两个基于混酸配体的十二核锰配合物的合成和结构
2012-09-09周爱菊梁晶晶郑彦臻张婷沈毅童明良
周爱菊梁晶晶郑彦臻张婷沈毅童明良
(1广州大学化学化工学院,广州510006)
(2中山大学化学与化学工程学院,广州510075)
两个基于混酸配体的十二核锰配合物的合成和结构
周爱菊*,1梁晶晶1郑彦臻2张婷1沈毅1童明良2
(1广州大学化学化工学院,广州510006)
(2中山大学化学与化学工程学院,广州510075)
应用乙酸锰、高锰酸钾和丙酸(或氯乙酸)混酸体系合成,得到了2个基于Mn12O12核心的十二核锰配合物,分子式分别为[Mn12O12(O2CMe)8(O2CEt)8(H2O)4]·2H2O(1)和[Mn12O12(O2CMe)12(O2CCl3)4(H2O)4]·4MeCO2H·8H2O(2)。X射线单晶衍射分析表明配合物1结晶于四方晶系I4空间群,晶胞参数为:a=b=2.650 5(3)nm,c=1.349 7(3)nm,Z=4;配合物2结晶于四方晶系I41/acd空间群,晶胞参数为:a=b=2.7067(1)nm,c=2.5880(2)nm,Z=8。这2个配合物结构都具备Mn12O12核心,与其他经典Mn12明星分子的核心相似。中心的Mn4O4立方核通过μ3-氧桥被非共面的Mn8O8环聚集在一起,而16个η2-μ-混合羧酸以不同方向在外围充当连接桥,同时提供了酸性环境。文中还分析了混酸体系的p K a值对配合物结构形成的影响。
十二核锰;簇合物;丙酸;氯乙酸
0 Introduction
The synthesis of discrete polymetallic complexes has been driving the last twenty years by the discovery that molecules with large numbers of unpaired electrons can behave as single-molecule magnets(SMMs)[1].SMMsexhibitpeculiar slowmagnetic relaxation and quantum tunneling of magnetization below a blocking temperature similar to those observed in classicalmagnets[2].Study of their unusual magnetic behavior will be not only beneficial for both physics and chemistry,but also potentially used in high-density information storage devices for quantum computing.Gradually,the vast majority of SMMs reported have contained manganese[3],cobalt[4],iron[5-6], nickel[7],chromium[8]or rare earth[9].Each of these SMMs has been synthesized using appropriate metal and coordinative flexible organic ligands.Their magnetic propertiesmay be altered depending on the intriguing superexchange coupling interaction propagated through the bridging bonds.It is known that the first SMM,[Mn12O12(O2CMe)16(H2O)4]·MeCO2H ·3H2O(complex 3,omitted as Mn12-OAc),was obtained and acetic acid in the system is not only a bridging ligand but a solventwith acidic conditions[10].At this time of day,there is a continuing need for new synthetic methods that can achieve novel SMMs. Following the above consideration,we choose mixed acid as solvents to provide acidic conditions and act as bridged ligands.In particular,adjusting the appropriate equivalence relation of the two acids,we obtained two discrete polymetallic complexes with Mn12O12core,formulated as[Mn12O12(O2CMe)8(O2CEt)8(H2O)4]·2H2O(1)and[Mn12O12(O2CMe)12(O2CCCl3)4(H2O)4]·4MeCO2H·8H2O(2).
1 Experimental
1.1 M aterials and physicalmeasurements
1.2 Synthesis of com plexes 1 and 2
[Mn12O12(O2CMe)8(O2CEt)8(H2O)4]·2H2O(1):Mn(O2CMe)2·4H2O(0.245 g,1 mmol)was dissolved in amixture of 10 mL propionate acid and 5 mL acetic acid and treated with a fine powder of KMnO4(0.060 g,0.38 mmol)with vigorous stirring to afford a dark brown solution.After 12 h of additional stirring,the impurities were removed by filtration.The filtrate was left undisturbed at room temperature in vacuum for several weeks.Large black cubic crystals of the productwere obtained with a yield of 72%after being washed with hexanes and dried in the vacuum.Anal. Calcd.for C40H76Mn12O50(%):C,23.75;H,3.72.Found (%):C,23.83;H,3.80.FT-IR(KBr,cm-1):3 375.6(w), 2975.5(m),2939.8(m),2881.6(w),1562.3(vs),1466.2 (s),1425.8(vs),1297.9(s),1079.6(w),1012.2(w),888.3 (w),813.2(w),564.1(vs),447.9(m).
[Mn12O12(O2CMe)12(O2CCCl3)4(H2O)4]·4MeCO2H· 8H2O(2):To a solution ofCl3CCO2H(2.608 g,16mmol) in 20 mL acetic acid was added Mn(O2CMe)2·4H2O (1.000 g,4.082 mmol).The reaction mixture was stirred for two hours.Then the solution was added KMnO4(0.240 g,1.519 mmol)and was stirred for another few hours.The result black solution was filtered and the filtrate was stored for a few days at room temperature.Rectangle black block crystals of the product were obtained with a yield of 82%after recrystalized in CH2Cl2.Anal.Calcd.for C40H76Mn12O64Cl12(%):C,18.00;H,2.79.Found(%):C,18.02;H, 2.87.FT-IR(KBr,cm-1):3389.8(w),2937.9(w),1754.3 (w),1718.4(w),1656.9(s),1571.5(s),1502.9(s),1446.7 (vs),1376.1(s),1353.3(s),1328.5(s),1240.8(w),1028.9 (w),960.6(w),841.4(s),746.8(m),716.8(w),675.3(s), 646.7(s),608.1(s),566.2(w),541.4(w),446.3(w).
1.3 X-ray crystallography
The diffraction data of 1 and 2 were collected on a Bruker Smart Apex CCD diffractometerwith graphitemonochromated Mo Kαradiation(λ=0.071 073 nm)at 293K.Thedataintegrationand reductionwereprocessed with SAINT software.An empirical absorptioncorrection was applied to the collected reflectionswith SADABS[11].The structures were solved by direct methods and refined by the full-matrix least-squares method based on F2using the SHELXTL program package[12].All non-hydrogen atoms were refined with anisotropic displacement parameters.The organic hydrogen atoms were generated geometrically(C-H, 0.096 nm);the aqua hydrogen atoms were included in themodels in calculated positions and were refined as constrained to bonding atoms.The details of crystallographic data is provided in Table 1.
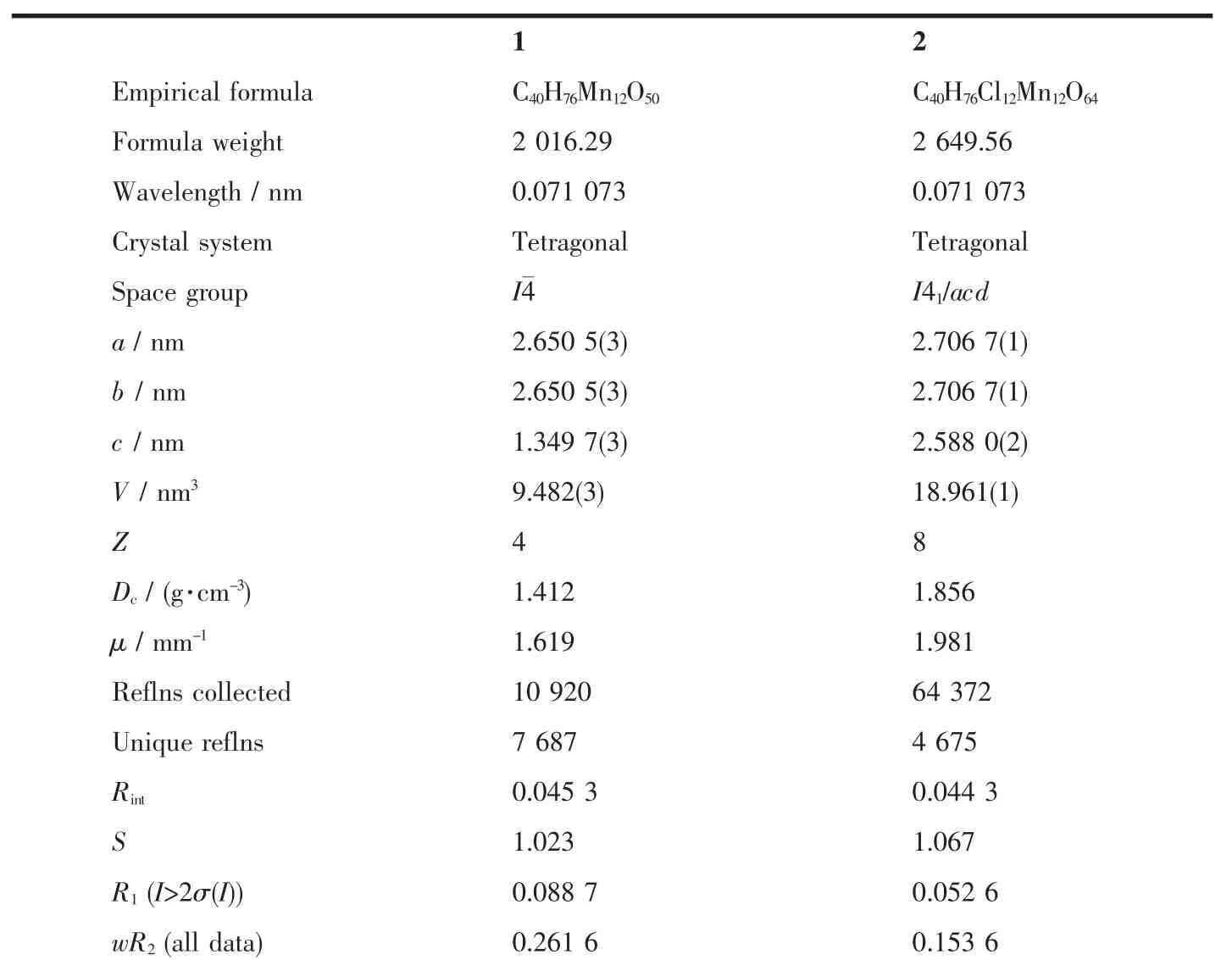
Table 1 Crystal Data and structure refinement for complex 1 and 2
CCDC:847334,1;847335,2.
2 Results and discussion
2.1 Synthesis
Enormous elaboration is being focused on constructing dodecanuclearmanganese complexes by choosing simplex carboxylate ligands or non-organic bridging ligands to completely replace the O2CMe-ligands of first SMM[Mn12O12(O2CMe)16(H2O)4]·MeCO2H·3H2O (omitted as Mn12-OAc).In this case,the previous reported synthetic strategies to achieve Mn12complexes are using RCO2-to reaction with Mn12-OAc in polar and anhydrous solvents,such as MeCN,EtOH, CH2Cl2,CHCl3,and so on[13-19].In our investigation, however,two new derivatives1 and 2 of the Mn12family were prepared by the partial substitution of O2CMewith EtCO2-and Cl3CCO2-,respectively.Encouraged by our preliminary experimentation,the satisfying molar ratios of the MnⅡ∶MnⅦhave been found to give consistently pure and high yield products.Moreover,small changes to the MnⅡ∶MnⅦratios have no noticeable effect on the identity of the products and yields.A MnⅡ∶MnⅦratio of 3.67∶1.33 has become our routinely used stoichiometry and has provided access to complexe 1 and 2.The ratio provides theoretically an average metal oxidation of+3.33.Potassium permanganate in our experiments commonly has been a little excessive comparative to the ratios in theory.
Previous development and use of ligand substitution reactions on Mn12-OAc complex have access tomany[Mn12O12(O2CR)16(H2O)4]derivativeswith a large variety of R groups[2,5,13-21].The ligand substitution reaction according to eq.(1)needs a carboxylic acid with a lower p Kaand an excessive amount to remove the acetic acid.[Mn12O12(O2CMe)16(H2O)4]+16RCO2H=
[Mn12O12(O2CR)16(H2O)4]+16MeCO2H(1) When a conjugate acid of the carboxylate group owns a p Kacomparable to,or even higher than that of acetic acid(p Ka=4.76),such as EtCO2H(p Ka=4.88),it is difficult to complete substitution reactions.And even if a carboxylic acid with a much lower p Ka, Cl3CCO2H(p Ka=0.65),cannot obtain the complete substitution productswithout removing the acetic acid. That is,above two strategies usually need to be taken into account at the same time.In complex 1,EtCO2H and MeCO2H have a similar p Kaand both they are liquid.Therefore,they have similar chances and finally only partial replacements of acetate groups. Excess acid could not be added.We attempted double propanoic acid dosage than that of in Experimental Section and the similar result was obtained.For complex 2,four to eight double in the Cl3CCO2H dosage got the same product and only the yield had little difference.The procedures described in Experimental Section have been found to give consistently pure and high yield products.We haven′t explored all possible solvents,reagent ration and stoichiometric relation,so do not claim that procedures optimization.Both the two compounds are soluble in acetone,MeCN,EtOH,CHCl3and CH2Cl2.
2.2 Description of structures
Labelled ORTEP plots of 1 and 2 are given in Fig.1 and Fig.2.Crystallographic information for 1 and 2 is given in Table 1.
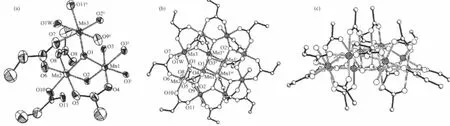
Fig.1(a)Coordination environments ofMn atoms in 1 with thermal ellipsoids at30%;(b)View along the c axis of theMn12molecule in 1;(c)Side view of the Mn12molecular in 1
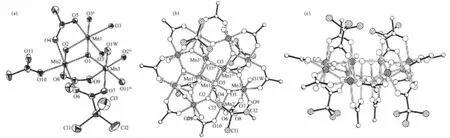
Fig.2(a)Coordination environments ofMn atoms in 2 with thermal ellipsoids at30%;(b)View along the c axis of the Mn12molecule in 2;(c)Side view of the Mn12molecular in 2
Complex 1 crystallized in the tetragonal space group I4 with the asymmetric unit containing two 0.25 Mn12molecules of the formula unit,which are related by a crystallographically inversion center,and twohalf-occupancy water guest molecules.The two Mn12molecules in 1 are structurally equivalent,so that only one of them is described here.Fig.1 shows a perspective view of one Mn12molecule with atom labeling. The Mn12molecule consists of a central[Mn4ⅣO4] cubane surrounded by a nonplanar Mn8O8ring,which are bridged by and connected to the cube viaμ3-O2-ions similar to complex 2 which crystallized in the tetragonal space group I41/acd(Fig.2).Aswe know,they have the same Mn12O12core,which are also found in other reported Mn12 clusters.The Mn-O bond distancesmake it clear that all of the atoms in the central cube are MnⅣ,and the Mn8O8ring consists of eight MnⅢ.These assignments are supported by marked Jahn-Teller elongation in the axial Mn-O acetates bonds(0.2037~0.2216 nm)in complex 1.From Fig.1b,we can see that eight propionate ligands are all at the equatorial MnⅢ-MnⅢcarboxylate sides while the remaining eight axial MnⅢ-MnⅢsites are occupied by acetates.So it possesses a structure similar to that reported in the literature[13].The occupation of the axial positions by the MeCO2-rather than the Et-CO2-groups is rationalized on the basis of relative basicities.The acetate acid has a lower p Ka(4.76)while EtCO2H has a higher p Ka(4.88).The more basic, stronger donor EtCO2-ligands favor occupation of the equatorial siteswhere shorter and stronger Mn-O bonds can be formed[13,18].Moreover,theMeCO2-groups are all bridging MnⅢ/MnⅣpairs with only one O atom on a Jahn-Teller(JT)elongation site,and these are the(Mn1/ Mn2,Mn1i/Mn2i,Mn1ii/Mn2ii,Mn1iii/Mn2iii,Mn2/Mn3, Mn2i/Mn3i,Mn2ii/Mn3iiand Mn2iii/Mn3iii)pairs,respectively(Fig.1b),which are different from those reported in literature[18].In complex 2,only four Cl3CCO2-groups replace the MeCO2-site in Mn12-OAc in the four of eight axial sites and they are all bridging MnⅢ/MnⅢpairs,which are analogical to NO3-groups in complexes reported by literature[16].Similarly,the p Kaof Cl3CCO2H (0.65)is close to that of inorganic acid(p Kais lower than 2),for example HNO3,which ismuch lower than that of ButCH2CO2H(p Kais higher or equal to 5). Therefore,in 2 the remaining four axial MnⅢ-MnⅢand the eight equatorial MnⅢ-MnⅢcarboxylate sites are occupied by acetates(Fig.2b),resulting in a[Mn12O12]16+core similar to that of 1 and with a significant distortion reflecting the increase of bond distances equal to 0.0289 nm(longer than the standard distance,0.01 nm),even longer than that of Mn12-O2CEt(complex 4) and all of the MnⅢ-MnⅢdistances in 2(Table 2). Compared to complex 1,Jahn-Teller elonga-tion axes are even longer and all the MnⅢ-O bonds in the Cl3CCO2-groups and four of the MnⅢ-O bonds in the MeCO2-which are bridging MnⅢ/MnⅣpairs are showing strong Jahn-Teller elongation in complex 2.There are 0.028 9 nm longer for Jahn-Teller axes on average than MnⅢ-O bonds without Jahn-Teller effects.Besides of these,Mn…Mn bond distances in complex 2 are longer than that of 1,whichmeans there is an apparent distortion in[Mn12O12]16+ring for 2.Additional differences are reflected in the linearity of the Mn4 units that span the core of the cluster,with 1 and 2 having angles of the type MnⅢ…MnⅣ…MnⅣequal to almost 180°while the corresponding angle in Mn12-OAc is only/171°.However,the angles of the type MnⅢ…MnⅣ…MnⅣin complex 3 are in the range of173.81° ~177.88°(Table 2).That is to say,the plates consist of the[Mn12O12]16+rings in complexes 1,2,and 4 are not somore turn up than complex 3.The similarity of complexes 1 and 2,and the difference between them and complex 3 are likely reflective of the distributionof ligands around the cluster.Furthermore,there are three styles of the four coordinated H2O being arranged to coordinate to MnⅢions(Fig.3).In the type of(a),each H2O ligand bonds to a MnⅢions[5,15];in the type of(b),each two H2O ligands bond to a MnⅢion and them have been arranged between the plate of [Mn12O12]16+[17];and the type of(c),has two H2O ligands bonded to one MnⅢion and one H2O ligand on each of two other MnⅢions[2].Both the cores of[Mn12O12]16+in complexes 1 and 2 are belong to the type of(a), and here the p Kaof the substitution acid,RCO2H maybe play an important role.
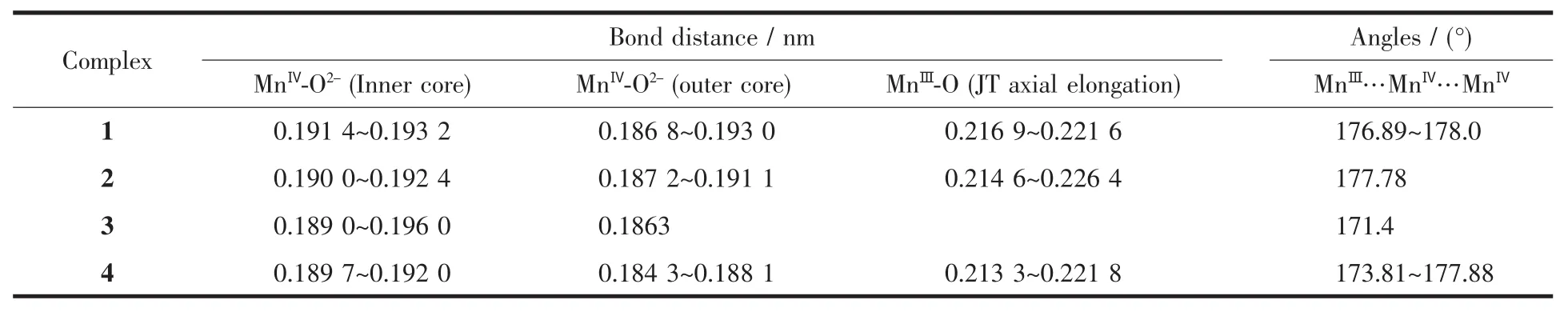
Table 2 Com parison of M nⅢ-O bond lengths and selected M nⅢ…M nⅣ…M nⅣangles in comp lex 1~4
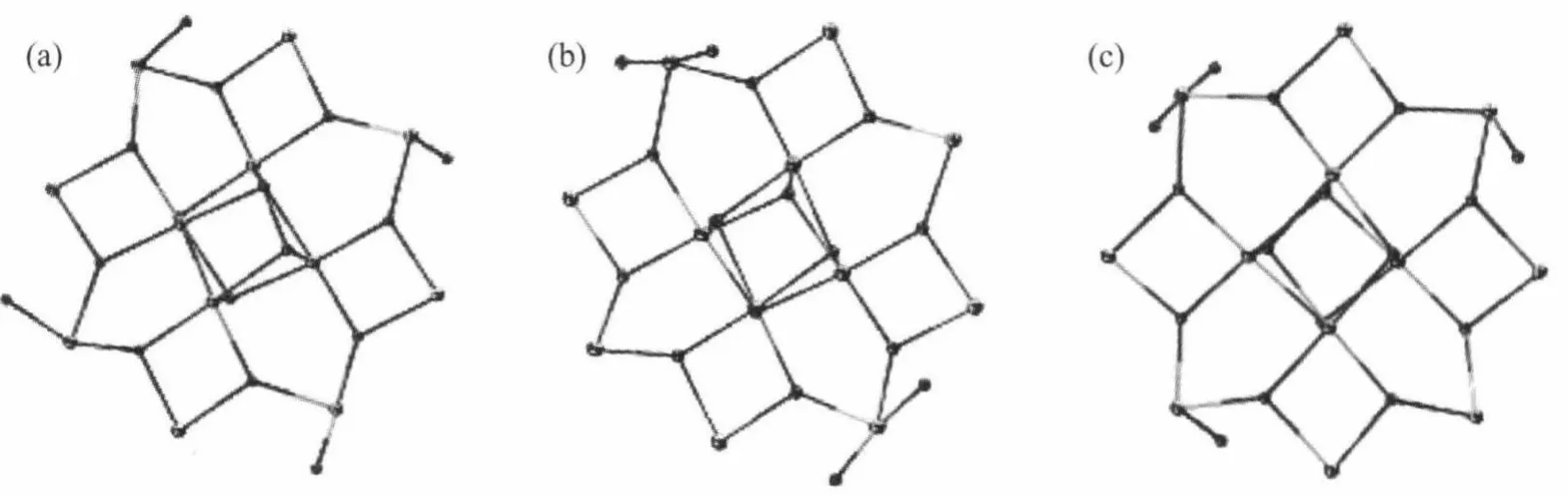
Fig.3(a),(b)and(c)are the three types of the coordinated H2Omolecules in the [Mn12O12(O2CR)x((O2CR′)16-x(H2O)4)](from literatures)
In conclusion,we synthesized two complexes, compound 1 and 2,involve two mixed bridging ligands.The different strategy in our synthesis is thatwe choose mixed acid as the solvents to provide acid condition,which change the idea that replace the MeCO2-group in the Mn12-OAc with other groups and its condition of non-acid organic solvents.
[1]Leuenberger M N,Loss D.Nature,2001,410:789-793
[2]Eppley H J,TsaiH L,Vries N,etal.J.Am.Chem.Soc., 1995,117:301-317
[3]Stamatatos TC,Abboud K A,WernsdorferW,etal.Angew. Chem.Int.Ed.,2008,47:6694-6698
[4]Jurca T,Farghal A,Lin P,etal.J.Am.Chem.Soc.,2011, 133:15814-15817
[5]Accorsi S,Barra A,Caneschi A,etal.J.Am.Chem.Soc., 2006,128:4742-4755
[6]Boudalis A K,Donnadieu B,Nastopoulos V,et al.Angew. Chem.Int.Ed.,2004,43:2266-2270
[7]AromíG,Parsons S,WernsdorferW,etal.Chem.Commun., 2005,40:5038-5040
[8]Rinck J,NovitchiG,HeuvelW V,etal.Angew.Chem.Int. Ed.,2010,49:7583-7587
[9]Feng X,Zhou W,Li Y,etal.Inorg.Chem.,2012,51:2722-2724
[10]Lis T.Aca Crystallogr.Sect.B,1980:2042
[11]Sheldrick GM.SADABS 2.05,University of Göttingen, Göttingen,Germany.
[12]SHELXTL 6.10;Bruker Analytical Instrumentation:Madison, WI,2000.
[13]Sessoli R,TsaiH L,Schake A R,etal.J.Am.Chem.Soc., 1993,115:1804-1816
[14]Chakov N E,Abboud K A,Zakharov L N,etal.Polyhedron, 2003,22:1759-1763
[15]Boskovic C,Pink M,Huffman JC,etal.J.Am.Chem.Soc., 2001,123:9914-9915
[16]Kuroda-Sowa T,Fukuda S,Miyoshi S,et al.Chem.Lett., 2002:682-683
[17]Artus P,Boskovic C,Yoo J,et al.Inorg.Chem.,2001,40: 4199-4210
[18]Soler M,Artus P,Folting K,etal.Inorg.Chem.,2001,40: 4902-4912
[19]Wei Y G,Zhang SW,Shao M C,etal.Polyhedron,1997, 16:1471-1478
[20]An J,Chen ZD,Zhang X X,etal.J.Chem.Soc.,Dalton Trans.,2001:3352-3356
[21]Aubin SM,Sun Z,Guzei IA,et al.Chem.Commun.,1997, 17:2239-2240
Synthesis and Structure of Two Dodecanuclear M anganese Clusters Based on M ixed Acids
ZHOU Ai-Ju*,1LIANG Jing-Jing1ZHENG Yan-Zhen2ZHANG Ting1SHEN Yi1TONGMing-Liang2
(1School of Chemistry and Chemical Engineering,Guangzhou University,Guangzhou 510006,China)
(2School of Chemistry&Chemical Engineering,Sun Yat-Sen University,Guangzhou 510275,China)
Two new Mn12O12cores based dodecanuclearmanganese complexes formulated with[Mn12O12(O2CMe)8(O2CEt)8(H2O)4]·2H2O(1)and[Mn12O12(O2CMe)12(O2CCl3)4(H2O)4]·4MeCO2H·8H2O(2)have been synthesized by the reaction of Mn(O2CMe)2·4H2O,KMnO4and mixed acid of RCO2H and MeCO2H(R=Et(1),Cl3CCO2H(2))in acidic conditions.X-ray diffraction crystal structure analysis shows that the complex 1 crystallizes in the tetragonal space group I4 has a=b=2.650 5(3)nm,c=1.349 7(3)nm and Z=4.The complex 2 crystallizes in tetragonal space group I41/acd has a=b=2.706 7(1)nm,c=2.588 0(2)nm and Z=8.In the two complexes,the structure of the Mn12O12core is similar to that found in all classic Mn12derivatives and comprises a central Mn4O4cubane core surrounded by a nonplanar Mn8O8ring held together withμ3-oxo bridges,while the peripheric bridging is ensured by sixteenη2-μ-mixed carboxylate anions in different orientations which provide an acidic condition.The effect of pKa data in eachmixed acid system for obtaining the two complexes are also investigated in this article.CCDC:847334,1;847335,2.
dodenuclearmanganese,cluster;propionic acid;chloroethanoic acid
;all preparations and manipulations were performed under aerobic conditions.The C,H, microanalyses were carried out with a Elementar Various-EL CHNS elemental analyzer.The FT-IR spectra were recorded from KBr pellets in the range 4 000~400 cm-1on a Bruker-EQUINOX 55 FT-IR spectrometer.
book=2017,ebook=70
O641.71+1
A
1001-4861(2012)11-2425-06
2011-10-16。收修改稿日期:2012-07-09。
国家自然科学青年基金(No.21001032),广东省高等学校人才项目育苗工程(No.LYM10102),广东省大学生创新实验项目(No.1107811002)资助项目。
*通讯联系人。E-mail:ahcfzhouaiju@163.com,Fax:86-20-39366908
