含喹啉氧基乙酰胺型配体的锌配合物的合成、晶体结构及荧光性质
2012-12-11吴伟娜
吴伟娜 王 元 唐 宁
(1河南理工大学物理化学学院,焦作 454000)
(2兰州大学化学化工学院和应用有机重点实验室,兰州 730000)
含喹啉氧基乙酰胺型配体的锌配合物的合成、晶体结构及荧光性质
吴伟娜*,1,2王 元1唐 宁2
(1河南理工大学物理化学学院,焦作 454000)
(2兰州大学化学化工学院和应用有机重点实验室,兰州 730000)
合成并通过单晶衍射表征了一个配合物[ZnL(NO3)2]·CH3CN(1,L=N-(1-萘基)-2-(8-喹啉氧基)乙酰胺)。在配合物1中,金属锌离子采取扭曲的四方锥的配位构型。来自配体L的1个氧原子,2个氮原子及来自2个硝酸根的2个氧原子和中心锌离子配位。配合物通过分子间的N-H…O氢键作用构筑成沿a轴的链状结构。乙腈溶液中配合物在414.8 nm处有强荧光发射。
锌配合物;晶体结构;荧光
The amide type ligands with nitrogen and oxygen as electron donor atoms,are effective chelating agents that can form complexes with different metal ions.Recently,bi-,tri-,tetra-and hexapodal amide type ligands have been investigated by Liu and his coworkers[1-4].Such ligands,which are flexible in structure,could shield the encapsulated ion from interaction with the surroundings effectively.They have strong antenna effect to Eu(Ⅲ)and Tb(Ⅲ)[2-4].We have also reported some quinolin-8-yloxyl substitutedacetamide ligands and their fluorescence properties with the rare earth ions[5-8].However,the investigations on the complexes of amide ligands with transition metal ions are relatively few.Thus,in this work,a zinccomplex containing an amide type ligand was synthesized and characterized by X-ray diffraction.In addition,the fluorescence spectra of the ligand and the complex in CH3CN solution were investigated.
1 Experimental
1.1 Materials and measurements
Solvents and starting materials for synthesis were purchased commercially and used as received.Elemental analyses were carried out on an Elemental Vario EL analyzer.The infrared spectra(IR,ν=4000~400 cm-1)were determined by the KBr disc method on a Nicolet-170SX FTIR spectrophotometer.The UV spectra were recorded on a Shimadzu UV-240 spectrophotometer.Fluorescence spectra were determined on a Hitachi F-4500 FL spectrophotometer.In the measurements of emission and excitation spectra the pass width was 5 nm.
1.2 Preparations of[ZnL(NO3)2]·CH3CN(1)
The ligand N-(naphthalen-1-yl)-2-(quinolin-8-yloxy)acetamide(L)[5](0.38 g,1 mmol)was dissolved in acetonitrile(10 mL),then an acetonitrile solution(10 mL)containing zinc nitrate hexehydrate(0.295 g,1 mmol)was added dropwise at room temperature.After stirring for 2 h,the mixture was filtered and set aside to crystallize at room temperature for 1 d,giving colorless block crystals,which were collected by filtration,washed with Et2O and dried in air.Yield ca.45%based on L.Anal.Calcd.for C23H19N5O8Zn(%):C,49.43;H,3.43;N,12.53.Found(%):C,49.08;H,3.67;N,12.20.
1.3 X-ray crystallography
A colorless block crystal with dimensions of 0.20 mm×0.18 mm×0.18 mm was put on Bruker SMART APEXⅡCCD diffractometer equipped with a graphite monochromatized Mo Kα radiation(λ=0.071 073 nm)by using φ-ω scan mode at 294(2)K.Total of 5 962 reflections were collected in the range of 1.88°≤θ≤25.50°,of which 4 264 were independent with Rint=0.027 4,and 2523 with I>2σ(I)were considered as observed.Semi-empirical absorption correction was applied to the intensity data using the SADABS program[9].The structure was solved by direct methods and refined by fullmatrix least-square on F2using the SHELXTL-97 program[10].All non-hydrogen atoms were refined anisotropically.All H atoms bounded to C and N atoms were generated geometrically and refined isotropically using the riding mode.A summary of crystal data and details of the structure refinements are listed in Table 1.
CCDC:848089.

Table 1 Crystal data and structure refinement for the title complex
2 Result and discussion
2.1 Crystal structure of complex 1
As shown in Fig.1,the title complex contains one solvate acetonitrile molecule,with a composition of[ZnL(NO3)2]·CH3CN.Selected bond lengths and angles are summarized in Table 2.It can be confirmed that the Zn2+center coordinates with one nitrogen atom from the L ligand and four oxygen atoms,two of which are from Lligand and the others are from two nitrate ligands,respectively.The distances of Zn1…O1 and Zn1…O4 are both 0.254 9 nm,indicating that O1 and O4 atoms do not take part in coordination.The maximal two angles between the coordinate atoms and Zn2+ion are 146.85(19)°and 137.03(19)°,respectively.According to the Addison rule[11],the geometric index is 0.16,indicating that the Zn2+ion possesses a coordination geometry closer to a distorted square-pyramidal.However,most bond angles are highly deviated from those of the ideal geometry.Its structure is different from that of aqua[N-phenyl-2-(quinolin-8-yloxy)-acetamide]dinitratozinc(Ⅱ),in which the six-coordinated Zn atom is in a distorted octahedral geometry and an additional water O atom takes part in the coordination[12].The basal plane of the square-pyramid is made up of N4,O5,O7 and O8.The bond lengths from Zn2+center to these atoms are in the range of 0.2035(4)~0.2224(4)nm.The fifth coordination site is occupied by O3 located axially at 0.204 2(5)nm.The N-O bond lengths in the two nitrate groups are different.It is obvious that the two nitrate groups lie in dissimilar coordination environ-ments.In the crystal,intramolecular N-H…O hydrogen bonds link the complex molecules into chains along the a axis(Fig.2).

Table 2 Selected bond lengths(nm)and angles(°)in the title complex
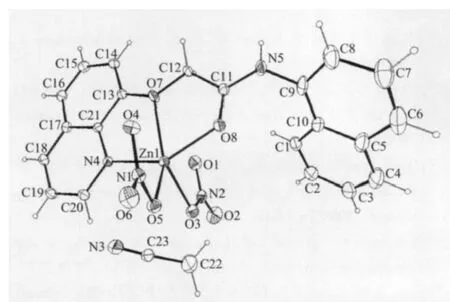
Fig.1 Molecular structure of the title complex shown with 30%probability displacement ellipsoids

Fig.2 Intramolecular hydrogen bonds of extended chainlike structure along a axis
2.2 IR spectra
The IR spectra of L show strong band at 1 694 cm-1,which are attributable to stretch vibrations of the carbonyl group of amide(ν(C=O)).The peak at 1 630 cm-1should be assigned to the ν(C=N),and the peak at 1 262 cm-1to ν(Ar-O-C).Upon coordination with Zn2+,the ν(C=O),ν(C=N)and ν(Ar-O-C)shift by 44,59 and 5 cm-1,respectively;indicating that carbonyloxygen atom,ethereal oxygen atom and quinoline nitrogen atom takepart in coordination to the metal ion[13-16].In addition,two intense absorption bands in the spectra associated with the asymmetric stretching appear at 1 319 cm-1(ν4)and 1 477 cm-1(ν1),clearly establishing that the NO3-groups(C2v)take part in coordination.The difference between the two bands is 158 cm-1,suggesting that the NO3-groups in the complexes are monodentate ligands[17].It is in accordance with the result of the crystal structure study.
2.3 UV spectra
The UV spectra of L and 1 in CH3CN solution(concentration:1×10-5mol·L-1)was measured at room temperature(Fig.3).The spectra of L feature three main bands located around 200(ε=89 553 L·mol-1·cm-1,),221(ε=82 308 L·mol-1·cm-1,)and 292 nm(ε=14 322 L·mol-1·cm-1)[18].The bands could be assigned to characteristic π-π*transitions centered on naphthalene,quinoline ring and the acetamide unit,respectively.They shift to 202(ε=115 032 L·mol-1·cm-1),218(ε=107 785 L·mol-1·cm-1)and 292 nm(ε=15 413 L·mol-1·cm-1)in complex 1,respectively.The hyperchromicities indicate that the ligand L takes part in the coordination in complex 1.
2.4 Fluorescence spectra
The fluorescence spectra of L and 1 in CH3CN solution(concentration:1×10-5mol·L-1)was measured at room temperature.The excitation wavelengths are both at 314 nm.The emission peak of the complex 1 is at 414.8 nm,but that of the ligand L is at 364.2 nm(Fig.4).It also can be seen that the emission intensity of the complex is much higher than that of the ligand.This is probably due to the energy transfer from the ligand to the Zn(Ⅱ)ion[19].The behavior of Zn2+coor-dinated to the ligand is in general as that of emissive species leading to a CHEF effect(chelation enhance-ment of the fluorescence emission).

Fig.3 UV spectra of L(dashed line)and complex 1

Fig.4 Fluorescence emission spectra of L(dashed line)and complex 1
[1]Song X Q,Yu Y,Liu W S,et al.J.Solid State Chem.,2007,180:2616-2624
[2]Tang Y,Zhang J,Liu W S,et al.Polyhedron,2005,24:1160-1166
[3]Song X Q,Wen X G,Liu W S,et al.J.Solid State Chem.,2010,183:1-9
[4]Tang Y,Liu D B,Liu W S,et al.Spectrochim.Acta A,2006,63:164-168
[5]Wu W N,Yuan W B,Tang N,et al.Spectrochim.Acta A,2006,65:912-918
[6]Wu W N,Tang N,Yan L.J.Fluoresc.,2008,18:101-107
[7]Wu W N,Cheng F X,Yan L,et al.J.Coord.Chem.,2008,61:2207-2215
[8]Wu W N,Tang N,Yan L.Spectrochim.Acta A,2008,71:1461-1465
[9]Sheldrick G M.SADABS,University of Göttingen,Germany,1996.
[10]Sheldrick G M.SHELX-97,Program for the Solution and the Refinement of Crystal Structures,University of Göttingen,Germany,1997.
[11]MA Wei-Xing(马卫兴),QIAN Bao-Hua(钱保华),CHENG Qing-Fang(程青芳),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2006,22:2101-2104
[12]Wu W N,Wang Y,Zhang A Y,et al.Acta Cryst.E,2010,66:m288-
[13]HU Xiao-Li(胡晓黎),FAN Li-Yan(范丽岩),YAN Lan(闫兰),et al.Chin.J.Applied Chem.(Yingyong Huaxue),2002,19:727-729
[14]Zhang Y L,Liu W S,Dou W,et al.Spectrochim.Acta A,2004,60:1707-1711
[15]JIANG Yi-Hua(江以桦),YANG Ru-Dong(杨汝栋),YAN Lan(闫兰)et al.J.Chin.Rare Earth Soc.(Zhongguo Xitu Xuebao),2002,20:474-477
[16]ZHOU Yu-Ping(周毓萍),YANG Zheng-Yin(杨正银),YU Hong-Jun(于红娟),et al.Chin.J.Applied Chem.(Yingyong Huaxue),1999,16:37-41
[17]Nakamato K.Infrared and Raman Spectra of Inorganic and Coordination Compounds.New York:John Wiley,1978:227
[18]Song X Q,Zang Z P,Liu W S,et al.J.Solid State Chem.,2009,182:841-848
[19]Wang Y,Yang Z Y.J.Lumin.,2008,128:373-376
Zinc Complex with Acetamide Type Ligand Bearing Quinolinyloxy Unit:Synthesis,Crystal Structure and Fluorescence Spectra
WU Wei-Na*,1,2WANG Yuan1TANG Ning2
(1Department of Physics and Chemistry,Henan Polytechnic University,Jiaozuo,Henan 454000,China)
(2College of Chemistry and Chemical Engineering and State Key Laboratory of Applied Organic Chemistry,Lanzhou University,Lanzhou 730000,China)
A complex,[ZnL(NO3)2]·CH3CN(1,L=N-(naphthalen-1-yl)-2-(quinolin-8-yloxy)acetamide),was synthesized and characterized by X-ray diffraction.In complex 1,the Zn2+center,possessing a coordination geometry closer to a distorted square-pyramidal,coordinates with one nitrogen atom from the L ligand and four oxygen atoms,two of which are from L ligand and the others are from two nitrate ligands,respectively.In the crystal,intramolecular N-H…O hydrogen bonds link the molecules into chains along the a axis.In CH3CN solution,the complex 1 exhibits strong emission band at 414.8 nm.CCDC:848089.
zinc complex;crystal structure;fluorescence
O614.24+1
A
1001-4861(2012)02-0425-04
2011-06-05。收修改稿日期:2011-09-11。
国家自然科学基金(No.21001040)资助项目。
*通讯联系人。E-mail:wuwn08@hpu.edu.cn
