5-氨基双四唑富氮配位化合物的合成、结构及其对高氯酸铵的热分解影响
2012-12-11夏正强陈三平高胜利
谢 钢 夏正强 陈三平 高胜利
(合成与天然功能分子教育部重点实验室,西北大学化学与材料科学学院,西安 710069)
5-氨基双四唑富氮配位化合物的合成、结构及其对高氯酸铵的热分解影响
谢 钢 夏正强 陈三平*高胜利*
(合成与天然功能分子教育部重点实验室,西北大学化学与材料科学学院,西安 710069)
水热条件下合成了两个5-氨基双四唑配位化合物Cu(bta)(bpy)(H2O)(1)和Pb2(bta)2(en)2·4H2O(2)(H2bta=5-氨基双四唑,bpy=2,2′-联吡啶,en=乙二胺),并借助单晶X-射线衍射技术对其结构进行了表征。在配合物1中,5-氨基双四唑配体以双齿螯合模式与铜离子配位形成离散的分子,并通过H键作用进一步形成了三维的超分子结构。在配合物2中,强的R22(8)氢键环作用将双核的Pb2(bta)2(en)2单元连接成一维的链,这些链通过与水分子氢键作用被进一步组装成三维的超分子结构。另外,通过DSC技术探究了它们作为添加剂对高氯酸铵的热分解催化影响。研究发现,铅基化合物2的催化效果较铜基化合物1要好。
5-氨基双四唑;配位化合物;高氯酸铵;晶体结构
Energetic metal salts are employed as attractive alternatives in view that they ballistically modify the combustion pattern of propellants without much negative effect on energetics[1].Of those energetic metal salts,rich-nitrogen energetic compounds as environmentally benign explosives are employed as the ligands tosynthesize the energetic metal salts.As one of the energetic materials,tetrazoles with an outstanding property of high nitrogen content,high positive heat of formation and good thermal stability,owing to their aromatic ring system[2],have promised their complexes interesting for energetic materials.Furthermore,metal cations complexes are sought components for pyrotechnical mixtures,by the combination of the energetic nitrogen rich ligands and the colorant metal cations[3].Until now,many metal-tetrazole derivatives complexes have been synthesized and characterized to be the potential energetic materials,components for pyrotechnical mixtures,additives in pyrotechnics and AP based propellants for their characteristics of high energy and thermal stability[3-8].In addition,the application field of tetrazoles are involved of magnetic[9-11]and catalytic application[12],as well as adsorption[13-14],topology[15-17]and energetic materials[18-21].
N,N-bis(1H-tetrazole-5-yl)-amine(H2bta)has been widely reported as energetic materials with high nitrogen and high thermal stability.As the derivative of tetrazole,H2bta possesses versatile coordination modes due to the nine electron-donating nitrogen atoms are the potential coordination sites,which promises the H2bta to be an intriguing ligand in coordination chemistry.Moreover,the nitrogen atoms are predicted to be involved in the hydrogen bonds motif to construct supramolecules and capture guest molecules[22].Above all,the H2bta complexes with the metal ions could not only give an interesting structure but also provide the active metals or metal oxides at the molecule level on the propellant surface to improve combustion reaction when the compounds are used as the additives in the propellant[1,23].Therefore,both in theoretical prospect and practical application,the syntheses and structures of H2bta complexes are of significance.
In this paper,we report the syntheses and structures of two new energetic coordination compounds based on N,N-bis(1H-tetrazole-5-yl)-amine,Cu(bta)(bpy)(H2O)(1)and Pb2(bta)2(en)2·4H2O(2).Because the thermal decomposition of AP directly influences the combustion behavior of solid propellants[24-25],compounds 1 and 2 as the additives on the decomposition of AP were explored by the differential scanning calorimetry(DSC)techniques.
1 Experimental
1.1 Materials and instruments
All reagents were purchased commercially and used without further purification.H2bta·H2O was synthesized according to the reference[4].Elemental analyses were carried out with an Elementar Vario EL Ⅲ analyzer.IR spectra were recorded with a Tensor 27 spectrometer(Bruker Optics,Ettlingen,Germany).Thermogravimetric measurements were performed with a Netzsch STA449C apparatus under a nitrogen atmosphere with a heating rate of 10℃·min-1from 30 to 900℃.DSC experiments were performed with a thermal analyzer of Perkin-Elmer Pyris 6 DSC with heating rates of 5,10,15 and 20℃·min-1from 30 to 500℃.
1.2 Preparation
Preparation of Cu(bta)(bpy)(H2O)(1):A mixture of Cu(NO3)2·3H2O(0.048 8 g,0.2 mmol),H2bta·H2O(0.034 3 g,0.2 mmol),2,2′-bipyridyl(0.031 2 g,0.2 mmol)and H2O(7 mL)was sealed in a 10 mL Teflonlined stainless autoclave and heated at 130℃under autogenous pressure for 3 d and then cooled at a rate of 5℃·min-1to room temperature;green block crystals were obtained.Yield:0.022 5 g(28.9%based on Cu).Anal.calcd.for C12H11ON11Cu(Mr=388.87)(%):C 37.03,H 2.83,N 39.60;found(%):C 36.68,H 2.76,N 39.04.IR(KBr,cm-1):3 527(s),3 458(s),3 394(m),3195(w),2819(m),2364(m),1 652(vs),1515(vs),1 456(s),1 338(m),1 247(m),1 145(m),1 147(m),1 097(w),1 051(s),1002(s),854(s),810(m),734(s),686(s).
Preparation of Pb2(bta)2(en)2·4H2O(2):A mixture ofPb(NO3)2(0.066 2 g,0.200 mmol),H2bta·H2O(0.034 7 g,0.2 mmol),ethylenediamine(30 μL)and H2O(7 mL)was sealed in a 10 mL Teflon-lined stainless autoclave and heated at 120℃under autogenous pressure for 3 d and then cooled at a rate of 5℃·h-1to room temperature;colorless block crystals were obtained in the solution of the reactors after 1 week.Yield:0.0620 g(34.1%based on Pb).Anal.calcd.for C8H26O4N22Pb2(Mr=908.91)(%):C 10.57,H 2.88,N 33.91;found(%):C 10.34,H 2.67,N 33.68.IR(KBr,cm-1):3 357(w),3 284(w),3 143(w),2914(m),1 616(vs),1 525(s),1 496(s),1 419(s),1 398(s),1 313(s),1 236(s),1124(s),995(s),781(s),694(s),559(s).
1.3 X-ray crystallography
All single-crystal X-ray diffraction experiments were performed with a Bruker Smart Apex CCD diffractometer equipped with graphite monochromated Mo Kα radiation(λ=0.071 073 nm)using φ-ω scan mode.The single-crystal structures of compounds were solved by direct methods and refined with full-matrix least-squares techniques based on F2using SHELXS-97 and SHELXL-97[26-27].All non-hydrogen atoms were refined anisotropically.The H atoms attached C or N atoms were placed at calculated positions in the ridingmodelapproximation, with their displacement parameters set to 1.2 times Ueqof the parent atoms.The water H atoms were located in difference Fourier maps,and then refined with isotropic thermal parameters 1.5 times those of O atoms.Crystal data and structure refinements parameters for 1 and 2 are listed in Table 1.The selected bond distances and angles are shown in Table 2 and hydrogen bonding interactions for 1 and 2 are shown in Tables 3.
CCDC:855627,1;774052,2.

Table 1 Crystal data and structure parameters for 1 and 2

Table 2 Selected bond lengths(nm)and angles(°)for 1 and 2

Continued Table 1

Table 3 Hydrogen bonding interactions in 1 and 2
2 Results and discussion
2.1 Description of crystal structures
Single-crystal X-ray diffraction studies reveal that 1 and 2 both crystallize in the triclinic crystal system,space group P1.The molecular structure of 1 is shown in Fig.1.There are two crystallographically unique Cu(Ⅱ)ions,two bta2-ligands,two bpy co-ligands and two coordinated water molecules in the asymmetric unit.The Cu1(Ⅱ)ion is five-coordinated by four N atoms from one bta2-ligand(N1 and N2)and one bpy molecule(N3 and N8),and one coordinated water molecule(O1).The coordination environment of Cu2(Ⅱ)center is exactly similar with that of Cu1(Ⅱ)in 1,while there are some subtle differences in the bond lengths and angles.The Cu-N bond distances vary from 0.1960(3)to 0.205 6(4)nm with the mean of 0.199 7(8)nm,while the Cu-O bond distance is slightly longer,0.2234(3)nm.

Fig.1 ORTEP drawing of asymmetric unit of compound 1 with 30%probability level
It is worth noting that the tetrazole nitrogen atoms and coordinated water molecules are involved in the extensive strong,very directional hydrogen bonds(Fig.2 and Table 3),which connect the discrete Cu(Ⅱ)coordination units to generate a 3D supramolecular structure.
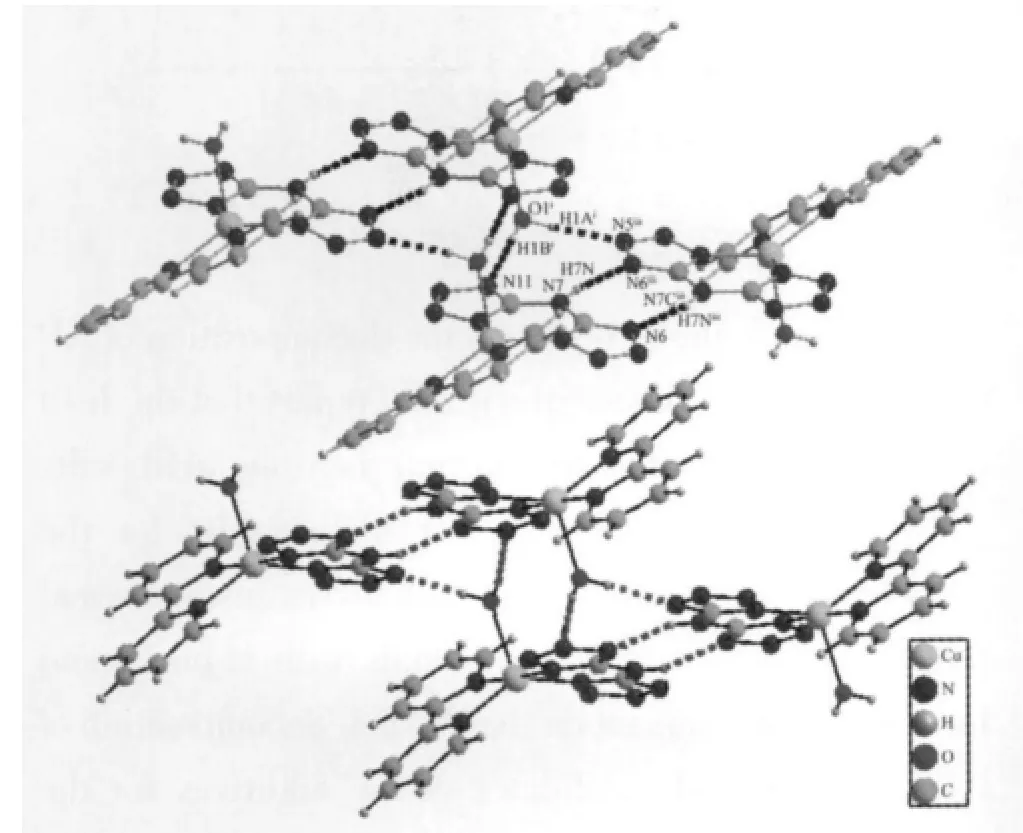
Fig.2 Intermolecular hydrogen bonding interactions in compound 1
As shown in Fig.3,2 is a binuclear molecule with two bta2-and ethylenediamine,which is just like the reported Pb2(bta)2(bpy)2(2′)and[Pb2(bta)2(phen)2]·2H2O(2″)[28].The similar structures are due to the same coordination modes of bta2-and the auxiliary ligands.However,the length of the bridge bonds N-Pb and Pb…Pb are different(0.289 2 nm for 2,0.279 1 nm for 2′,0.2848 nm for 2″).The strong hydrogen bond ring motif(N5…N6i0.29543 nm,i2-x,1-y,1-z)is identified as the very commonwhich bridges the binuclear units to the 1D chain(Fig.4 and Table 3),where the Pb2N4ring planes are parallel.The free water molecules construct the 1D chain to a 3D supramolecule through the hydrogen bonds.
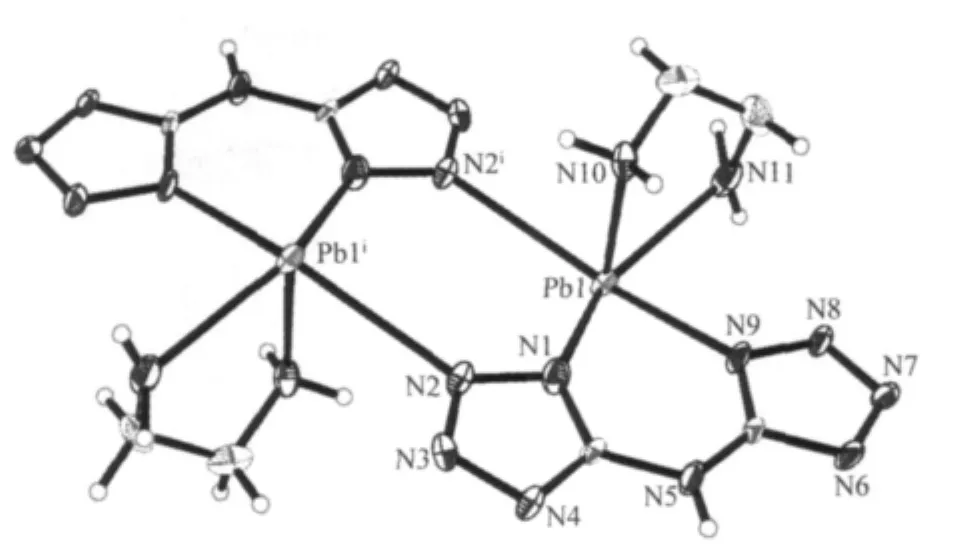
Fig.3 Coordination environment of Pb2+ion in 2 with 30%thermal ellipsoids probability level

Fig.4 1D chain afforded by hydrogen bonds in 2
2.2 Thermal gravimetric analyses
Thermal gravimetric(TG)analyses were carried out between 30 and 800℃(Fig.5)under nitrogen atmosphere.In the TG curve,1 undergoes a dehydration process during the temperature range from 100 to 150℃with a weight loss of 4.81%corresponding to the loss of one water molecule(calcd.4.62%).Then the compound are stable until 200℃and loses 72.81%(calcd.74.81%),which is due to the decomposition of ligand ejecting nitrogen gas[30].For 2,it firstly loses the lattice water between 50 and 110℃,and then loses en molecules at around 220℃,after that a series of weight losses up to 700℃are found.

Fig.5 TG curves of 1(left)and 2(right)
2.3 Effects on thermal decomposition of ammonium perchlorate
To check the effects of thermal decomposition of AP after adding the as-synthesized compounds,compounds 1 and 2 and AP were mixed at a mass ratio of 1∶3 to prepare the target samples for thermal decomposition analyses.A total sample mass used was less than 1.0 mg for all runs.The Kissiger′s method was used to investigate the apparent activate energy(Ea)and the pre-exponential factor(A)by four different heat rates of 5,10,15 and 20℃·min-1[28].DSC curve of AP has three peaks under our experiments condition.The first endothermic peak at 242℃is attributed to the crystallographic transition of AP from orthorhombic to cubic.The second peak at 288℃is exothermic,which is corresponding to the low-temperature decomposition(LTD).The third peak following the LTD instantly is an endothermic peak which is assigned to the hightemperature decomposition(HTD).The HTD process either exothermic or endothermic process,depending on the competition between sublimation and thermal decomposition[31-32].
After adding the compounds 1 and 2 as the additives to the AP,the first endothermic peak of crystallographic transition was not affected.But the decomposition of AP has changed,the endothermic HTD process change to the exothermic or convert to one exothermic peak with the LTD.Compared to the HTD of the pure AP,the decomposition temperature is lower,as shown in Fig.6.For AP with complexes 1 and 2 as the additives respectively,the two decomposition process become to a broad exothermic band with one or several peaks,which is due the compounds decompose earlier than the pure AP.

Fig.6 DSC curves for AP,AP after adding 1 and 2 respectively
Comparing the DSC curves of 1 and 2 with the AP,the decomposition peaks temperature(Tp)of the mixture for adding 2 are lower than that for 1 as well as the activation energy of the mixture,as illustrated in Table 4.Therefore,2 shows better on the decomposition of AP than 1,which is consistent with the report that the lead energetic 4-(2,4.6-trinitroa-nilino)benzoic acid salts performs better than its cobalt or nickel salts for the propellants[23].In summary,the analyses results reveal that both the two metal compounds with H2bta ligand have significant impact on the thermal decomposition of AP,and are good candidates of the additives for the AP-based propellants.
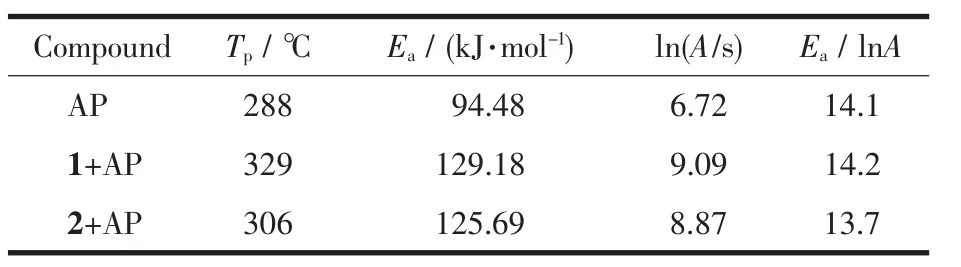
Table 4 Kinetic parameters of thermal decomposition for AP and AP with additives
[1]Kulkarni P B,Reddy T S,Nair J K,et al.J.Hazard.Mater.,2005,123:54-60
[2]Stierstorfer J,Tarantik K R,Klaptke T M.Chem.Eur.J.,2009,15:5775-5792
[3]Hartdegen V,Klapötke T M,Sproll S M.Inorg.Chem.,2009,48:9549-9556
[4]Friedrich M,Galvez-Ruiz J C,Klaptke T M,et al.Inorg.Chem.,2005,44:8044-8052
[5]Klapötke T M,Sabate C M,Welch J M.Eur.J.Inorg.Chem.,2009:769-776
[6]Klapötke T M,Sabate C M,Rasp M.Dalton Trans.,2009:1825-1834
[7]Karaghiosoff K,Klaptke T M,Sabate C M.Chem.-Eur.J.,2009,15:1164-1176
[8]ZHAO Feng-Qi(赵凤起),CHEN San-Ping(陈三平),FAN Guang(范广),et al.Chem.J.Chinese Universities(Gaodeng Xuexiao Huaxue Xuebao),2008,29:1519-1522
[9]Gao E Q,Liu N,Cheng A L,et al.Chem.Commun.,2007:2470-2472
[10]Lu Y B,Wang M S,Zhou W W,et al.Inorg.Chem.,2008,47:8935-8942
[11]Janiak C,Scharmann T G,Brzezinka K W,et al.Chem.Ber.,1995,128:323-328
[12]Horike S,Dinca M,Tamaki K,et al.J.Am.Chem.Soc.,2008,130:5854-5855
[13]Li J R,Tao Y,Yu Q,et al.Chem.-Eur.J.,2008,14:2771-2776
[14]DincǎM,Yu A F,Long J R.J.Am.Chem.Soc.,2006,128:8904-8913
[15]Zhang X M,Jiang T,Wu H S,et al.Inorg.Chem.,2009,48:4536-4541
[16]Chen Q Y,Li Y,Zheng F K,et al.Inorg.Chem.Commun.,2008,11:969-971
[17]Janiak C,Scharmann T G.Polyhedron,2003,22:1123-1133
[18]Klapötke T M,Mayer P,MiróSabaté C,et al.Inorg.Chem.,2008,47:6014-6027
[19]Steinhauser G,Klapötke T K.Angew.Chem.,Int.Ed.,2008,47:3330-3338
[20]Singh R P,Verma R D,Meshri D T,et al.Angew.,Chem.Int.Ed.,2006,45:3584-3601
[21]Joo Y H,Twamley B,Garg S,et al.Angew.Chem.,Int.Ed.,2008,47:6236-6239
[22]Zheng L L,Li H X,Leng J D,et al.Eur.J.Inorg.Chem.,2008:213-217
[23]Pundlik S M,Palaiah R S,Nair J K,et al.J.Energ.Mater.,2001,19:339-347
[24]Cui P,Li F S,Zhou J,et al.Propellants,Explos.Pyrotech.,2006,31:452-455
[25]Chen L J,Li L P,Li G S.J.Alloys Compd.,2008,464:532-536
[26]Sheldrick G M.SHELXS-97,Program for Solution of Crystal Structures,University of Gottingen,Germany,1997.
[27]Sheldrick G M.SHELXL-97,Program for Refinement of Crystal Structures,University of Gottingen,Germany,1997.
[28]Wang W T,Chen S P,Gao S L.Eur.J.Inorg.Chem.,2009:3475-3480
[29]Etter M C.Acc.Chem.Res.,1990,23:120-126
[30]Poturovic S,Lu D,Heeg M J,et al.Polyhedron.,2008,27:3280-3286
[31]Lang A J,Vyazovkin S.Combust.Flame.,2006,145:779-790
[32]Vyazovkin S,Wight C A.Chem.Mater.,1999,11:3386-3393
Nitrogen-Rich Coordination Compounds with N,N-Bis(1H-tetrazole-5-yl)-amine:Synthesis,Structure and Effect on the Thermal Decomposition of Ammonium Perchlorate
XIE GangXIA Zheng-QiangCHEN San-Ping*GAO Sheng-Li*
(Key Laboratory of Synthetic and Natural Functional Molecule Chemistry of Ministry of Education,College of Chemistry and Materials Science,Northwest University,Xi′an 710069,China)
Two coordination compounds with N,N-bis(1H-tetrazole-5-yl)-amine(H2bta),Cu(bta)(bpy)(H2O)(1)and Pb2(bta)2(en)2·4H2O(2)(bpy=2,2′-bipyridyl and en=ethylenediamine),have been hydrothermally synthesized and structurally characterized by single crystal X-ray diffraction.In 1,the H2bta ligands coordinate with Cu2+ions in the bidentate chelate mode to form discrete molecular structures,which are assembled through extended hydrogen bonds to generate a 3D architecture.2 presents a 3D supramolecule stucture constructed from 1D chains,in which the binuclear Pb2(bta)2(en)2units are bridged by the strong hydrogen bond ring motif R22(8).Furthermore,compounds 1 and 2 were explored as additives to promote the thermal decomposition of ammonium perchlorate(AP)by the differential scanning calorimetry techniques(DSC).DSC curves reveal that lead-based compound 2 shows better performance than the copper-based compound 1.CCDC:855627,1;774052,2.
N,N-bis(1H-tetrazole-5-yl)-amine;coordination compounds;ammonium perchlorate;crystal structure
O614.43+3;O614.121
A
1001-4861(2012)02-0367-07
2011-11-01。收修改稿日期:2011-12-05。
国家自然科学基金(No.21073142,21173168,21127004),陕西省自然科学基金(No.09JS089)和陕西省教育厅科技专项基金(No.2010JK882,2010JQ2007)资助项目。
*通讯联系人。E-mail:gaoshli@nwu.edu.cn,sanpingchen@126.com
