A Novel Preparation of Artificial Bile Ducts for Clinical Application of Biliary Diseases
2012-02-07SHITongna史同娜YANGQingSHAOMeiling邵梅玲ZHANGHongrui张洪瑞
SHI Tong-na(史同娜),YANG Qing(杨 庆),SHAO Mei-ling(邵梅玲),ZHANG Hong-rui(张洪瑞)
College of Material Science and Engineering,Donghua University,Shanghai 201620,China
Introduction
In the current treatment for an extra-hepatic bile duct affected by cancer or stenosis,the traditional treatment is to resect the affected duct portion and then anastomose the rest of hilar bile duct to the small intestine,which is called biliaryenteric anastomosis. In many cases,however,the postoperative course is complicated by retrograde infections via the intestine or stenosis at the anastomosis,requiring reanastomosis[1].Sometimes,bile stagnation due to impaired bile flow may require liver transplantation even when the impairment is just limited to the bile duct[2].In the worst case,after patients receive new livers,chronic rejection of the transplanted organ may result in bile duct stenosis,which may lead to liver graft failure and then require retransplantation[3,4].Therefore,in recent years,surgeons have been anticipating the advent of artificial bile ducts that have the same functions as the natural bile duct and can smoothly drain bile into the intestinal tract.Although there have been many artificial organs used in clinical applications[5-8],few reports have been reported on attempts to create artificial bile ducts[9-14].In this paper,an artificial bile duct has been fabricated which can resemble the native duct in both function and morphology.
In 1978, Hartung[15]repaired bile duct with polytetrafluoroethylene(PTFE)and found that inflammation caused by the material was slight.PTFE is a hydrophobic material which is interesting for biological applications[16].In this work,PTFE was used to fabricate artificial bile duct.As PTFE is a porous material,some plugging agents must be used to sealup the microporesto preventbile leaking out.Fluoroelastomer-246B is a kind of excellent elastic material and can be used as suitable biomaterial for making artificial bile ducts[17].Because of its low surface energy,the PTFE surface is with poor wettability and very difficult to bond with other materials including fluoroelastomer-246B.The application of plasma treatmentsisparticularly usefulforfluoropolymer materials[18]to improve the bonding ability between PTFE and fluoroelastomer-246B.In addition,plasma treatment has also been used to modify PTFE for its medical application[19].In this study,a PTFE raw tube was used to form the inner tube with PTFE film winded on the outer surface.The outer surface of PTFE film was then modified by He-plasma to facilitate the coating of fluoroelastomer-246B onto the PTFE film surface.
1 Experimental
1.1 Materials
PTFE raw tube with wall thickness of 1 mm and density of 2.2 g/cm3was commercially available in medical materials market.The acetone(AR)was obtained from Shanghai Chemical ReagentPurchaseand Supply Wulian Chemical Factory,China.Fluoroelastomer-246B with density of 1.86 g/cm3was purchased from Shanghai 3F New Material Co.,Ltd.(referred as“3F”,Shanghai,China).
1.2 Preparation of artificial bile duct
The inner tube was prepared by stretching PTFE raw tube at 260℃,winding PTFE films on the stretched PTFE then heatshaping PTFE tube at 340℃.The sample was cleaned with acetone for 24 h and dried at room temperature before plasma treatment.Different treatment conditions were used.Fluoroelastomer-246B was dissolved in acetone with the mass ratio of fluoroelastomer-246B to acetone 1∶20.After the surface modification by He-plasma treatment,the fluoroelastomer-246B solution was coated on the surface to seal up the micro-pores in the PTFE material and the artificial bile duct was prepared(Fig.1).

Fig.1 Artificial bile duct
1.3 X-Ray diffraction measurements
To evaluate the crystallinity changes of the PTFE samples,X-Ray diffraction(XRD)measurements were carried out by using a Rigaku DMAX-2000 high-resolution XRD(at 40 kV and 30 mA)with sealed-tube Cu Kα (1.542Å)radiation collimated by a graphite monochromator.
1.4 Scanning electron microscope (SEM)observation
The surface microstructure changes were characterized by using a JSM-5600 LV SEM(JEOL,Japan)with voltage of 15 kV.Prior to SEM examination,a conductive thin gold film was deposited on the specimen surface.
1.5 Water osmosis test
In order to examine the surface sealing of tubes,the osmosis test needs to be done.Distilled water was used to replace bile in the duct permeability test.The water permeability of the tubular samples was characterized by measuring the volume of water permeating through the wall in unit time under the pressure of 10 kPa.The sample tube was fixed to the ends of two glass tubes and suffused with running water(shown in Fig.2).The flow rate has to be well adjusted to expel the air bubbles and the vacuum pump was adjusted to keep the pressure around 10 kPa.The value was calculated by measuring the volume of water leaking from unit area of tube wall per unit time.

Fig.2 The instrument of the water osmosis test
1.6 Animal experiment
The artificial bile ducts shown in Fig.1 were implanted into six white pigs(Shanghai)with the weight of 40-50 kg and the diameter of bile duct being about 3.5-5.0 mm.All six of the recipient pigs survived up to their sacrifices at 90 days after implantation.All of them gained weight after operation with no evidence of jaundice.The artificial bile ducts were removed from the recipient pigs after 90 days,the morphology of artificial bile ducts was examined by SEM,and the growth of bile duct epithelial cells in the tissues around artificial bile duct was observed through transmission electron microscope(TEM).
2 Results and Discussion
2.1 Crystallization properties of PTFE sample
The XRD patterns of different samples are shown in Fig.3.For PTFE raw tube,obvious diffraction peaks are shown at 18.1°,31.7°,36.7°,and 41.3°in Fig.3(a).For PTFE sample being stretched 4 times at 260℃,the diffraction peaks at 18.1°,31.7°,and 36.7°weakened significantly while the peak at 41.3°almost disappeared,as shown in Fig.3(b).From Fig.3(c),after the stretched PTFE tube being heatshaped at 340℃,all the diffraction peaks strengthened,especially for the peak at 18.1°.Figure 3 indicated that stretching the PTFE raw tube by 4 times at 260℃made the crystalloid structure broken and the crystallinity declined,however,after the stretched PTFE tube being heat-shaped at 340℃ and cooling at room temperature,the crystallinity recovered again and the crystalloid structure became more perfect.It was clear that temperature played an important role in the process of PTFE crystallization.
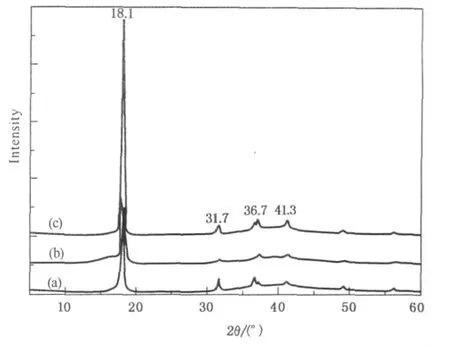
Fig.3 XRD spectra of different PTFE samples:(a)PTFE raw tube;(b)PTFE raw tube being stretched 4 times at 260℃;(c)stretched PTFE tube being heatshaped at 340℃and cooling at room temperature
2.2 SEM studies
SEM photos in Fig.4 are shown the morphologies of different PTFE samples.From the SEM images,the island nodes connected by micro-fibers are revealed in Figs.4(a),(b),and(c),respectively.The nodes on the surface are connected by micro-fibers,therefore pores are formed among the microfibers.Microfibers are oriented along the drawing direction,while the“knots”are arranged perpendicular to the drawing direction.
Compared with Fig.4(a),the pores in Fig.4(b)are larger,the width of nodes is decreased,the length of fibril becomes greater,and the micro-fibers become more complete and neat.Such phenomena may be due to the internal stress being accumulated inside the molecules after the PTFE raw tube being stretched by 4 times at 260℃.When the stretched tube was heat-shaped at a higher temperature(340℃),the inner stress was bound to be released.It was found that,if the heating shape was carried out under a tension-free state,the molecular chains of the specimen would produce a strong contraction due to the stress yield generated from the heating process.In order to avoid contraction of the molecular chains and ensure the stability of the formation of pores,the heat-shaping experiments need to be done.Meanwhile,under the heat treatment at 340℃,the adjacent micro-fibers were fused,and stress yield of the molecularchainswasalso inhibited,leading to the increment of porosity and the interface between the fiber and node becoming blurred.
The arrangement direction of the micro-fibers in Fig.4(c)is different from that in Fig.4(b).It seems that both of them are almost perpendicular to each other.The arrangement directions of the micro-fibers shown in Figs.4(a)and(b)are observed to be arrayed in the axial direction,while the direction of the micro-fibers in Fig.4(c)seems to be arrayed along the radial direction.This is because the PTFE film is wrapped around the surface of the stretched PTFE tube,and the winding direction is perpendicular to the axial direction,shown in Fig.4(c).The role of the winding film is to increase the radial strength of PTFE bile duct to withstand a certain radial pressure.In addition,the surface of PTFE film also has mesh pore structure,which can be seen from Fig.4(c).
As shown in Fig.4(d),the pores on the PTFE surface are completely covered by fluoroelastomer-246B after coating 5 layers on the plasma treated PTFE surface.It was reported that the adhesion of PTFE surface could be increased after plasma treatment[20].

Fig.4 SEM photos of the surface structures of samples:(a)PTFE raw tube being stretched 4 times at 260℃;(b)stretched PTFE tube being heat-shaped at 340℃then cooling at room temperature;(c)stretched tube with PTFE films wrapped outside being heatshaped at 340℃then cooling at room temperature;(d)coating 5 layers of fluoroelastomer-246B on He-plasma treated PTFE tube
2.3 Water permeability test
According to SEM analysis in Fig.4,the water osmosis of samples upon the average pore diameter was calculated and listed in Table 1,and the wall thickness was measured by slide caliper rule.
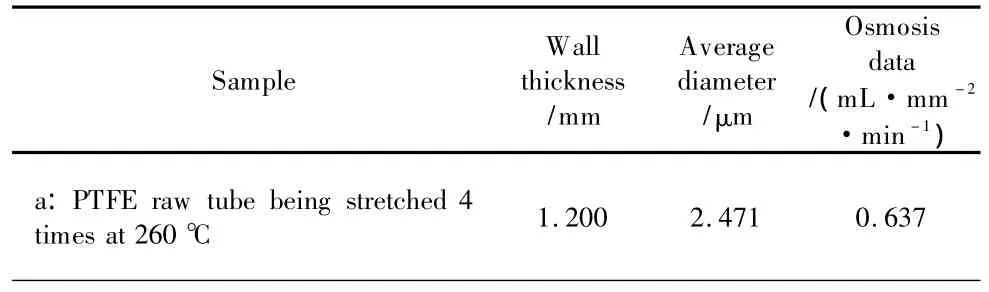
Table 1 The water osmosis testing results of different samples
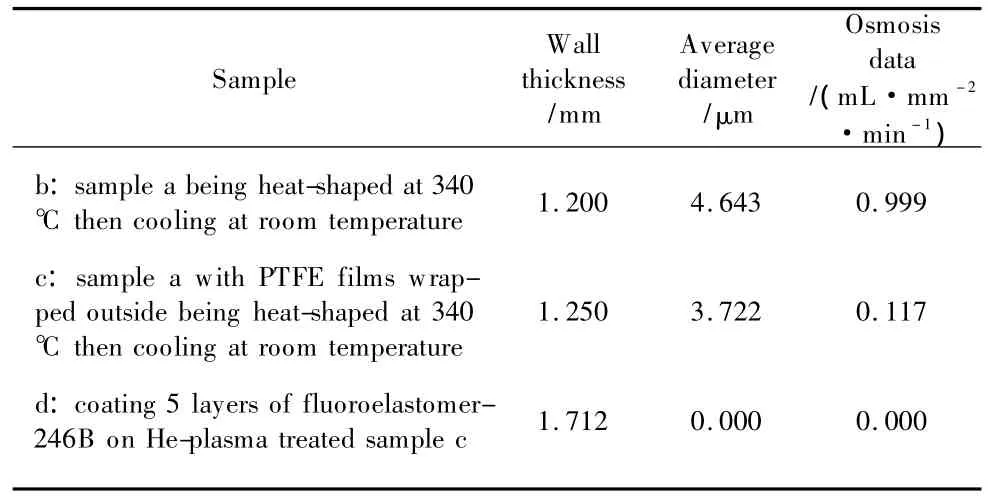
(Table 1continued)
As shown in Table 1,the osmosis increases first and then decreases to 0.000.The data of the water osmosis test increase from 0.637 mL·mm-2·min-1to 0.999 mL·mm-2·min-1.It might be attributed to the heat-shaped process at 340℃ which expanded the pores of the stretched PTFE tube.The results were consistent with the SEM photos shown in Figs.4(a)and(b).Similarly,with the pore diameter in Fig.4(c)becoming smaller than the ones in Fig.4(b)and the wall thicknessincreasing,the waterosmosis value obviously decreases to 0.117 mL·mm-2·min-1.After coating fluoroelastomer-246B on the He-plasma treated PTFE surface,the pores were gradually sealed to achieve the purpose of inhibiting the waterpenetration,which caused the wallthickness increasing and then the osmosis value being continually declined to 0.000.In short,the water osmosis values decrease with the pore diameter reducing and wall thickness increasing.
2.4 Analysis of animal experiment
The artificial bile ducts were implanted in pigs and the relevant tests were shown in Figs.5-8.As shown in Fig.5,90 days after implantation,the artificial bile duct has been completely wrapped by the fibrous tissues,which indicated there was no leakage of bile and the tissues around the artificial bile duct grew well.It shows that the materials used for the preparation of artificial bile duct have good compatibility with peripheral tissue.In Fig.6,the inside wall of artificial bile duct is smooth and clean,no sediments are attached on the inside wall.This indicated the bile was flowing smoothly in the artificial bile duct and no bile deposition occurred.As seen in Fig.7,compared with the original artificial bile duct,there are no significant changes on the surface morphologies of the ones being removed from the pigs.In Fig.8(a),the glands with a few chronic inflammatory cells generate arrange in rules,and the lobular structure of liver is normal,which shows there is no significant change in periportal area.The growth of bile duct epithelial cells through TEM observation is shown in Fig.8(b).The bile duct epithelial cells arrange well in the tissues around the artificial bile duct and no inflammatory cells occur in the tissues.

Fig.5 The appearance of artificial bile duct after implantation:(a)the implantation of artificial bile duct;(b)90 days after implantation
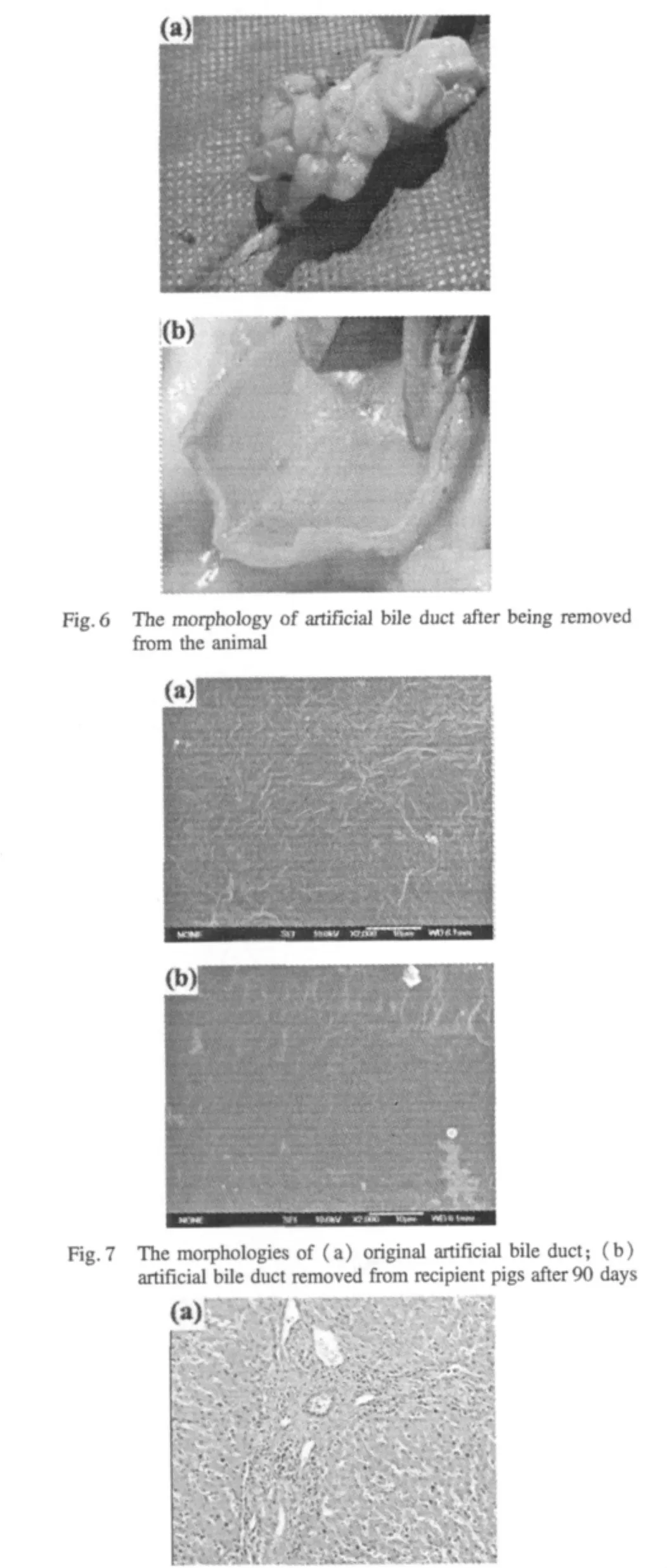

Fig.8 The compatibility testbetween artificialbile ductand surrounding tissues:(a)light microscope:anatomy of the liver pathology(original magnification×100);(b)TEM:the growth of bile duct epithelial cells(original magnification×4 000)
3 Conclusions
In this paper,the artificial bile duct was fabricated from the PTFE raw tube byheat stretching,heat setting,plasma treatment,and fluorine rubber coating process. The crystallization properties and water osmosis test of different samples were characterized,and the surface structures of the sample tubes had been observed by SEM.In order to evaluate the tissue compatibility and clinical application,animal test was done.The test results show that:(1)the crystallinity of the PTFE is affected by heat treatment process;(2)pores on the PTFE surface can be completely covered by fluorine rubber after coating 5 layers of fluorine rubber on the plasma treated PTFE surface;(3)the data of water osmosis test decrease with the pore diameter reducing and wall thickness increasing;(4)the results of animal test show that the artificial bile duct can avoid the leakage of bile and the compatibility with peripheral tissue is good.These results represent this novel artificial bile duct has a great potential for the development of a new form of treatment for the biliary diseases.
[1]Ammori B J,Joseph S,Attia M,et al.Biliary Strictures Complicating Pancreaticoduodenectomy[J].International Journal of Gastrointestinal Cancer,2000,28(1):15-22.
[2]Egawa H,Inomata Y,Uemoto S,et al.Biliary Anastomotic Complications in 400 Living Related Liver Transplantations[J].World Journal of Surgery,2001,25(10):1300-1307.
[3]Sugawara Y,Makuuchi M,Takayama T,et al.Small-for-Size Grafts in Living-Related Liver Transplantation[J].Journal of the American College of Surgeons,2001,192(4):510-513.
[4]Halme L,Hockerstedt K,Lautenschlager I.Cytomegalovirus Infection and Development of Biliary Complications after Liver Transplantation[J].Transplantation,2003,75(11):1853-1858.
[5]Visconti R P,Kasyanov V,Gentile C,et al.Towards Organ Printing:Engineering an Intra-organ Branched Vascular Tree[J].Expert Opinion on Biological Therapy,2010,10(3):409-420.
[6]Norotte C,Marga F S,Niklason L E,et al.Scaffold-Free Vascular Tissue Engineering Using Bioprinting [J].Biomaterials,2009,30(30):5910-5917.
[7]Shimizu T,Sekine H,Yamato M,et al.Cell Sheet-Based Myocardial Tissue Engineering:New Hope for Damaged Heart Rescue[J].Current Pharmaceutical Design,2009,15(24):2807-2814.
[8]Gojo S,Toyoda M,Umezawa A.Tissue Engineering and Cell-Based Therapy toward Integrated Strategy with Artificial Organs[J].Journal of Artificial Organs,2011,14(3):171-177.
[9]Shimono K,Nosé Y.The Need to Develop Artificial Bile Ducts[J].Artificial Organs,1995,19(2):115-116.
[10]Xu J H,Sun S M,Zhang Q Y,et al.Experiment for a Polyurethane Replacement of the Common Bile Duct[J].Chinese Medical Journal,1998,111(1):86-87.
[11]Rosen M,Ponsky J,Petras R,et al.Small Intestinal Submucosa as a Bioscaffold for Biliary Tract Regeneration [J].Surgery,2002,132(3):480-486.
[12]Mitsuo M,Takahiro T,Yasuko T,et al.A Tissue-Engineered Artificial Bile Duct Grown to Resemble the Native Bile Duct[J].American Journal of Transplantation,2005,5(6):1541-1547.
[13]Meng B,Wang J,Zhu N,et al.Study of Biodegradable and Self-expandable PLLA Helical Biliary Stent in vivo and in vitro[J].Journal of Materials Science:Materials in Medicine,2006,17(17):611-617.
[14]Aikawa M,Miyazawa M,Okada K,et al.Regeneration of Extrahepatic Bile Duct Possibility to Clinical Application by Recognition of the Regenetive Process[J].Journal of Smooth Muscle Research,2007,43(6):211-218.
[15]HartungH,KirchnerR,Baba N,etal. Histological,Laboratory,and X-Ray Findings after Repair of the Common Bile Duct with a Teflon Graft[J].World Journal of Surgery,1978,2(5):639-642.
[16]Vandencasteele N,Nisol B,Viville P,et al.Plasma-Modified PTFE for Biological Applications:Correlation between Protein-Resistant Properties and Surface Characteristics[J].Plasma Processes and Polymers,2008,5(7):661-671.
[17]Liu S Y,Wang G Y,Liu K,et al. Feasibility of Fluoroelastomer-246B as the Substitute of Bile Duct[J].Journal of Clinical Rehabilitative Tissue Engineering Research,2008,12(1):80-84.
[18]Nitschke M,König U,Lappan U,et al.Low Pressure Plasma-Based Approaches to Fluorocarbon Polymer Surface Modification[J].Journal of Applied Polymer Science,2007,103(1):100-109.
[19]Onder S,Kazmanli K,Kok F N.Alteration of PTFE Surface to Increase Its Blood Compatibility[J].Journal of Biomaterials Science-Polymer Edition,2011,22(11):1443-1457.
[20]Lin T K,Wu S J,Peng C K,et al.Surface Modification of Polytetrafluoroethylene Films by Plasma Pretreatment and Graft Copolymerization to Improve Their Adhesion to Bismaleimide[J].Polymer International,2009,58(1):46-53.
杂志排行
Journal of Donghua University(English Edition)的其它文章
- Service Robot Localization Based on Global Vision and Stereo Vision
- Synthesis and Characterization of Novel Fluoroalkyl Unsaturated Multi-carboxylic Acid Esters
- Efficient Rate Control Algorithm for Hierarchical Video Coding
- Effects of Pre-oxidation Conditions on Adsorption Performance of Activated Carbon Fibers
- A Modified Differential Evolution for Uniform Parallel Machines Scheduling Problem with Minimizing Makespan
- Sorption Kinetics and Capacity of Composite Materials Made up of Polymeric Fabric and Expanded Perlite for Oil in Water
