石蒜属植物生物碱成分研究进展
2012-09-15王跃虎陈丽娟龙春林
王 欢,王跃虎,陈丽娟,龙春林,3*
1中国科学院昆明植物研究所,昆明 650204;2云南农业大学农学与生物技术学院,昆明,650201;3中央民族大学生命与环境科学学院,北京,100081
石蒜属植物生物碱成分研究进展
王 欢1,2,王跃虎1,陈丽娟2,龙春林1,3*
1中国科学院昆明植物研究所,昆明 650204;2云南农业大学农学与生物技术学院,昆明,650201;3中央民族大学生命与环境科学学院,北京,100081
石蒜属为东亚特有属。该属植物富含生物碱,且此类次生代谢产物具有细胞毒、抗疟疾、抗病毒以及对乙酰胆碱酯酶的抑制作用等活性。本文概述了该属中生物碱类成分的化学结构、药理活性及其生源途径。
石蒜科;石蒜属;生物碱
石蒜科(Amaryllidaceae)石蒜属(LycorisHerb.)为东亚特有属,是一类兼具观赏价值与药用价值的经济植物[1]。该属植物约有20多种,主要分布于中国和日本,少数产于缅甸和朝鲜。我国有16种,主要分布在长江以南地区[2]。民间将其鳞茎捣碎、敷治肿毒[3]。植物化学成分分析和现代药理实验表明,石蒜属植物含有多种具有药理活性的生物碱类次生代谢产物。
1 生物碱类次生代谢产物
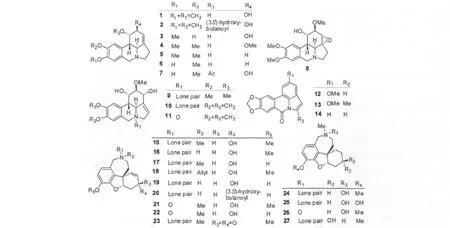
国内外学者对石蒜属的生物碱类成分进行了系统性的研究,共从中得到54个生物碱(见表1及图1)。根据这些产物结构的不同,可将其划分为十种骨架类型(图2):石蒜碱型(Lycorine-type,Ⅰ),加兰他敏型(Galanthamine-type,Ⅱ),文殊兰碱型(Crinine-type,Ⅲ),水仙环素型(Narciclasine-type,Ⅳ),石蒜宁碱型(Lycorenine-type,Ⅴ),水仙碱型(Tazettine-type,Ⅵ),高山网球花碱型(Montanine-type,Ⅶ),孤挺花定型(Belladine-type,Ⅷ),雪花吲哚碱型(Galanthindole-type,Ⅸ)和艾斯敏型(Ismine-type,Ⅹ)。
2 药理活性
石蒜属生物碱类次生代谢产物具有多种药理活性,主要为细胞毒、抗疟疾以及对乙酰胆碱酯酶(Acetylcholinesterase,AChE)的抑制作用。
2.1 细胞毒活性
前人对石蒜碱型、加兰他敏型、文殊兰碱型、水仙环素型、石蒜宁碱型、水仙碱型和艾斯敏型生物碱的细胞毒活性进行了研究。结果表明:石蒜碱(1)和伪石蒜碱(3)对多种癌细胞系以及人类白血病细胞系具有较强的抑制活性[4-8],Lamoral-Theys等研究发现1通过抑制肿瘤细胞生长而不是毒杀细胞发挥抗肿瘤作用,且对肿瘤细胞的抑制活性是正常细胞的15倍[7];网球花胺(29)不仅对Hela和Vero细胞系具有较强的抑制活性[4,5],其浓度为10 μM时也能够诱导人 T淋巴细胞白血病细胞株(Jurkat cells)凋亡且对健康细胞无毒害[9];石蒜西定(32)和水仙环素(33)对60多种人类肿瘤细胞具有强烈的抑制活性,其IC50为0.01~0.41 μM[4,5,10,11];高石蒜碱(40)对鼠癌细胞系(L5178)具有弱的活性(IC50=28.5 μM),但能够提高阿霉素的抗肿瘤活性(Fix为0.63±0.05)[12];小星蒜碱(43)不仅对Hela和Vero细胞系具有较强的抑制活性,还可以抑制胶原蛋白Ⅰ的侵染[4,5];漳州水仙碱(46)对人类癌细胞系(MCF-7,HeLa和A431)以及鼠淋巴瘤细胞系(L5178)具有强烈的抗增殖活性(IC50为0.8~8.8 μM)[12];而加兰他敏型[6]、石蒜宁碱型[4,5]和艾斯敏型[12]生物碱的细胞毒活性较差。

表1 石蒜属植物生物碱成分Table 1 Alkaloids fromLycoris

30 11-Hydroxyvittatine(O-Demethylhaemanthamine) Ⅲ L.squamigera[43]31 Vittatine(维他廷;Crinine,文殊兰碱) Ⅲ L.radiata[38,41,49];L.guangxiensis[36]32 Lycoricidine(石蒜西定) Ⅳ L.radiata[22,24];L.sanguinea[48];L.squamigera[43];L.traubii[16]33 Narciclasine(水仙环素;Lycoricidinol,石蒜西定醇) Ⅳ L.chinensis[35];L.radiata[22,24];L.sanguinea[48];L.squamigera[43];L.traubii[16]34 Arolycoricidine Ⅳ L.sanguinea[48]35 Arolycoricidinol(Narciprimine,水仙明) Ⅳ L.sanguinea[48]36 Lycorenine(石蒜伦碱,石蒜宁碱) Ⅴ L.albiflora[32];L.radiata[40]37 O-Methyllycorenine Ⅴ L.aurea[30];L.radiata[24,41]38 O-Methyllycorenine N-oxide Ⅴ L.radiata[41]39 Radiatine Ⅴ L.radiata[50]40 Homolycorine(高石蒜碱;Narcipoetine) Ⅴ L.aurea[30];L.radiata[38,40,41];L.albiflora[32]41 9-Demethylhomolycorine Ⅴ L.radiata[24,40,41]42 Homolycorine N-oxide Ⅴ L.radiata[41]43 Hippeastrine(小星蒜碱) Ⅴ L.aurea[30];L.radiata[24,38,40,41]44 Hippeastrine N-oxide Ⅴ L.radiata[41]45 Tazettine(水仙碱) Ⅵ L.aurea[34];L.radiata[38,41];L.sanguinea[48];L.squamigera[43]46 Pretazettine(漳州水仙碱) Ⅵ L.radiata[39,40]47 8-O-Methylpretazettine Ⅵ L.squamigera[43]48 3-O-Ethyltazettinol Ⅵ L.aurea[51]49 Squamigine Ⅶ L.squamigera[43]50 Montanine(山小星蒜碱) Ⅶ L.squamigera[43]51 2R-Hydroxy-O,N-dimethylnorbelladine Ⅷ L.squamigera[43]52 Lycosinine A Ⅸ L.aurea[30]53 Lycosinine B Ⅸ L.aurea[30]54 Ismine(艾斯敏) Ⅹ L.squamigera[43]
2.2 乙酰胆碱酯酶(AChE)抑制活性
加兰他敏(15)对AChE具有长期的竞争性可逆抑制作用(IC50= 1.9 μM)[13],对老年性痴呆(Alzheimer's disease,AD)具有特殊的疗效,其盐酸盐Reminyl®已批准用于治疗中早期的老年痴呆症[14]。López等对隶属于石蒜碱型、石蒜宁碱型、文殊兰碱型、加兰他敏型以及水仙碱型的22个生物碱进行体外AChE抑制活性筛选,结果表明仅属于石蒜碱型和加兰他敏型的生物碱显示有活性,其中Sanguinine(17)的活性是阳性对照加兰他敏的十倍[15]。
2.3 抗疟疾活性
体外实验表明,LT1(2)具有显著的抗疟疾活性,对恶性疟原虫(Plasmodium falciparum)K1和FCR3株系的IC50值分别为0.60 μg/mL和0.45 μg/ mL,选择指数(SI)分别为13.5和18.0[16]。Sener等对石蒜碱型、文殊兰碱型、水仙碱型和加兰他敏型生物碱的抗疟疾活性进行了评价,石蒜碱(1)和网球花胺(29)对恶性疟原虫(P.falciparum)T9.96和K1株系的活性最高,其IC50值为0.38~1.03 μg/ mL;除加兰他敏(15)外,其它生物碱的活性均高于阳性对照氯喹(IC50=6.06 μg/mL)和甲氟喹(IC50=3.94 μg/mL)[17]。Campbell等的研究也证实石蒜碱(1)具有很好的抗疟疾活性[18]。
2.4 作用于心血管系统
Schmeda-Hirschmann等研究了隶属于石蒜碱型、石蒜宁碱型、加兰他敏型和文殊兰碱型的20个生物碱对正常小鼠血压的影响。在15 mg/kg的剂量下,加兰他敏(15)、石蒜宁碱(36)、高石蒜碱(40)、9-O-去甲高石蒜碱(41)和艾斯敏(54)对血压的降低率超过20%,持续时间为2.0~36.0 min[19]。
2.5 抗病毒活性
Szlávik等研究了石蒜碱(1)、网球花定(28)、网球花胺(29)、高石蒜碱(40)和水仙碱(45)对反转录病毒HIV的抑制活性,结果表明1、29和40具有较强的抗反转录病毒活性(TC50=0.75~1.0 μg/mL; ID50=0.4~7.3 μg/mL),但治疗指数较低(TI50= 1.3~1.9);而28和45没有活性[20]。Li等的研究证实石蒜碱(1)对SARS冠状病毒有很好的活性,其EC50为15.7±1.2 nM,CC50为14980.0±912.0 nM,选择指数(SI)高达900[21]。
2.6 植物生长调节剂
Okamoto等研究发现石蒜(L.radiata)的甲醇提取物对燕麦生长和水稻种子的萌发具有强烈的抑制作用,并从中分离得到石蒜西定(32)和水仙环素(33);这两个生物碱在浓度大于1 ppm时对燕麦生长和水稻的萌发具有抑制作用,并强烈抑制烟草愈伤组织的分化[22]。Iqbal等发现石蒜碱(1)作为化感物质对周围植物的生长具有抑制作用[23]。
2.7 昆虫拒食活性
Numata等研究表明石蒜碱(1)和9-O-去甲高石蒜碱(41)对Eurema hecabe mandarina的幼虫具有较强的拒食活性,浓度为0.05%时进食率小于30%;石蒜宁碱(36)、6-O-甲基石蒜宁碱(37)和小星蒜碱(43)具有微弱的拒食活性[24]。
2.8 其它活性
Toriizuka等研究发现 LT1(2)具有杀锥体虫(Trypanosoma brucei)的活性,其 IC50值为1.9 μg/ mL,选择指数(SI)为4.3[16]。Evidente等的研究表明水仙环素(33)对抗坏血酸(Ascorbic acid,AA)的生物合成具有强烈的抑制活性,当其浓度为5 μM时抑制率为100%;石蒜碱(1)对AA的生物合成具有一定的抑制活性,当其浓度为20 μM时抑制率为88%[25]。McNulty等研究了天然和半合成石蒜属生物碱对细胞色素酶CYP450 3A4的抑制活性,结果表明加兰他敏(15)和水仙环素(33)在低浓度(0.21~0.63 μM)时即对CYP450 3A4有抑制活性[26]。
3 生源途径
从石蒜属植物中已分离得到的10种骨架类型均是由苯丙氨酸和酪氨酸的代谢产物经过一系列的生物化学反应产生的[27]。2,3-二羟基苯甲醛与酪胺缩合生成孤挺花定型生物碱去甲孤挺花定(Norbelladine),在儿茶酚O-甲基转移酶的作用下,由S-腺苷甲硫氨酸(SAM)提供甲基生成4'-O-methylnorbelladine,再以之为前体进行酚-酚偶联生成各类石蒜属生物碱(图3)[28]。A为对-邻位(para-ortho')偶联,之后由N原子提供电子形成石蒜碱型[28];随后,C-6与N原子断开形成雪花吲哚碱型[29],C-6再通过O原子与C-1相连则形成石蒜宁碱型[30];B为对-对位(para-para')偶联,经N原子提供电子形成文殊兰碱型[28];随后,失去C-11和C-12生成水仙环素型[11,29],进而C-6与N原子断裂形成艾斯敏型;文殊兰碱型的C-6与N断开再通过O与C-11相连形成水仙碱型[31]。C为邻-对(ortho-para')偶联,经氧化、亲核攻击和还原等反应生成加兰他敏型[28]。在D中4'-O-methylnorbelladine经车瑞灵型(Cheryline-type)转化为高山网球花碱型[29]。因此,石蒜属生物碱具有相同的起源,其间生源关系如图3所示。
4 展望
国内外学者对石蒜属植物中的生物碱成分进行了大量的研究,但仍不全面有待进一步研究。我国石蒜属植物资源丰富,应充分利用其资源优势从中寻求新的抗癌、抗病毒、抗疟疾以及能够治疗AD的药物或前导药物;同时,深入了解石蒜属生物碱的生物合成途径,通过代谢工程提高药效成分含量降低成本,从而推动我国该领域制药业的蓬勃发展。

图3 石蒜属植物生物碱成分的可能生源途径[28-31]Fig.3 Proposed biosynthetic pathways for theLycorisalkaloids
1 Zhao TR(赵天荣),et al.The Headway of Research inLycoris.No Hort(北方园艺),2008,65-69.
2 Linghu YW(令狐昱慰),Li DW(李多伟).Advances in the Research ofLycoris.Subtrop Plant Sci(亚热带植物科学).2007,36:73-76.
3 Editorial Committee of the Flora of China of Chinese Academy of Science(中国科学院植物志编辑委员会).Flora of China(中国植物志).Beijing:Science Press,1985,16:16-17.
4 Evidente A,Kornienko A.Anticancer evaluation of structurally diverse amaryllidaceae alkaloids and their synthetic derivatives.Phytochem Rev,2009,8:449-459.
5 Evidente A,et al.Biological Evaluation of structurally diverse amaryllidaceae alkaloids and their synthetic derivatives:discovery of novel leads for anticancer drug design.Planta Med,2009,75:501-507.
6 Jokhadze M,et al.In vitroCytotoxicity of some medicinal plants from georgian Amaryllidaceae.Phytother Res,2007,21:622-624.
7 Lamoral-Theys D,et al.Lycorine,the main phenanthridine amaryllidaceae alkaloid,exhibits significant antitumor activity in cancer cells that display resistance to proapoptotic stimuli: an investigation of structure-activity relationship and mechanistic insight.J Med Chem,2009,52:6244-6256.
8 Liu XS,et al.Lycorine induces apoptosis and down-regulation of Mcl-1 in human leukemia cells.Cancer Lett,2009,274:16-24.
9 Griffin C,et al.Selective cytotoxicity of pancratistatin-related natural amaryllidaceae alkaloids:evaluation of the activity of two new compounds.Cancer Cell Int,2007,7:10.
10 Pettit GR,et al.Antineoplastic agents,256.cell growth inhibitory isocarbostyrils fromHymenocallis.J Nat Prod,1993,56: 1682-1687.
11 Ingrassia L,et al.Amaryllidaceae isocarbostyril alkaloids and their derivatives as promising antitumor agents.Translat Oncol,2008,1:1-13.
12 Zupkó I,et al.Antitumor activity of alkaloids derived from amaryllidaceae species.In Vivo,2009,23:41-48.
13 Elgorashi EE,et al.Acetycholinesterase enzyme inhibitory effects of amaryllidaceae alkaloids.Planta Med,2004,70: 260-262.
14 Howes MJR,Houghton PJ.Plants used in Chinese and Indian traditional medicine for improvement of memory and cognitive function.Pharmacol Biochem Behav,2003,75:513-527.
15 López S,et al.Acetylcholinesterase inhibitory activity of some amaryllidaceae alkaloids andNarcissusExtracts.Life Sci,2002,71:2521-2529.
16 Toriizuka Y,et al.New lycorine-type alkaloid fromLycoris traubiiand evaluation of antitrypanosomal and antimalarial activities of lycorine derivatives.Bioorg Med Chem,2008,16:10182-10189.
17 Sener B,et al.Antimalarial activity screening of some alkaloids and the plant extracts from amaryllidaceae.Phytother Res,2003,17:1220-1223.
18 Campbell WE,et al.Alkaloids from south african amaryllidaceae-part 7-bioactive alkaloids fromBrunsvigia radulosa.Phytochemistry,2000,53:587-591.
19 Schmeda-Hirschmann G,et al.Activity of amaryllidaceae alkaloids on the blood pressure of normotensive rats.Pharm Pharmacol Commun,2000,6:309-312.
20 Szlávik L,et al.Alkaloids fromLeucojum vernumand antiretroviral activity of amaryllidaceae alkaloids.Planta Med,2004,70:871-873.
21 Li SY,et al.Identification of natural compounds with antiviral activities against SARS-associated coronavirus.Antiviral Res,2005,67:18-23.
22 Okamoto T,et al.Lycoricidinol and lycoricidine,new plantgrowth regulators in bulbs ofLycoris radiataHerb.Chem Pharm Bull,1968,16:1860-1864.
23 Iqbal Z,et al.Plant growth inhibitory activity ofLycoris radiataHerb.and the possible involvement of lycorine as An allelochemical.Weed Biol Manage,2006,6:221-227.
24 Numata A,et al.Antifeedants for the larvae of the yellow butterfly,Eurema Hecabe Mandarina,inLycoris radiata.Chem Pharm Bull,1983,31:2146-2149.
25 Evidente A,et al.Further experiments on structure-activity relationships among the lycorine alkaloids.Phytochemistry,1986,25:2739-2743.
26 McNulty J,et al.Selective cytochrome P450 3A4 inhibitory activity of amaryllidaceae alkaloids.Bioorg Med Chem Lett,2009,19:3233-3237.
27 Cedrón JC,et al.Pancratium canarienseas an important source of amaryllidaceae alkaloids.J Nat Prod,2009,72: 112-116.
28 Dewick PM.Medicinal Natural Products.A Biosynthetic Approach 3rd.West Sussex:John Wiley&Sons Ltd,2009.364-366.
29 Jin Z.Amaryllidaceae and sceletium alkaloids.Nat Prod Rep,2007,24:886-905.
30 Yang Y,et al.Alkaloids from the bulbs ofLycoris aurea.Helv Chim Acta,2005,88:2550-2553.
31 Zheng Y(郑颖),et al.Progress on Phytochemical Research for Benzylphenethylamine Alkaloids.Nat Prod Res Dev(天然产物研究与开发),2009,21:171-176.
32 Boit HG,et al.Amaryllidaceen alkaloide.22.alkaloide ausHippeastrum rutilum,Lycoris albiflora,Zephyranthes andersonianaundSternbergiafischeriana.Naturwissenschaften,1958,45:390.
33 Boit HG,Ehmke H.Amaryllidaceae alkaloids.XVI.alkaloids ofNerine corusca,N.flexuosa,Pancreatium illyricum,Lycoris aurea,andL.incarnata.Chem Ber,1957,90:369-373.
34 Wang XY(王晓燕),et al.GC-MS Analysis of chemical constituent inLycorisaurea.Chin Trad Herb Drug(中草药),2007,38:188,217.
35 Ma GE(马广恩),et al.Alkaloids ofLycoris.XI.Antitumor principles and the alkaloids ofLycoris chinensis.Chin Trad Herb Drug(中草药),1987,18:342-345.
36 Li HY,et al.Alkaloids ofLycoris guangxiensis.Planta Med,1987,53:259-261.
37 Kihara M,et al.Isolation and structure elucidation of a novel alkaloid,incartine,a supposed biosynthetic intermediate,from flowers ofLycoris incarnate.Chem Pharm Bull,1994,42: 289-292.
38 Takagi S,et al.Gas liquid chromatography of alkaloids.I.separation of alkaloids of amaryllidaceae.Chem Pharm Bull,1968,16:1116-1120.
39 Kobayashi S,et al.Alkaloids of amaryllidaceae.A new alkaloid,sanguinine,fromLycoris sanguineaMaxim.Var.kiushianaMakino,and pretazettine fromLycoris radiataHerb.Chem Pharm Bull,1976,24:1537-1543.
40 Kobayashi S,et al.Isolation ofO-demethyllycoramine from bulbs ofLycoris radiataHerb.Chem Pharm Bull,1980,28: 3433-3436.
41 Kihara M,et al.Alkaloidal constituents of the flowers ofLycoris radiataHerb.(Amaryllidaceae).Chem Pharm Bull,1991,39:1849-1853.
42 Abdallah OM.Minor Alkaloids fromLycoris sanguinea.Phytochemistry.1995,39:477-478.
43 Kitajima M,et al.Two New Alkaloids from Bulbs ofLycoris squamigera.Heterocycles.2009,77:1389-1396.
44 Kihara M,et al.Incartine,A biosynthetic intermediate,from the flowers ofLycoris incarnate.Heterocycles,1992,34:1299-1301.
45 Wang L,et al.Two new amaryllidaceae alkaloids from the bulbs ofLycoris radiata.Chem Pharm Bull,2009,57:610-611.
46 Noshita T,et al.Isolation of hippadine from the rroots ofLycoris radiata.Nat Med,2002,56:216.
47 Kobayashi S,et al.AlkaloidN-oxides fromLycoris sanguinea.Phytochemistry,1991,30:675-677.
48 Takagi S,Yamaki M.On the constituents of the bulbs ofLycoris sanguinea.Yakugaku Zasshi,1974,94:617-622.
49 Uyeo S,et al.Occurrence of alkaloids vittatine and haemanthamine inLycoris radiataHerb.Chem Pharm Bull,1966,14:793-794.
50 Uyeo S,Yamato Y.The structure of radiatine,A new alkaloid occurring inLycoris radiataHerb.Yakugaku Zasshi J Pharm Soc Jap,1965,85:615-623.
51 Pi HF,et al.A new alkaloid fromLycoris aurea.Chin Chem Lett,2009,20:1319-1320.
Research Progress of Alkaloids from Lycoris
WANG Huan1,2,WANG Yue-hu1,CHEN Li-juan2,LONG Chun-lin1,3*
1Kunming Institute of Botany,Chinese Academy of Sciences,Kunming 650204,China;2College of Agronomy and Biotechnology,Yunnan Agricultural University,Kunming 650201,China;3College of Life and Environmental Sciences,Minzu University of China,Beijing 100081,China
Lycorisis an endemic genus to East Asia.The genus is rich in alkaoids,and these secondary metabolites have cytotoxic,antimalarial,antiviral,anticholinesterase,and other activities.In this paper,chemical structures,pharmacological activities,and biogenesis of alkaloids fromLycorishave been discussed.
Amaryllidaceae;Lycoris;Alkaloid
R282.71
A
1001-6880(2012)05-0691-08
2010-07-05 接受日期:2010-08-25
*通讯作者 E-mail:long@mail.kib.ac.cn
