Suspended Sediment Concentration Distribution in the Bohai Sea, Yellow Sea and East China Sea
- Role of Ekman Transport Versus Ekman Pumping in Driving Summer Upwelling in the South China Sea
