Sorption Kinetics and Capacity of Composite Materials Made up of Polymeric Fabric and Expanded Perlite for Oil in Water
2012-02-07QIPeishi祁佩时LINNaLIUYunzhi刘云芝ZHAOJunjie赵俊杰
QI Pei-shi(祁佩时),LIN Na(林 娜),LIU Yun-zhi(刘云芝),ZHAO Jun-jie(赵俊杰)
State Key Laboratory of Urban Water Resource and Environment,Harbin Institute of Technology,Harbin 150090,China
Introduction
With the development of petrochemicals industry along river and oil transportation as well as the laying of oil pipes through the river,accidental oil spills are not unexpected consequences which not only can cause loss of energy source,but also have a threat to the security of drinking water of inhabitants[1]. Oil spills in the river have different characteristics compared with oil spill at sea,such as fast oil spreading due to the high flow velocity,and contaminative area will be larger which can cause trans-boundary pollution and international dispute[2].Therefore,it is important to develop fast and efficient techniques of oil removal from the water environment.Among existing techniques for the oil removal,mechanical recovery by sorbents is considered to be one of the most efficient techniques[3].
There are many superior oil sorption materials currently on the market.However,they are very costly(such as production cost,post-treatment cost)and storage is limited,and they can not meet emergency oil spill cleanup in river.Perlite is a glassy volcanic rock of rhyolitic composition,usually contains between 2%and 6%of water.Raw perlite can be heated rapidly at 700-1 200℃,to expand as much as 8-15 times of their original volume[4].Expanded perlite(EP)is available in the large scale and has excellent properties,such as low density,high porosity,thermal and chemical inertness,and relatively low price,which make EP useful in many applications including combating oil spills[5].In practical application,the major problem is the bulky,fragile nature,and poor oil/water selectivity of EP[4].One technique used for overcoming the problem is to fill bulky sorbent into a bag forming composite material.Inagaki et al.studied the oil sorption and recovery using exfoliated graphite packed into a plastic bag and found the materials composing the bag strongly affected the sorption behavior when oil layer on the water surface was 1 mm thick[6].Hussein et al.studied the oil sorption behavior of carbonized pith bagasse packed into polypropylene bag[7].It showed that pads containing carbonized pith bagasse exhibited high oil retention ability and a high selectivity for the oils over the water. However,few works involved the oilsorption mechanism,especially about the sorption kinetics and the sorption equilibrium of composite materials,have been published.
Commercial acrylic fabric(AF),polypropylene nonwoven(PP),and silk stocking(SS)are three of the most widely used fabrics which are air-stable,durable,cheap,and available in the large scale.For this reason,in this work,AF,PP,and SS polymers were used for plastic bags to make composite materials with EP and investigate the oil sorption behavior of them.Their oilsorption capacity,retention capacity,and oil/water selectivity were determined. Meanwhile,the oilsorption kinetics and isotherms were analyzed.
1 Experimental
1.1 Materials
EP was purchased from Perlite Products Factory in Harbin,China.The chemical constituent of the EP is given in Table 1.Different polymeric fabrics were used in the composition bags,namely,AF,PP,and SS.The main characteristics of the polymeric fabrics are given in Table 2.In order to overcome the bulky and fragile nature of EP and improve its oil sorption capacity,EP was packed into a polymeric fabric bag forming a composite material for the subsequent oil sorption studies.Polymeric fabrics were cut into small square bags with the size of 25 cm2,and 0.5 g of EP was packed into a bag.It would be worth noting that all sorption materials were used as received without further treatment.

Table 1 Chemical composition of EP

Table 2 Characteristics of studied polymeric fabrics
Diesel oil was obtained from gas station in Harbin,China.Diesel oil is a less volatile oil,which has better compositional uniformity and minimizes transient change in its physicochemical properties during experiments[8].The physical properties of the diesel oil are given in Table 3.

Table 3 Characteristics of diesel oil
1.2 Sorption experiment
All tests were performed at(15±1)℃.Sorption of composite materials was done by using oil/water system and pure oil system.The sorption test was firstly performed over oil floating on water.The desired amount of diesel oil was taken in a 250 mL beaker containing 150 mL of tap water.The weighed composite material was immersed horizontally in the oil and offered a certain period of sorption time to adsorb oil.Then adsorbed sorbent was hung in the air for 5 min to allow the excess oil to drip from the bag.Water content of the sorbent was analyzed by distillation,according to ASTM D95-83[9].For comparison,the weight of the oil and water adsorbed by the polymeric fabrics without EP was measured.
The oil sorption capacity of composite materials was determined according to Eq.(1):

where q is the oil sorption capacity of composite materials(g/g);STis the total weight of oil,water,and composite materials(g);SWis the water weight(g);SPOis the weight of the empty polymeric fabrics and oil sorbed(g);SEPis the dry EP weight(g).
The oil removal efficiency can be written as:
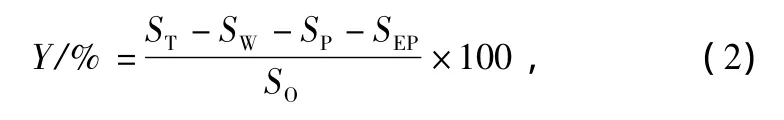
where Y is the oil removal efficiency of composite materials(%);SOis the initial(added)weight of oil in water(g);SPis the weight of the empty polymeric fabrics(g).
For determination of oil retention capacity of composite materials,sorption test without water was performed.For this,150 mL of diesel oil was taken in a 250 mL beaker.The weighed composite material was immersed horizontally in the oil.After saturated with oil,the adsorbed sorbent was lifted above the pure oil system,allowing free oil dripping out from the sorbent.The transient weight was recorded using a digital balance after 1,2,3,5,10,and 20 min.The oil retention capacity of composite materials can be calculated by Eq.(1)with SW=0.
2 Results and Discussion
2.1 Effect of sorbent dosage
The interaction between the oil and the sorbent could be affected by the sorbent dosage.The results shown in Fig.1 reveal that the effect of EP dosage varies from 0.1 g to 1.5 g and polymeric fabrics remain unchanged for oil sorption in the water.In Fig.1,the oil removal efficiency increases as EP weight increases,however,oil sorption capacity of composite materials decreases at the same situations.The results are consistent with sorption capacity of oil dependent on the bulk density of bulky sorbent[6].This can be attributed to the change of pores volume and surface area of sorbent materials for oil sorption as bulk densityofbulkysorbent increases.The optimum quantity of EP is between 0.5 g to 1.0 g for 25 cm2polymeric fabric bag,and oil removal efficiency for 6 L/m2oil amount in the water is 52%-72%for AF composite material,44%-63%for PP composite material,and 37%-48%for SS composite material,respectively.

2.2 Complementary effect of EP and polymeric fabric
Figure 2 shows the oil sorption results for bulky EP,empty polymeric fabrics,and their composite materials.The practical sorption amount was defined as the oil left in the sorbent materials after the 5 min free drop period and represented the oil that could be recovered in the field practice[10].In Fig.2,the amount of adsorbed oil by AF composite material is 7.18 g which is 2.19 g higher than the 5.62 g adsorbed amount by 0.5 g EP(2.99 g)alone and AF polymeric fabric(2.63 g)alone.Meanwhile,the adsorbed amount of PP composite material and SS composite material is 6.08 g and 5.65 g,which are higher than the sum amount of EP alone and corresponding polymeric fabric alone,respectively.These observations suggest that EP and polymeric fabrics can complement each other when used together for oil sorption.Different composite materials have different oil increment amount which can be ascribed to two effects:(1)huge numbers of voids among EP particles provide large amounts of storage volume for adsorbed oil acting as oil reservoir;(2)different polymeric fabrics provide differentoilpenetrability and oilretention capacity for composite materials.

Fig.2 Comparison of sorption capability of bulky EP(0.5 g),empty polymeric bag(AF,PP,SS,25 cm2bag),and composite material(25 cm2bag containing EP 0.5 g)
2.3 Effect of desorption time and oil retention
Oil retention capacity isan importantparameterin evaluating the ability of sorbent to retain the adsorbed oil during transfer and handling operations[11]. Figure 3 shows the dependence between retention capacity and draining time of different composite materials.It reveals that the rate of desorption for various composite materials follows almost the same trend.There are three zones in each curve.The first zone is the initial stage of release,which lasts over 1 min.The rate of release is very high during this period.Here the retention capacity drop is very noticeable.This can be explained by the fact that instantaneous dripping of oil out from external surfaces of composite materials.The second or transition zone occurs from 1 min to 5 min,during which the rate of release is reduced as result of effect of capillary force.The third zone represents the steady-state period,during which the rate of release tends to begin a descent towards a steady-state and additional time will not release any significant amount of oil[12].

Fig.3 Effect of desorption time on oil sorption capacity of composite materials
2.4 Effect of oil amount in the water and sorption isotherms
Sorption selectivity is the ability of a sorbent material to preferentially adsorb one material over another.Oil selectivity over water makes sorbents attractive in oil spill cleanup.
Figure 4 shows the sorption capacity of composite materials for oil and water when oil amount on the water surface changes.It is clear that as oil amount in the water is increased,oil sorption capacity of composite materials increases until the maximum sorption capacity is achieved.This result is the same trend with previous research[13].When the oil film on water surface is thick enough(more than 3 L/m2),three composite materials have good sorption selectively between oil and water,and water sorption capacity of them is about 0.1-0.2 g/g.However,when oil film on water surface is very thin(1 L/m2),PP and SS composite materials can adsorb water.The results are attributed to the surface characteristic of polymeric fabric which affects significantlythe sorption behavior of composite material.AF,PP,and SS polymeric fabrics surface are hydrophobic(contact angles > 90°),and the surface properties of them are supposed to be different from raw fibers since they have undergone different mechanical and chemical changes during the material production[14]. Hydrophobic surface tends not to adsorb water or be wetted by water in oil/water system.However,PP and SS composite materials can adsorb water when oil amount in the water is very low or none.The results can be explained with the theory proposed by Miller and Tyomkin[15],and the water penetration need not be restricted to contact angles less than 90°when the pore wells are curved.Moreover,PP composite material can adsorb water,and this is possibly due to the continuous water migration through the finer capillaries in the melt-blown layer of the PP polymeric fabric.As Sarmadi et al.showed through electron micrograph,the open capillary spaces of the melt-blown layer could be directly contacted by water[16].The results indicate that composite materials containing hydrophobicpolymeric fabrics can have water penetrability which is related with polymeric fabrics physical construction.Therefore,it can be concluded that chemical and physical characteristics of polymeric fabrics co-together influenced on oil sorption behavior of composite materials.
In order to investigate the process of sorption of oil on composite materials surface,isotherm data were evaluated.The analysis of the isotherm data is important to develop an analytic equation which accurately represents the results and can be used for design purposed[17].The most well known isotherms are Langmuir and Freundlich theoretical equilibrium isotherms for solid-liquid sorption systems.Langmuir isotherm theory assumes a homogeneous sorption surface that has equal sorption sites with equal sorption energies.Theoretically,the sorbent has finite capacity for the sorbate.Therefore,a saturation value is reached where no further adsorption can occur[18].It can be described as:

where qeis the oil sorption capacity of sorbent(g/g)at equilibrium;Ceis the oil amount in the water(L/m2);KLand aLare Langmuir isotherm constants.

Rearrangement of the Eq.(3)yields:

When Ce/qeis plotted against Ce,a straight line with slope of aL/KLand intercept 1/KLis obtained,as shown in Fig.5(a).The isotherm constants and their correlation coefficients(R2)are listed in Table 4,and the values of R2are between 0.996 and 0.999,indicating that the Langmuir isotherm is favorable atthe concentrationsstudied.The equation is thermodynamically consistent and follows Henry's Law at low concentration.Therefore,as Cebecomes lower,aLCeis much less than unity and qe,that is,analogous to Henry's Law[5].

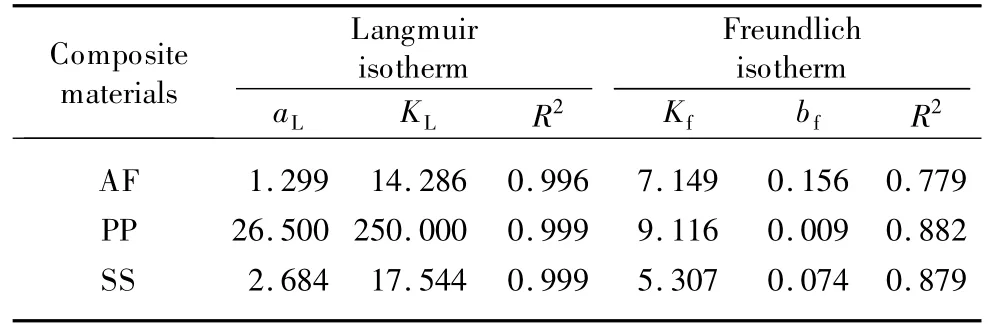
Table 4 Isotherms parameters for diesel oil sorption on composite materials
Freundlich isotherm theory considersa heterogeneous sorption surface involving multilayer sorption that has unequal sorption sites with different sorption energies[19].It can be described as:

where Kfand bfare Freundlich isotherm constants.
Rearrangement of the Eq.(5)yields:

Obviously,a plot of lnqeversus lnCeyields a straight line of slope bfand intercept lnKf.The plot isotherm fit is shown in Fig.5(b)and parameter values are presented in Table 4.From R2values,it is clear that the Freundlich isotherm fit is relatively poor for Langmuir isotherm.
2.5 Effect of sorption time and sorption kinetics
Sorption kinetic is an important characteristic in defining the efficiency of oil sorption of composite materials.Therefore,the effect of sorption time on the oil sorption capacity was investigated.The results are shown in Fig.6 for AF,PP,and SS composite materials.The overall trend is the same for three composite materials.As expected,oil sorption capacity of different composite materials increases significantly with time,and the use of the sorption process over 5 min has no significant influence on oil sorption.Therefore,the sorption time is set at 5 min.

Fig.6 Effect of sorption time on oil sorption capacity of composite materials
In order to investigate the mechanism of sorption kinetics of oil on composite materials,kinetics constants,and intra-particle diffusion were determined using pseudo-first order,pseudosecond order,and the intra-particle diffusion models.Lagergren proposed a method for sorption analysis which was pseudo-first order kinetic equation[20].And it can be described as:
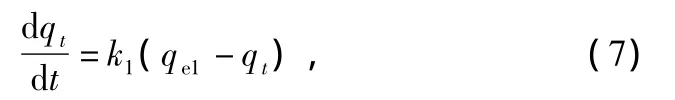
where qe1and qtare the oil sorption capacities of sorbent(g/g)at equilibrium and at time t,respectively.k1is the pseudo-first order sorption rate constant(min-1).Integrating the equation above with the limit q=0 at time t=0 gives:

It is clear that from Eq.(8)that the representation of ln(qe1-qt)versus t allows us to work out qe1and k1from the intercept and slop,respectively(Fig.7(a)).The correlation coefficients,R2,for the application of the pseudo-first order model are extremely low.All of calculated qe1from the intercepts of the straight lines plots are far from the expected qe1value as depicted in Table 5.Therefore,the sorption process of studied composite materials is not a pseudo-first order kinetic.

Ho and McKay proposed another sorption kinetic which was the pseudo-second order model[21].This model contains all possible sorption processes,i.e.,external liquid membrane diffusion,surface sorption,and intra-particle diffusion.It can be given as follows:
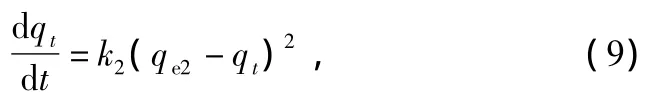
where qe2and qtare the oil sorption capacities of sorbent(g/g)at equilibrium and at time t,respectively.k2is the pseudosecond order sorption rate constant (g·g-1·min-1).Integrating Eq.(9)and rearranging with the limit q=0 at time t=0 give:

As shown in Fig.7(b),the values of qe2and k2could be calculated from the plots of t/qtversus t.The correlation coefficient for pseudo-second order kinetic model were 0.999 for three composite materials as indicated in Table 5.Moreover,the value of calculated qe2is close to the experimental data.Therefore,the sorption process of studied composite materials is a pseudo-second order kinetic.
The pseudo-second order kinetic model can not give a definite mechanism of sorption.Therefore,the model proposed by Weber and Morris has been applied in which intra-particle diffusion is considered as a rate-limiting[22].It can be described as:

where qtis the oil sorption capacity of sorbent(g/g)at time t,and kSis the intra-particle diffusion sorption rate constant(g·g-1· min-1/2).Such a plot may present a multilinearity,indicating that a few steps take place.The first sharper portion is attributed to the diffusion of the oil molecule from the oil/water system to the external surface of the sorbent particle(Fig.8)[23].The second portion describes the gradual adsorption stage,i.e.,the oil molecule migrates from the relatively small area of the external surface to the pores within each sorbent particle,where intra-particle diffusion is rate limiting.The third portion is attributed to the final adsorption-desorption equilibrium stage for which oil layer is formed on the sorbent surface[23].
The intra-particle diffusion model constants are calculated and given in Table 5.The correlation coefficients(R2)for the intra-particle diffusion model are between 0.929 and 0.949,which are lower than the pseudo-second order kinetic model,but they indicate that sorption of oil on composite materials may be followed by intra-particle diffusion model.

Fig.8 Mechanism of adsorption

Table 5 Kinetic parameters for diesel oil sorption on composite materials
Figure 7(c)shows the fitting of intra-particle sorption kinetic model with experimental data.As shown from the plots of qtversus t1/2,the plots are not linear over the whole time range,implying that more than one process affects the oil sorption.For three composite materials,the sorption of given oil gives a similar pattern.The multiple natures of these plots can be explained by the diffusion of adsorbate to the external surface of the sorbent particle and the intra-particle diffusion that gives further two linear portions.If the intra-particle diffusion is the only rate-controlling step,the plot passes through the origin;if not,the diffusion of adsorbate to the external surface of the sorbent particle controls the sorption to some degree[24].It can be deduced from Fig.7(c)that there are three processes that control the rate of sorption but only one is the rate-limiting step.One can observe that instantaneous sorption process,i.e.,diffusion of oil molecule to the external surface of composite materials,is the fastest.The second portion of plots seems to refer the diffusion of oil molecule into pores of composite materials.This implies that the intra-particle diffusion of oil molecules into small pores is the rate-limiting step in sorption process.Moreover,the third portion of plots is nearly parallel,which achieves the final adsorption-desorption equilibrium.As shown in Fig.7(c),there is a small difference in the slope of plots.For three composite materials this slope increases in the order SS<PP<AF which corresponds to an enhanced diffusion of oil molecules through pores.This might arise from difference in the polymeric fabrics surface properties.
3 Conclusions
The use of three different composite materials as sorbents of diesel oil was investigated in this study.The following conclusions were drawn based on the experimental studies.
(1)The EP dosage influenced significantly oil sorption capacity of composite materials and oil removal efficiency in the water.More EP dosage,higher oil removal efficiency,and loweroilsorption capacity ofcomposite materialswere investigated.The optimum quantity of EP was between 0.5 g and 1.0 g for 25 cm2polymeric fabrics bags.
(2)EP and polymeric fabrics could complement each other when used together for oil sorption.Oil sorption capacity of composite materials was higher than the sum amount of adsorbed oil of EP alone and polymeric fabric alone.
(3)The isotherms determined for three composite materials were found to fit the Langmuir equation very well,indicating thatcomposite materials showed homogeneous sorption surface with having equal sorption energies.The sorption capacity of composite materials followed the general trend:SS<PP<AF.AF composite material showed an excellentoilsorption capacity of10.17 g/g with high hydrophobicity and low water uptake of approximately 0.1-0.2 g/g.When oil film on the water surface was very thin(1 L/m2),PP and SS composite materials could adsorb water due to the water penetrability of their polymeric fabrics surface constructions.The results suggested that chemical and physical characteristics of polymeric fabrics co-together influenced oil sorption behavior of composite materials.
(4)The composite materials only required 5 min to achieve the sorption equilibrium,and the pseudo-second order kinetic model could describe very well the sorption of oil in the water.The correlation coefficients were all 0.999.There were three processes that controlled the rate of sorption but only intra-particle diffusion was the rate-limiting step.For three composite materials,the sorption rate of intra-particle diffusion increased in the order SS<PP<AF due to the difference in the polymeric fabrics surface properties.
[1]Bolognesi C,Perrone E,Roggieri P,et al.Bioindicators in Monitoring Long Term Genotoxic Impact of Oil Spill:Haven Case Study [J].Marine Environmental Research,2006,62(Supplement 1):S287-S291.
[2]Shen H T,Yapa P D.Oil Slick Transport in Rivers[J].Journal of Hydraulic Engineering,1988,114(5):529-543.
[3]Adebajo M O,Frost R L,Kloprogge J T,et al.Porous Materials for Oil Spill Cleanup:a Review of Synthesis and Absorbing Properties[J].Journal of Hazardous Materials,2003,10(3):159-170.
[4]Roulia M,Chassapis K,Fotinopoulos C,et al.Dispersion and Sorption of Oil Spills by Emulsifie-Modified Expanded Perlite[J].Spill Science and Technology Bulletin,2003,8(5/6):425-431.
[5]Alihosseini A,Taghikhani V,Safekordi A A,et al.Equilibrium Sorption of Crude Oil by Expanded Perlite UsingDifferent Adsorption Isotherms at 298.15 k [J].International Journal of Environmental Science and Technology,2010,7(3):591-598.
[6]Inagaki M,Shibata K,Setou S,et al.Sorption and Recovery of Heavy Oils by Using Exfoliated Graphite Part III:Trials for Practical Applications[J].Desalination,2000,128(3):219-222.
[7]Hussein M,Amer A A,Sawsan I I.Oil Spill Sorption Using Carbonized Pith Bagasse:Trial for Practical Application [J].International Journal of Environmental Science and Technology,2008,5(2):233-242.
[8]Abdullah M A,Rahmah A U,Man Z.Physicochemical and Sorption Characteristics of Malaysian Ceiba pentandra(L.)Gaertn.as a Natural Oil Sorbent[J].Journal of Hazardous Materials,2010,177(1/2/3):683-691.
[9]ASTM D95-83.Standard Test Method for Water in Petroleum Products and Bituminous Materials by Distillation[S].Annual Book of American Society for Testing and Materials Standards,ASTM Committee on Standards,West Conshohocken,PA,1983.
[10]Lin C,Hong Y J,Hu A H.Using a Composite Material Containing Waste Tire Powder and Polypropylene Fiber Cut End to Recover Spilled Oil[J].Waste Management,2010,30(2):263-267.
[11]Lim T T,Huang X F.Evaluation of Kapok(Ceiba pentandra(L.)Gaertn.)as a Natural Hollow Hydrophobic-Oleophilic Fibrous Sorbent for Oil Spill Cleanup[J].Chemosphere,2007,66(5):955-963.
[12]Garg V K,Moirangthem A,Kumar R,et al.Basic Dye(Methylene Blue)Removal from Simulated Wastewater by Adsorption Using Indian Rosewood Sawdust:a Timber Industry Waste[J].Dyes and Pigments,2004,63(3):243-250.
[13]Bastani D,Safekordi A A,Alihosseini A,et al.Study of Oil Sorption by Expanded Perlite at 298.15K [J].Separation and Purification Technology,2006,52(2):295-300.
[15]Miller B,Tyomkin I.Spontaneous Transplanar Uptake of Liquids by Fabrics[J].Textile Research Journal,1984,54(11):706-712.
[16]Sarmadi A M,Kwon Y A,Young R A.Wettability of Nonwoven Fabrics. 1. Effectof FluorochemicalFinishes on Water Repellency[J].Industrial& Engineering Chemistry Research,1993,32(2):279-287.
[17]Pandey P K,Sharma S K,Sambi S S.Kinetics and Equilibrium Study of Chromium Adsorption on ZeoliteNaX[J].International Journal of Environmental Science and Technology,2010,7(2):395-404.
[18]Langmuir I.The Adsorption of Gases on Plane Surfaces of Glass,Mica and Platinum [J].Journal of the American Chemical Society,1918,40(9):1361-1403.
[19]Freundlich H.Über die Absorption in Lösungen[J].Zeitschrift fur Physikalicshe Chemie,1906,57:385-470.(in German)
[20]Lagergren S.About the Theory of So-Called Adsorption of Soluble Substances[J].Kungliga Svenska Vetenskapsakademiens Handlingar,1898,24(4):1-39.
[21]Ho Y S,McKay G.A Kinetic Study of Dye Sorption by Biosorbent Waste Product Pith[J].Resources,Conservation and Recycling,1999,25(3/4):171-193.
[22]Weber W J,Morris J C.Kinetics of Adsorption on Carbon from Solution[J].Journal of the Sanitary Engineering Division,1963,89(2):31-60.
[23]Karan C P,Rengasamy R S,Das D.Oil Spill Cleanup by Structured Fibre Assembly[J].Indian Journal of Fibre& Textile Research,2011,36(2):190-200.
[24]Lorene-Grabowska E,Gryglewicz G.Adsorption of Lignite-Derived Humic Acids onCoal-Based Mesoporous Activated Carbons[J].Journal of Colloid and Interface Science,2005,284(2):416-423.
猜你喜欢
杂志排行
Journal of Donghua University(English Edition)的其它文章
- A Novel Preparation of Artificial Bile Ducts for Clinical Application of Biliary Diseases
- Service Robot Localization Based on Global Vision and Stereo Vision
- Synthesis and Characterization of Novel Fluoroalkyl Unsaturated Multi-carboxylic Acid Esters
- Efficient Rate Control Algorithm for Hierarchical Video Coding
- Effects of Pre-oxidation Conditions on Adsorption Performance of Activated Carbon Fibers
- A Modified Differential Evolution for Uniform Parallel Machines Scheduling Problem with Minimizing Makespan
