Preparation of high performance Zn4Sb3bulk thermoelectric materials
2011-12-28CHENZhongchunJunichiTsujimuraRyoKuramoto
CHEN Zhong-chun,Junichi Tsujimura,Ryo Kuramoto
(1.Department of Mechanical and Aerospace Engineering,Graduate School of Engineering,Tottori University,Koyama-minami 4-101,Tottori 680-8552,Japan;2.Department of Metallurgy,Graduate School of Engineering,Tohoku University,Aoba-yama 6-6-02,Sendai 980-8579,Japan)
Preparation of high performance Zn4Sb3bulk thermoelectric materials
CHEN Zhong-chun1,Junichi Tsujimura2,Ryo Kuramoto2
(1.Department of Mechanical and Aerospace Engineering,Graduate School of Engineering,Tottori University,Koyama-minami 4-101,Tottori 680-8552,Japan;2.Department of Metallurgy,Graduate School of Engineering,Tohoku University,Aoba-yama 6-6-02,Sendai 980-8579,Japan)
A“reaction-extrusion process”has been developed to prepare Zn4Sb3bulk materials with high thermoelectric performance.The synthesis,densification,and shape-forming of Zn4Sb3bulk materials were realized simultaneously in one hot-extrusion process,and the resulting extrudates had high density with single β-Zn4Sb3phase.A large extrusion ratio and a small punch speed are advantageous to enhance thermoelectric performance.The extruded Zn4Sb3materials exhibited excellentthermoelectric performance,forexample,the dimensionless thermoelectric figure of merit is 1.77 at 623 K,which is 36%higher compared to conventional hot-pressed materials.On the other hand,the incorporation of 1%SiC nanosized particles into Zn4Sb3matrix leads to improvements in both thermoelectric and mechanical properties.
thermoelectric materials;thermoelectric power generation;extrusion;reactive synthesis
As energy and global warming issues have been growing problems,thermoelectric power generation,which directly converts waste heat from automobiles,garbage incinerators,geothermal heat,and various industrial processes into electrical energy,has attracted much attention as an energy-saving technique.Even though different kinds of thermoelectric materials are necessary depending on the temperature of a waste-heat source,the present work focuses on Zn4Sb3thermoelectric compound,which is one of the promising thermoelectric materials applicable to an intermediate temperature range of 473~773 K.
It has been found that β-Zn4Sb3semiconducting compound has excellent thermoelectric performance in the intermediate temperature range,for example,the dimensionless thermoelectric figure of merit(ZT)is 1.3 at 673 K[1].The thermoelectric figure of merit is defined as

where α is the Seebeck coefficient,ρ the electrical resistivity,κ the thermal conductivity,and T the thermodynamic temperature.
So far,several melting methods[2,3]and hotpressing techniques[1,4~7]have been developed to prepare Zn4Sb3bulk materials.However,there exist some problems,for example,in cast ingots,a large number of cracks are formed due to mismatch in coefficients of thermal expansion and volume change during phase transformation at 765 K[8],while in the case of hot pressing,compositional change occurs easily due to decomposition of Zn4Sb3phase at high temperatures.
In the present work,we tried to prepare Zn4Sb3bulk materials through a“reaction-extrusion process”using two kinds of metal powders,Zn and Sb,as the starting materials.The synthesis,densification,and shape-forming of Zn4Sb3bulk materials were realized simultaneously in one hot-extrusion process.Furthermore,to improve the mechanical properties of Zn4Sb3compound,nanosized SiC parti-cles have been incorporated into Zn4Sb3matrix as a reinforcement.The objective of the present work was to clarify the effects of some processing parameters and SiC reinforcing phase on extrusion behavior,thermoelectric,and mechanical properties of the extruded Zn4Sb3bulk materials.
1 Experimental procedure
In this work,high-purity Zn and Sb powders,with average particle sizes of 36 μm and 11 μm respectively,were used as the starting materials.Besides,SiC powder with an average particle size of 100 nm was used as a reinforcement for the purpose of improving mechanical properties of Zn4Sb3compound.Zn and Sb powders with a stoichiometric composition of Zn4Sb3were mixed by ball milling.In the case of SiC addition,before the ball milling,Zn,Sb,and nanosized SiC powders were treated in ethanol by an ultrasonic dispersion method.The obtained powder mixtures were compacted by uniaxial pressing up to ~80% of theoretical density.The prepared green compact was vacuum-encapsulated into an Al alloy can(sheath),followed by consolidation using a hot-extrusion technique.Prior to the extrusion operations,the vacuum-encapsulated billet was heated to a given temperature and held for 20 min in the extrusion container.Subsequently,the billet was extruded at a temperature ranging from 673 K to 723 K with different extrusion ratios of 5~25.The extrusion was conducted under a punch speed of 1 or 10 mm/min.
The bulk density of the extruded samples was determined by the Archimedes method.The phase identifications were performed by X-ray diffraction (XRD)with CuKα radiation.The microstructure was characterized using optical microscopy(OM) and scanning electron microscopy(SEM).The Seebeck coefficient and electrical resistivity were measured using a Seebeck coefficient/electrical resistivity measuring system from room temperature to 673 K under He atmosphere.The thermal conductivity was calculated from specific heat,density,and thermal diffusivity measured by a laser-flash method.The dimensionless thermoelectric figure of merit(ZT) was calculated using Eq.(1).In addition,the Vickers hardness was measured under a load of 200 g,and flexural strength was measured by fourpoint bending tests.
2 Results and discussion
2.1 Synthesis,densification,and extrusion behavior ofβ-Zn4Sb3compound
Fig.1 shows the appearance of an extrusion billet on the longitudinal section after heating in the container at 723 K.The area marked by a dotted frame shown in Fig.1 corresponds to the initial position of the green compact,which has a density value of 80%of the theoretical density.After heating,the green compact expanded largely along the longitudinal direction,and its relative density decreased from 80%to 60%.This indicates that the chemical reactions between Zn and Sb occur during the heating period immediately before extrusion operations(see Fig.2).The volume change is attributed to density differences between Zn(Sb)and Zn4Sb3before and after reactions.
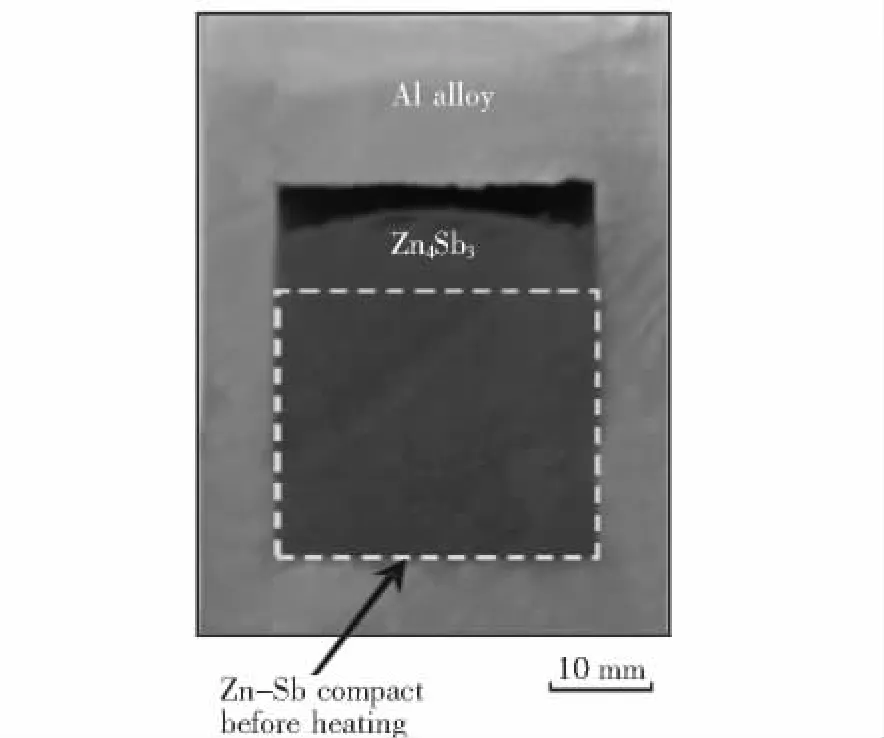
Fig.1 A picture showing the longitudinal section of a billet after heating in the container at 723 K for 20 min.The dotted frame indicates the position of initial green compact.
The X-ray diffraction patterns of hot-extruded Zn4Sb3samples are illustrated in Fig.2.As a reference,the XRD pattern of the green compact of Zn and Sb powder mixture was also included in Fig.2.The compact showed well-defined peaks (Fig.2(a))of Zn and Sb as expected.The extruded samples with different extrusion temperatures showed almost the same patterns(Fig.2(b)and (c)),which consisted of peaks of β-Zn4Sb3single phase,and no other unexpected phase could be detected.These results reveal that the reactions between Zn and Sb powders,which are encapsulated within the Al alloy can,do occur during the hotextrusion process.It is reasonable to consider that the chemical reactions between Zn and Sb take place during the heating period prior to extrusion operations.The reactions between Zn and Sb can be expressed as

The resultant reaction product(Zn4Sb3) is subsequently densified through hydrostatic pressure and large shear deformation generated during the extrusion.In addition,as shown in Fig.2(d),the extruded sample containing in volume fraction 1% SiC particles exhibited the XRD pattern almost the same as those without SiC addition.Clear peaks of SiC phase cannot be confirmed,presumably due to its small average size(100 nm)and low amount added.

Fig.2 XRD patterns of(a)Zn-Sb green compact and hot-extruded Zn4Sb3bulk samples at(b)673 K and(c)723 K.(d)1%SiC nanosizd particles were added(T=723 K).
Fig.3 showstypicalextrusion pressure vs.stroke curves,when vacuum-encapsulated billets were extruded with an extrusion ratio of R=7 at 723 K.Both curves with and without SiC addition are similar to each other,although the addition of 1%SiC particles caused a slightly higher extrusion pressure relative to the sample without SiC addition.It appears that there are different behaviors around points B,C,D,and E in the extrusion pressure vs.stroke curves.In the initial extrusion period up to point B shown in Fig.3,the pressure gradually rose with extrusion stroke.This corresponds to the compaction of Zn4Sb3and plastic deformation of the Al alloy.During the heating stage prior to extrusion operations,as mentioned above,Zn and Sb react with each other and,hence,Zn4Sb3is generated.Accordingly,the compact inside the Al alloy can is gradually consolidated during the initial period of extrusion,thus resulting in pressure increase.At point B where the slope of the pressure vs.stroke curve becomes small,the Al alloy in the front end of the billet starts to be extruded out of the die.When the pressure reaches point C,Zn4Sb3compound starts to be formed with Al alloy sheath on its surface layer.As the extrusion proceeds,the extrusion pressure is reduced,because of the decrease in friction areas between the billet and extrusion container.The extrusion of Zn4Sb3continues up to point D which corresponds to the completion of extrusion of Zn4Sb3compound.After point D,the extrusion is only associated with the Al alloy remaining in the billet.As a result,the extrusion pressure abruptly falls to a lower level(E).

Fig.3 Extrusion pressure vs.stroke curves under the conditions of R=7 at 723 K.The solid and dotted lines correspond to the curves without and with 1%SiC addition,respectively.
It has been confirmed that the extrusion pressure rises with increasing extrusion ratio or decreasing extrusion temperature.Moreover,defect-free and sound extrudates with greater than 99%of theoretical density were obtained under the conditions of T=723 K and R≥7,regardless of the presence of SiC particles.
With regard to microstructures of hot-extruded Zn4Sb3samples,our previous investigation[9]has showed that the extruded sample with an extrusion ratio of R=7 has a microstructural feature of fine equiaxed grains with an average grain size of 5 μm.No evident elongated grains are observed in the extrusion direction,although heavy plastic deformation is introduced during hot-extrusion process.It is believed that the finely grained microstructure is likely to be due to dynamic recrystallization during hot extrusion and static recrystallization after extru-sion.Fig.4 shows the SEM images of fracture surfaces of the extruded samples after 4-point bending tests.The fracture surface of 0%SiC sample(without SiC addition)was relatively smooth,while the sample containing 1% SiC particles exhibited a rougher surface.It was seen from the magnified images(Fig.4(c)and(d))that the grain sizes in 1%SiC sample were smaller than those in 0%SiC sample.Furthermore,nanosized SiC particles were dispersed inside grains and at grain boundaries.The presence of these SiC particles and grain refinement contribute to the improvement of mechanical properties of extruded Zn4Sb3bulk materials,which will be described later.

Fig.4 SEM images of the fracture surfaces of extruded Zn4Sb3samples after 4-point bending tests.(a)0%SiC and(b)1%SiC.(c)and (d)are magnified images for the areas of the white frames shown in(a)and(b),respectively.

Fig.5 Temperature dependence of the Seebeck coefficient and electrical resistivity of the Zn4Sb3 samples extruded under different conditions.The filled and unfilled symbols indicate electrical resistivity and Seebeck coefficient,respectively.

Fig.6 Temperature dependence of dimensionless thermoelectric figure of merit of extruded Zn4Sb3 samples.The inserted curve indicates the results reported in Ref.[1].
2.2 Effect of extrusion parameters on thermoelectric properties
The temperature dependence of the Seebeck coefficient and electrical resistivity of extruded Zn4Sb3samples is shown in Fig.5.Both the Seebeck coefficient and electrical resistivity increased with increasing temperature.Although different extrusion conditions,such as extrusion ratio(R)and punch speed (V),were used in the experiments,almost no difference in the Seebeck coefficient was observed.With regard to the electrical resistivity,however,it seems sensitive to extrusion conditions.Under the condition of the same extrusion ratio,a smaller punch speed gave rise to a lower level of electrical resistivity.This is probably related to the dynamic recrystallization during hot extrusion,and static recrystallization and recovery after extrusion,as described previously.A low punch speed causes decrease in lattice defects,as a consequence,the electrical resistivity is reduced.
It is interesting to note that the extruded Zn4Sb3sample with a larger extrusion ratio(R=25)showed smaller electrical resistivity than that with a smaller extrusion ratio(R=7).As mentioned above,as a result of dynamic and static recrystallization,fine equiaxed grains are formed,and the grain size becomes small with increasing the extrusion ratio.It is well known that electrical resistivity increases with the decrease in grain size in polycrystalline materials,according to the grain-boundary scattering mechanism.In the case of the Zn4Sb3materials prepared by hot pressing,however,Ueno et al.[7]found that the contribution of grain boundaries to electrical resistance is negligibly small.It is considered that the reduction of electrical resistivity in hot-extruded samples with a larger extrusion ratio may be associated with various lattice defects generated during extrusion,such as point defects including vacancies and anti-site defects[10,11],dislocations,and deformation-induced structural defects[12].

Fig.7 Temperature dependence of the Seebeck oefficient and electrical resistivity of extruded Zn4Sb3 samples with different amounts of SiC particles.The filled and unfilled symbols indicate electrical resistivity and Seebeck coefficient,respectively.
Concerning the thermal conductivity,the extruded Zn4Sb3bulk sample prepared at a punch speed of 1 mm/min showed thermal conductivity values of around 0.75 W/(m·K)(see Fig.8).When punch speed increased,for example,10 mm/min,the thermal conductivity became larger at temperatures of>473 K.These results indicate that a small punch speed during extrusion is beneficial to the improvement in thermoelectric performance.
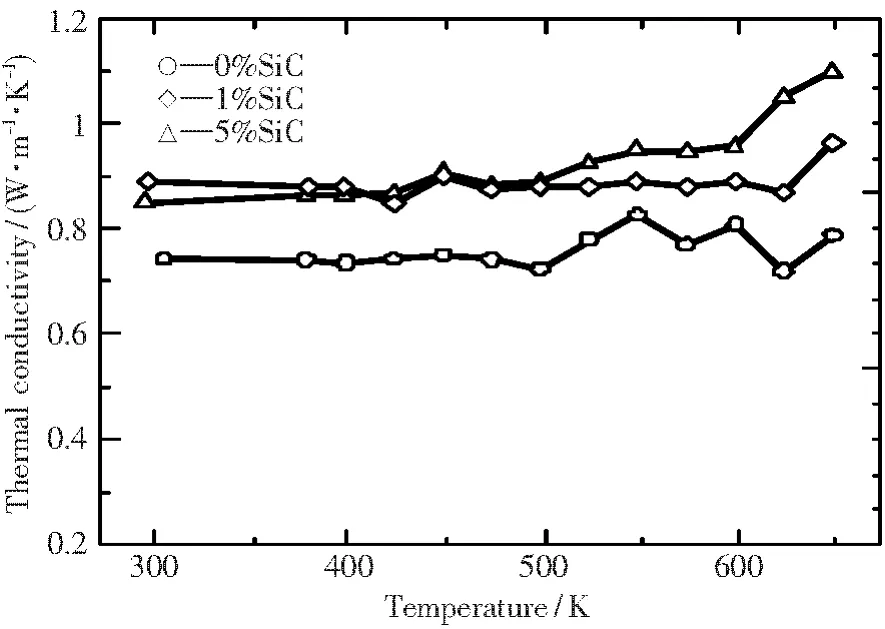
Fig.8 Temperature dependence of thermal conductivity of Zn4Sb3samples with different amounts of SiC particles.
The dimensionless thermoelectric figure of merit of the extruded Zn4Sb3samples with an extrusion ratio of R=7 is shown in Fig.6 as a function of temperature.In comparison with the reference data reported by Caillat et al.[1],the hot-extruded Zn4Sb3samples showed much higher ZT values in the whole temperature range of measurements.In particular,the sample prepared at a lower punch speed(V=1 mm/min)showed larger ZT values above 423 K.A largest ZT value,ZT=1.77,was achieved at 623 K.To our knowledge,this value is the largest so far in reported data for pure Zn4Sb3compound,which is 36%higher than that of hotpressed sample with ZT=1.3 at 673 K[1].The large ZT values of the extruded materials are attributed to their larger Seebeck coefficient and much lower electrical resistivity,which are believed to result from higher density.Another reason for larger ZT values is presumably concerned with lower oxygen concentration in extruded Zn4Sb3samples.In the“reaction-extrusion process”proposed in the current work,the oxidation of both Zn and Sb during preparation of the materials can be largely reduced due to vacuum-encapsulation and protection by the Al alloy sheath.This may be advantageous to improve transport properties such as electrical resistance.
2.3 Effect of SiC addition on thermoelectric and mechanical properties
From the above results,it can be found that the hot-extruded Zn4Sb3bulk materials have excellent thermoelectric performance.However,Zn4Sb3compound is extremely brittle,and its strength is not high.To improve the mechanical properties of Zn4Sb3bulk materials,nanosized SiC particles were incorporated into Zn4Sb3matrix as a reinforcement.Of course,it is necessary to understand the effect of SiC addition on thermoelectric properties.
Fig.7 shows the temperature dependence of the Seebeck coefficient and electrical resistance of hotextruded Zn4Sb3samples with different amounts of SiC particles.The Seebeck coefficient of the samples containing SiC particles showed higher values above 450 K,compared to pure Zn4Sb3sample(0% SiC).The increase in the Seebeck coefficient is thought to be due to(i)size reduction of Zn and Sb powders and(ii)grain refinement in the hot-extruded Zn4Sb3samples because of presence of SiC particles.In fact,it has been confirmed that the average particle sizes of Zn and Sb powders were largely reduced(about half of the initial average particle size)through ultrasonic dispersion and subsequent ball milling during the preparation of powder mixtures.With respect to electrical resistance,the sample with 1%SiC addition showed somewhat lower values in comparison with pure Zn4Sb3,whereas the addition of 5%SiC particles gave rise to an increase in electrical resistance.The increase in electrical resistance is the result of scattering from SiC particles and grain refinement(grain-boundary scattering).The reason for decrease in electrical resistance in 1%SiC sample is not clear,perhaps similar to the effect of extrusion ratio as shown in Fig.5,a further investigation is necessary.
From the results of thermal conductivity shown in Fig.8,it is evident that the incorporation of SiC particles leads to increases in thermal conductivity.SiC compound has a thermal conductivity value of>100 W/(m·K),which is much higher than that of Zn4Sb3(~0.75 W/(m·K)shown in Fig.8).Consequently,it seems that the influence of a high thermal conductivity value of SiC itself is larger than the contribution of grain refinement as a result of the presence of nanosized SiC particles.Fig.9 illustrates the temperature dependence of ZT for samples with different amounts of SiC particles.For 1%SiC sample,ZT was higher than that of pure Zn4Sb3above 500 K,and ZT reached 1.84 at 623 K.This arises mainly from larger Seebeck coefficient and smaller electrical resistance,even though there are higher thermal conductivity values,just as shown in Figs.7 and 8.Nevertheless,the addition of 5%SiC resulted in a decrease in ZT,especially at high temperatures.
In addition to thermoelectric properties,the effect of SiC nanosized particles on some mechanical properties has also been examined.Fig.10 shows Vickers hardness of the hot-extruded Zn4Sb3bulk materials as a function of volume fraction of SiC particles.The Vickers hardness increased with increasing the volume fraction of SiC particles.This is attributed to dispersion strengthening of SiC particles and grain refinement of Zn4Sb3matrix.The variation of flexural strength of the extruded Zn4Sb3samples with volume fraction of SiC particles is shown in Fig.11.The addition of 1%SiC particles caused a rapid increase in flexural strength.However,a further strength improvement cannot be found when more SiC particles were incorporated into Zn4Sb3matrix.This is primarily related to agglomeration of SiC particles.Since the average size of SiC particles is very small,particle agglomeration occurs easily with the increase in SiC amount.

Fig.9 Temperature dependence of dimensionless thermoelectric figure of merit of extruded Zn4Sb3 samples with different amounts of SiC particles.
From the above results,it is concluded that the addition of 1%SiC nanosized particles in Zn4Sb3contributes to the improvements in both mechanical and thermoelectric properties.Under the current experimental conditions,however,when more SiC particles are added in Zn4Sb3matrix,it appears there is a harmful effect on thermoelectric properties.
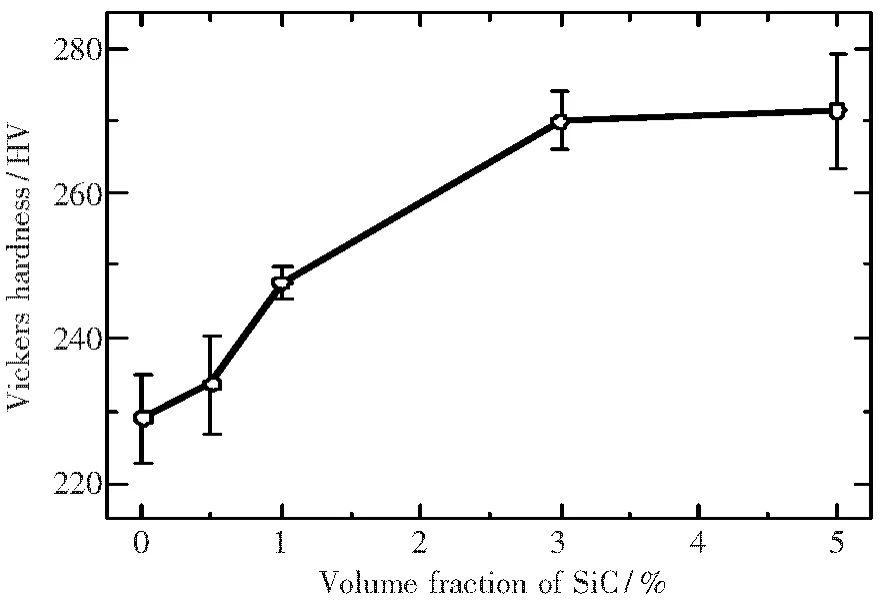
Fig.10 Vickers hardness of extruded Zn4Sb3samples as a function of volume fraction of SiC particles.

Fig.11 Variation of flexural strength of extruded Zn4Sb3samples with volume fraction of SiC particles.
3 Summary
In the present study,a“reaction-extrusion process”has been developed to prepare Zn4Sb3thermoelectric materials by using Zn and Sb powders as the starting materials.The effects of some processing parameters,such as extrusion ratio and punch speed,and SiC reinforcement on extrusion behavior,microstructure, thermoelectric, and mechanical properties of extruded Zn4Sb3bulk materials have been investigated.The synthesis,densification,and shape-forming of Zn4Sb3bulk materials were simultaneously achieved through hot extrusion of Zn-Sb powder mixture,which was vacuum-encapsulated into an Al alloy can.The extrudates had high density with single β-Zn4Sb3phase.The results showed that a large extrusion ratio and a small punch speed are advantageous to enhance thermoelectric performance.The extruded Zn4Sb3materials exhibited excellent thermoelectric performance,for example,the dimensionless thermoelectric figure of merit attains 1.77 at 623 K,which is 36%higher than that of conventional hot-pressed sample.Moreover,the addition of 1%SiC nanosized particles caused grain refinement of Zn4Sb3matrix,thus leading to improvements in both thermoelectric and mechanical properties(such as hardness and flexural strength).Acknowledgements
This work was supported in part by the Japan Science and Technology Agency(JST)and JFE 21st Century Foundation.
[1] Caillat T,Fleurial J-P,Borshchevsky A.Preparation and thermoelectric properties of semiconducting Zn4Sb3[J].J Phys Chem Solids,1997,58:1119-1125.
[2] Zhu T J,Zhao X B,Yan M,et al.Transport properties of β-Zn4Sb3prepared by vacuum melting[J].Mater Lett,2000,46:44-48.
[3] Souma T,Nakamoto G,Kurisu M.Low-temperature thermoelectric preperties of α-and β-Zn4Sb3bulk crystals prepared by a gradient freeze method and a spark plasma sintering method[J].J Alloy Comp,2002,340:275-280.
[4] Zhang L T,Tsutsui M,Ito K,et al.Effects of ZnSb and Zn inclusions on the thermoelectric properties of β-Zn4Sb3[J].J Alloy Comp,2003,358:252-256.
[5] Ur S-C,Kim I-H,Nash P.Thermoelectric properties of Zn4Sb3directly synthesized by hot pressing[J].Mater Lett,2004,58:2132-2136.
[6] Ueno K,Yamamoto A,Noguchi T,et al.Optimization of hot-press conditions of Zn4Sb3for high thermoelectric performance,I.Physical properties and thermoelectric performance[J].J Alloy Comp,2004,384:254-260.
[7] Ueno K,Yamamoto A,Noguchi T,et al.Optimization of hot-press conditions of Zn4Sb3for high thermoelectric performance III.Effect of starting particle size on thermoelectric and mechanical properties[J].J Alloy Comp,2005,392:295-299.
[8] Izard V,Record M C,Tedenac J C,et al.Discussion on the stability of the antimony-zinc binary phases[J].Calphad,2001,25:567-581.
[9] Chen Z-C,Tsujimura J,Fujita F.Processing and thermoelectric properties of Zn4Sb3compound by powder extrusion technique[C] //in:Powder Metallurgy&Particulate Materials,Proceedings of 2008 World Congress on Powder Metallurgy&Particulate Materials.2008:229-237.
[10] Miller G R,Li C Y.Evidence for the existence of antistructure defects in bismuth telluride by density measurements[J].J Phys Chem Solids,1965,26:173-177.
[11] Horá k J,Navrátil J,Star Z.Lattice point defects and freecarrier concentration in Bi2+xTe3and Bi2+xSe3crystals[J].J Phys Chem Solids,1992,53:1067-1072.
[12] Chen Z-C,Suzuki K,Miura S,et al.Microstructural features and deformation-induced lattice defects in hot-extruded Bi2Te3thermoelectric compound[J].Mater Sci Eng,2009,500:70-78.
TB 39
A
1671-6620(2011)01-0051-07
2010-05-20.
陈中春 (1963—),男,湖北应城人,日本鸟取大学教授,E-mail:chen@mech.to ttori-u.ac.jp.
