Over-expression of atf4 in Xenopus embryos interferes with neurogenesis and eye formation
2011-12-25LIUJianTaoYANGYongGUOXiaoGangCHENMiaoDINGHuanZhongCHENYongLongWANGMinRu
LIU Jian-Tao, YANG Yong, GUO Xiao-Gang, CHEN Miao, DING Huan-Zhong, CHEN Yong-Long,*, WANG Min-Ru
(1. Laboratory of Veterinary Pharmacology, College of Veterinary Medicine, South China Agricultural University, Guangzhou 510642, China; 2. Department of Veterinary Medicine, University of Science and Technology of Foshan, Foshan 528000, China; 3. Key Laboratory of Regenerative Biology, Guangzhou Institute of Biomedicine and Health, the Chinese Academy of Sciences, Guangzhou 510663, China)
Over-expression ofatf4inXenopusembryos interferes with neurogenesis and eye formation
LIU Jian-Tao1,2,#, YANG Yong3,#, GUO Xiao-Gang3, CHEN Miao3, DING Huan-Zhong1, CHEN Yong-Long3,*, WANG Min-Ru2,*
(1. Laboratory of Veterinary Pharmacology, College of Veterinary Medicine, South China Agricultural University, Guangzhou 510642, China; 2. Department of Veterinary Medicine, University of Science and Technology of Foshan, Foshan 528000, China; 3. Key Laboratory of Regenerative Biology, Guangzhou Institute of Biomedicine and Health, the Chinese Academy of Sciences, Guangzhou 510663, China)
Accumulated evidence indicates that the activating transcription factor 4 (atf4) is a developmentally relevant gene. Here, we report on the characterization ofatf4inXenopusembryos, which is differentially expressed in the central nervous system, eyes, blood, and the pronephros, as well as in developing endodermal organs such as the stomach, duodenum, liver, and pancreas. Ectopic expression ofatf4in the animal hemisphere ofXenopusembryos had no obvious effects on the induction of neural progenitors, but suppressed neurogenesis and eye formation without promoting apoptosis. Our data suggest that tightly controlledatf4activities may be crucial for normal neurogenesis and early eye patterning.
Xenopus;atf4; Eye; Neurogenesis; Apoptosis
Activating transcription factor 4 (atf4), also known as CREB-2, TAXREB67, or C/ATF, is a basic leucine zipper domain transcription factor that belongs to the cAMP responsive element binding (CREB) protein family and recognizes the ATF consensus siteTGACGT(C/A)(G/A) (Lee et al, 1987; Hai & Hartman, 2001). It is widely expressed in a variety of adult mouse and human tissues including the brain, heart, liver, spleen, thymus, lung, muscle, testis, and kidney (Tsujimoto et al, 1991; Karpinski et al, 1992; Vallejo et al, 1993). Gene targeting studies in mice revealed thatatf4plays specific roles in the development and physiology of a number oftissues and organs, such as lens formation, osteoclast differentiation, bone formation, synaptic plasticity, hematopoiesis, acinar cell viability, fertility, modulation of metabolic and oxidative stress, aspects of long-term memory, and lipid metabolism (Tanaka et al, 1998; Hettmann et al, 2000; Iida et al, 2007; Yang et al, 2004; Cao et al, 2010; Wang et al, 2010; Ameri & Harris, 2008). The stabilization of theatf4protein is required for the regulation of epithelial–mesenchymal transition of the avian neural crest (Suzuki et al, 2010). Althoughatf4is an oxidative stress- and endoplasmic reticulum stressinducible, prodeath transcription factor in neurons (Lange et al, 2008; Galehdar et al, 2010), little is known about its role in neurogenesis during early embryonic development.
Xenopusatf4cDNA has previously been cloned from stage 50 gonads (Akatsuka et al, 2004; Komatsuzaki et al, 2010). To screen novel pancreatic marker genes forXenopuspancreas development (Afelik et al, 2004), we identifiedatf4expressed in the developing pancreas of tadpole stage embryos. We report on the characterization of the embryonic expression pattern ofatf4and its novel function in neurogenesis and eye formation.
1 Materials and Methods
1.1 Isolation and construction of inducible Xenopusatf4
Xenopusatf4was first identified through the screening of an adultXenopuspancreas λZAP Express phage cDNA library (Afelik et al, 2004). The whole open reading frame ofXenopus laevisatf4was then subcloned into pGEM-T Easy vector (Promega) for generatingin situprobes or subcloned into the pCS2+ vector (Rupp et al, 1994) containing the human glucocorticoid receptor (GR) ligand-binding domain (Gammill & Sive, 1997) for generating capped mRNA. The latter construct was designated asatf4GR.
1.2 Embryo manipulation and mRNA injection
One thousand human chorionic gonadotropin IUs were injected into the dorsal lymph sacs of wild-typeXenopus laevisadult females to induce egg spawning. Eggs were squeezed and fertilizedin vitrowith minced testes, dejellied with 2% cysteine hydrochloride (pH 7.8-8.0) 30 min after fertilization and cultured in 0.1x MBS (1.76 mmol of NaCl, 48 mmol of NaHCO3, 20 mmol of KCl, 200 mmol of HEPES, 16 mmol of Mg2SO4, 8 mmol of CaCl2, 6 mmol of Ca(NO3)2, and pH 7.4) buffer at room temperature. Staging ofXenopus laevisembryos was conducted according to Nieuwkoop and Faber (1967). Embryos were fixed in MEMFA (0.1 mol of MOPS, pH 7.4, 2 mmol of EDTA and 4% formaldehyde) for 1 h at room temperature, washed in 100% ethanol three times for 10 min each, and stored at −20 °C in 100% ethanol. Gut dissection was carried out as reported previously (Chalmers & Slack, 1998).
To generate mRNA,atf4GRwas linearized by NotI and transcribed with a mMESSAGE Machine Sp6 kit (Ambion). Synthesized mRNA was purified using an RNAeasy kit (Qiagen). Up to 2 ng ofatf4GRmRNA either alone or together with 50 pg of β-galactosidaseRNA (Chitnis et al, 1995) was injected into one blastomere of 2-cell stage embryos from the animal pole. Dexamethasone was added at stage 12.5 to activate the fusion protein (Gammill & Sive, 1997). The uninjected side served as an internal control.
1.3 Whole-mount in situ hybridization
Whole-mountin situhybridization was performed as described by Harland (1991), with modifications as reported in Hollemann et al (1999). To generate digoxigenin-labeledatf4antisense probes, theatf4-pGEM-T plasmid was linearized with SalI and transcribed with T7 RNA polymerase. The digoxigeninlabeled antisense probes forsox3(Penzel et al, 1997),N-tubulin(Oschwald et al, 1991),pax6(Hirsch & Harris, 1997),otx2(Pannese et al, 1995),six3(Zhou et al, 2000) andrx1(Mathers et al, 1997) were prepared as described in the references. The antisense probe forpax2was directly transcribed with the RT-PCR product containing the T7 RNA polymerase promoter. Purification of digoxigenin-labeled RNA was performed using the Qiagen (Valencia, CA) RNAeasy kit according to the RNA cleaning protocol.
1.4 TUNEL assay
Whole-mount TUNEL assay was carried out as previously reported (Hensey & Gautier, 1998). Briefly, formaldehyde fixed embryos were washed in PBS and incubated in 150 U/mL terminal deoxynucleotidyl transferase (Takara), and 0.5 mmol digoxigenin–dUTP (Roche) overnight at room temperature. The reaction was then terminated in PBS/1 mmol EDTA, at 65 °C for 2 h followed by washes in PBS. Chromogenic reaction was carried out according to Harland (1991).
1.5 RNA extraction and RT-PCR
Total RNA from freshly collected embryos was extracted using the Trizol Reagent (Invitrogen) according to the manufacturer’s instructions and was then digested with DNaseI (Roche). First strand cDNA was synthesized using Superscript I M-MLV reverse transcriptase (Invitrogen), and then subjected to PCR analysis. The annealing temperatures, cycle numbers (in parentheses), and sequences of primers used in the RTPCR reactions are as follows:atf4(55℃, 24 cycles) forward 5′-GCATGAGCCCCTCTTACTTG-3′ and reverse 5′-GCTTTTGGCCTGTCGAACTT-3′,ornithine decarboxylase (ODC)(55℃, 23 cycles) forward 5'′-TGAATT GATGAAAGTGGCAAGG-3′ and reverse 5′-CAGGGCTGGGTTTATCACAGAT-3′.
2 Results
2.1 Spatial and temporal expression of atf4 in Xenopus embryos
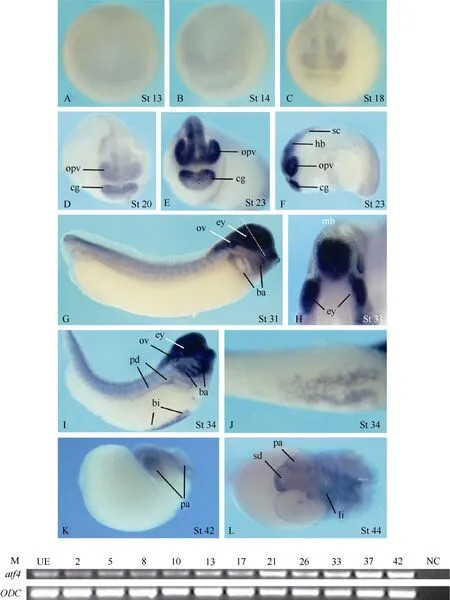
Fig. 1 Spatial and temporal expression of atf4 in Xenopus embryos
Theatf4expression was hardly detectable in embryos before neurulation by whole-mountin situhybridization (Fig. 1A, B). During neurulation,atf4was weakly expressed in the prospective midbrain, forebrain, and the cement gland anlage (Fig. 1C, D). At the tail bud stage, the expression levels increased and the signals in the central nervous system extended to the hindbrain and the anterior part of the neural tube. In addition, signals were also detected in the optic vesicles, branchial arches, otic vesicles, the pronephric ducts, the blood island, and the somites (Fig. 1E−J). At the transversal level of the eye and the midbrain,atf4expression obviously and uniformly covered the developing eyes and the whole developing neuroepithelium of the brain (Fig. 1H). At tadpole stage,atf4transcripts were detectable in the pancreas, the liver, and part of the stomach and duodenum (Fig. 1K, L).
Although no clearatf4expression signals were visualized by whole-mountin situhybridization analysis in embryos before neurulation (Fig. 1A, B and data not shown), RT-PCR analysis revealed thatatf4transcripts could be detected at all developmental stages examined, including the stages before the mid-blastula transition and unfertilized eggs (Fig.1M).
2.2 Overexpression of atf4 inhibits neurogenesis and eye anlage formation
Atf4knockout mice displayed microphthalmia with no recognizable lens (Tanaka et al, 1998; Hettmann et al, 2000; Masuoka & Townes, 2002), andatf4is specifically expressed in the eye vesicles and central nervous system ofXenopusembryos. We therefore investigated the effects of ectopicatf4activity on the morphology and expression of various molecular markers that reflect different aspects of neural development and eye formation inXenopusembryos (Fig. 2). Injections of a lower dose (1 ng per embryo) ofatf4GRmRNA led to microphthalmia (Fig. 2B) without affecting the gross morphology of the embryos, while a higher dose (2 ng per embryo) resulted in microcephaly with complete loss of eyes and slight tail deformation (Fig. 2C). We therefore chose the lower dose-injected embryos for checking marker gene expression.
Sox3expression identifies lens placodes and proliferating neural progenitors (Penzel et al, 1997; Bourguignon et al, 1998; Bellefroid et al, 1998; Ma et al, 2007). Ectopic expression ofatf4did not altersox3expression in the neural fold, but specifically blocked its expression in the lens placode (Fig. 2D, E). With respect to primary neurons,atf4exerted differential effects. While the lateral stripe and the trigeminal ganglion expression ofN-tubulin(Oschwald et al, 1991; Chitnis et al, 1995) were completely eliminated on theatf4GRinjected side, the medial stripe and part of the intermediate stripe were maintained (Fig. 2F, G). Overexpression ofatf4severely inhibitedpax2(Schlosser & Ahrens, 2004) expression in the midbrain-hindbrain boundary and completely abolished its expression in the placode, presumptive hindbrain, and spinal cord (Fig. 2H, I). Similarly,pax6(Hirsch & Harris, 1997) expression in the prospective hindbrain and spinal cord was completely lost and its expression in the eye anlage and presumptive forebrain and midbrain was drastically down-regulated uponatf4misexpression (Fig. 2J, K). Expression of other prospective lens and retinal markers likeotx2(Pannese et al, 1995),six3(Zhou et al, 2000), andrx1(Mathers et al, 1997) were also severely inhibited on the injected side (Fig.2 L-Q). As a result, at late stages of development, a microphthalmia phenotype was observed with no lens formed and only a tiny amount of retinal and retinal pigment epithelium cells developed (Fig. 3).

Fig. 2 Overexpression of atf4 interferes with neurogenesis and eye anlage formation
2.3 Overexpression of atf4 in Xenopus embryos did not promote apoptosis
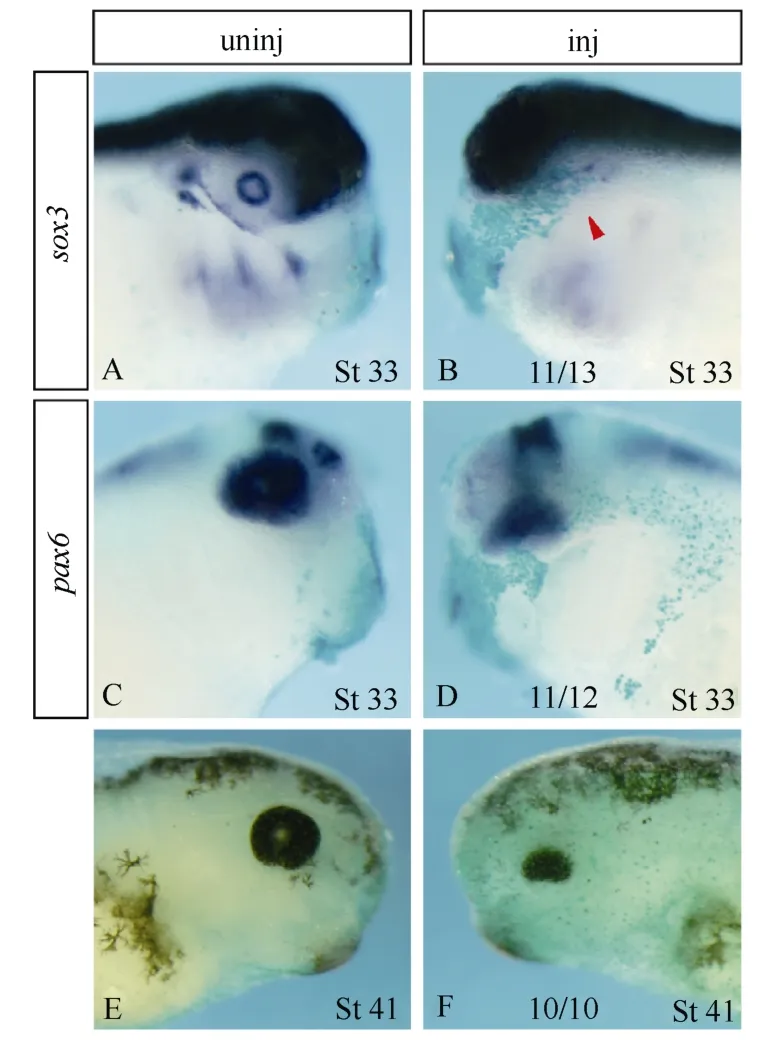
Fig. 3 Overexpression of atf4 led to microphthalmia
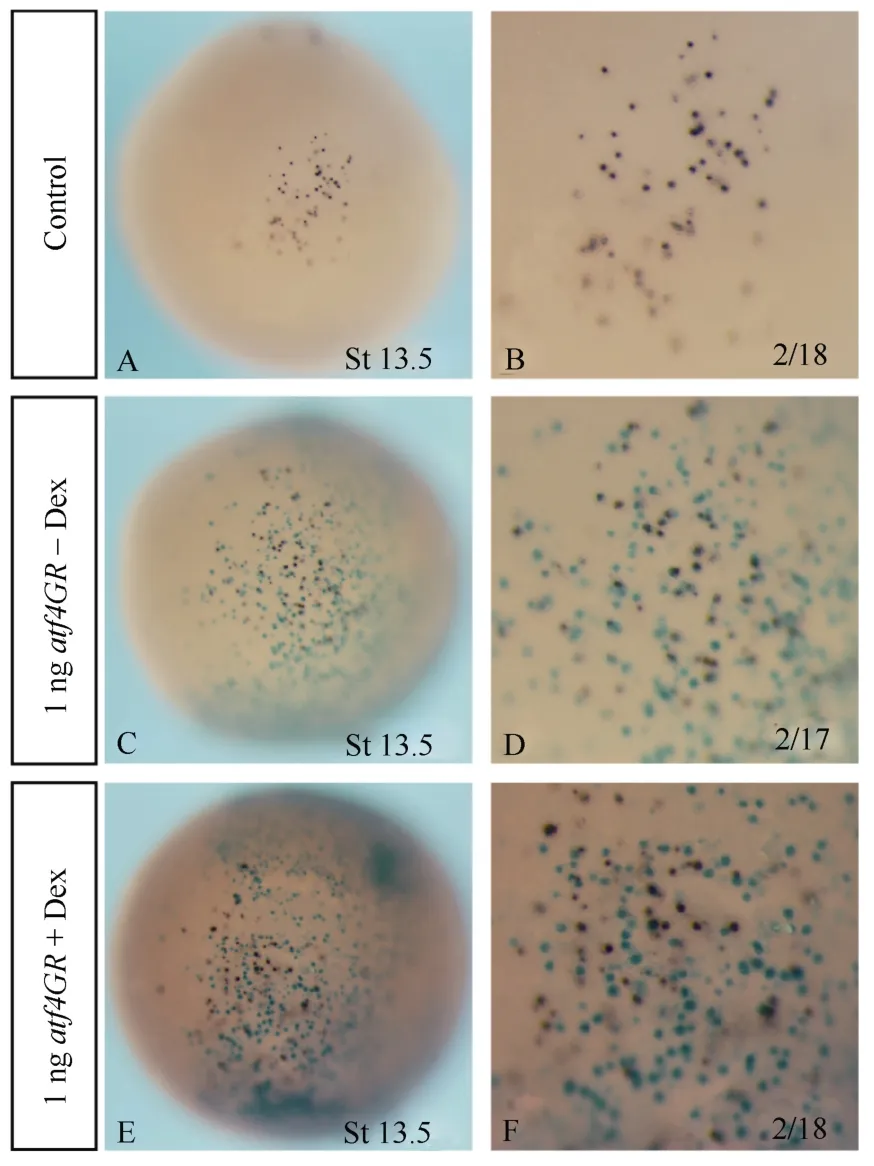
Fig. 4 Overexpression of atf4 in Xenopus embryos did not cause apoptosis
Forced expression ofatf4promoted the death of mouse cortical neurons in culture (Lange et al, 2008; Galehdar et al, 2010). We therefore checked apoptosis inatf4GRinjected embryos. TUNEL staining indicated there were no obvious signal differences between the control and the injected embryos (Fig. 4), suggesting that the elimination of primary neurons and inhibition of neural gene expression uponatf4overexpression was not attributed to increased apoptosis.
3 Discussion
Xenopusatf4displayed differential expression in tissues and organs derived from all three germ layers, which is consistent with data from mouse and quail studies (Murphy & Kolstø, 2000; Suzuki et al, 2010). Ectopic expression ofatf4inXenopusembryos did not affect initial neural induction, but interfered with neurogenesis by differentially blocking the primary neuron differentiation, completely inhibiting neural gene expression in the hindbrain, spinal cord, and lens placode, and partially eliminated prospective forebrain, midbrain, and retinal marker gene expression. Consequently, it led to microphthalmia with no lens formed and only a tiny amount of retinal and retinal pigment epithelium cells developed, which is reminiscent of the phenotype observed in ER81 injected embryos (Chen et al, 1999).
In mice,Atf4deletion did not affect the initial lens formation and the microphthalmia was caused by lens degeneration beginning at E14.5 (Tanaka et al, 1998; Hettmann et al, 2000; Masuoka & Townes, 2002). In this study, we found that ectopic expression ofatf4inXenopusembryos blocked initial lens placode formation.In vivo,atf4is weakly expressed at early neurula stages when early neural patterning and primary neurogenesis take place. Its expression becomes strong in the nervous system and optic vesicles at the tail bud stage. Our data suggests that a tight control of the level and timing ofatf4expression is required for proper neural development and eye formation inXenopusembryos. Further studies are required to determine howatf4interferes with early neurogenesis and retina development. Unlike in cultured mouse neurons, overexpression ofatf4did not promote apoptosis inXenopusembryos.
Acknowledgements:We thank Solomon Afelik and Marion Dornwell for the cDNA library screening at the University of Goettingen, Germany.
Afelik S, Chen Y, Pieler T. 2004. Pancreatic protein disulfide isomerase (XPDIp) is an early marker for the exocrine lineage of the developing pancreas inXenopus laevisembryos [J].Gene Expr Patterns, 4 (1): 71-76.
Akatsuka N, Kobayashi H, Watanabe E, Iino T, Miyashita K, Miyataa S. 2004. Analysis of genes related to expression of aromatase and estradiol-regulated genes during sex differentiation in Xenopus embryos [J].Gen Comp Endocrin, 136 (3): 382-388.
Ameri K, Harris AL. 2008. Activating transcription factor 4 [J].Int J Biochem Cell Biol, 40 (1): 14-21.
Bellefroid EJ, Kobbe A, Gruss P, Pieler T, Gurdon JB, Papalopulu N. 1998. Xiro3 encodes a Xenopus homolog of the Drosophila Iroquois genes and functions in neural specification [J].EMBO J, 17 (1): 191-203.
Bourguignon C, Li J, Papalopulu N. 1998. XBF-1, a winged helix transcription factor with dual activity, has a role in positioning neurogenesis in Xenopus competent ectoderm [J].Development, 125 (24): 4889-4900.
Cao HL, Yu SB, Yao Z, Galson DL, Jiang Y, Zhang XY, Fan J, Lu BF, Guan YF, Luo M, Lai YM, ZhuYB, Kurihara N, Patrene K, Roodman GD, Xiao GZ. 2010. Activating transcription factor 4 regulates osteoclast differentiation in mice [J].J Clin Invest, 120 (8): 2755–2766.
Chalmers AD, Slack JM. 1998. Development of the gut in Xenopus laevis [J].Dev Dyn, 212 (4): 509-521.
Chen Y, Hollemann T, Grunz H, Pieler T. 1999. Characterization of the Ets-type protein ER81 in Xenopus embryos [J].Mech Dev, 80 (1): 67-76.
Chitnis A, Henrique D, Lewis J, Ish-Horowicz D, Kintner C. 1995. Primary neurogenesis in Xenopus embryos regulated by a homologue of the Drosophila neurogenic gene Delta [J].Nature375 (6534): 761-766.
Galehdar Z, Swan P, Fuerth B, Callaghan SM, Park DS, Cregan SP. 2010. Neuronal apoptosis induced by endoplasmic reticulum stress is regulated by ATF4-CHOP-mediated induction of the Bcl-2 homology 3-only member PUMA [J].J Neurosci, 30 (50): 16938-16948.
Gammill LS, Sive H. 1997. Identification of otx2 target genes and restrictions in ectodermal competence during Xenopus cement gland formation [J].Development, 124 (2): 471-481.
Hai T, Hartman MG. 2001. The molecular biology and nomenclature of the activating transcription factor/cAMP responsive element binding family of transcription factors activating transcription factor proteins and homeostasis [J].Gene, 273 (1): 1-11.
Harland RM. 1991. In situ hybridization: an improved whole mount method for Xenopus embryos [M]//Danilchick M, Peng HB, Kay BK. Xenopus laevis: Practical uses in cell and molecular biology. Pictorial collage of embryonic stages. San Diego: Academic Press, 675-685.
Hensey C, Gautier J. 1998. Programmed cell death during Xenopus development: a spatio-temporal analysis [J].Dev Biol, 203 (1): 36-48.
Hettmann T, Barton K, Leiden JM. 2000. Microphthalmia due to p53-mediated apoptosis of anterior lens epithelial cells in mice lacking the CREB-2 transcription factor [J].Dev Biol, 222 (1): 110-123.
Hirsch N, Harris WA. 1997. Xenopus Pax-6 and retinal development [J].J Neurobiol32 (1): 45-61.
Hollemann T, Panitz F, Pieler T. 1999. In situ hybridization techniques with Xenopus embryos [M]//Richter JD. A Comparative Methods Approach to the Study of Oocytes and Embryos. New York: Oxford University Press, 279-290.
Iida K, Li YL, McGrath BC, Frank A, Cavener DR. 2007. PERK eIF2 alpha kinase is required to regulate the viability of the exocrine pancreas in mice [J].BMC Cell Biol, 8: 38.
Karpinski BA, Morle GD, Huggenvik J, Uhler MD, Leiden JM. 1992. Molecular cloning of human CREB-2: anATF/CREB transcription factor that can negatively regulate transcription from the cAMP response element [J].Proc Natl Acad Sci USA, 89 (11): 4820-4824.
Komatsuzaki E, Kitamura T, Murayama I, Saigo Y, Ojima K, Akatsuka N, Iwabuchi J, Miyata S. 2010. Characterization of an activating transcription factor 4 gene containing a consensus phosphorylation site for PKA in the gonads of Xenopus embryos [J].Zool Sci, 27 (1):19-23.
Lange PS, Chavez JC, Pinto JT, Coppola G, Sun CW, Townes TM, Geschwind DH, Ratan RR. 2008. ATF4 is an oxidative stressinducible, prodeath transcription factor in neurons in vitro and in vivo [J].J Exp Med, 205 (5): 1227-1242.
Lee KA, Hai TY, SivaRaman L, Thimmappaya B, Hurst HC, Jones NC, Green MR. 1987. A cellular protein, activating transcription factor, activates transcription of multiple E1A-inducible adenovirus early promoters [J].Proc Natl Acad Sci USA, 84 (23): 8355-8359.
Ma L, Zhao SH, Kong QH, Mao BY. 2007. Temporal and spatial expression patterns of Sox1 gene in Xenopus laevis embryo [J].Zool Res, 28 (4): 403-408.
Masuoka HC, Townes TM. 2002. Targeted disruption of the activating transcription factor 4 gene results in severe fetal anemia in mice [J].Blood, 99 (3): 736-745.
Mathers PH, Grinberg A, Mahon KA, Jamrich M. 1997. The Rx homeobox gene is essential for vertebrate eye development [J].Nature, 387 (6633): 603-607.
Murphy P, Kolstø A. 2000. Expression of the bZIP transcription factor TCF11 and its potential dimerization partners during development [J].Mech Dev, 97 (1-2): 141-148.
Nieuwkoop PD, Faber J. 1967. Normal Table of Xenopus Laevis (Daudin) [M]. Amsterdam: North-Holland Publishing Co.
Oschwald R, Richter K, Grunz H. 1991. Localization of a nervous system-specific classII beta-tubulin gene in Xenopus laevis embryos by whole-mount in situ hybridization [J].Int J Biochem Cell Biol, 35 (4): 399-405.
Pannese M, Polo C, Andreazzoli M, Vignali R, Kablar B, Barsacchi G, Boncinelli E. 1995. The Xenopus homologue of Otx2 is a maternal homeobox gene that demarcates and specifies anterior body regions [J].Development, 121 (3): 707-720.
Penzel R, Oschwald R, Chen Y, Tacke L, Grunz H. 1997. Characterization and early embryonic expression of a neural specific transcription factor xSOX3 in Xenopus laevis [J].Int J Dev Biol, 41 (5): 667-677.
Rupp R, Snider L, Weintraub H. 1994. Xenopus embryos regulate the nuclear localization of XMyoD [J].Genes Dev, 8 (11): 1311-1323.
Schlosser G, Ahrens K. 2004. Molecular anatomy of placode development in Xenopus laevis [J].Dev Biol, 271 (2): 439-466.
Suzuki T, Osumi N, Wakamatsu Y. 2010. Stabilization of ATF4 protein is required for the regulation of epithelial-mesenchymal transition of the avian neural crest [J].Dev Biol, 344 (2): 658-668.
Tanaka T, Tsujimura T, Takeda K, Sugihara A, Maekawa A, Terada N, Yoshida N, Akira S. 1998. Targeted disruption of ATF4 discloses its essential role in the formation of eye lens fibres [J].Genes Cells, 3 (12): 801-810.
Tsujimoto A, Nyunoya H, Morita T, Sato T, Shimotohno K. 1991. Isolation of cDNA for DNA-binding proteins which specifically bind to a tax-responsive enhancer element in the long terminal repeat of human T-cell leukemia virus type I [J].J Virol, 65 (3): 1420-1426.
Vallejo M, Ron D, Miller CP, Habener JF. 1993. C/ATF, a member of the activating transcription factor family of DNA-binding proteins, dimerizes with CAAT/enhancer-binding proteins and directs their binding to cAMP response elements [J].Proc Natl Acad Sci USA, 90 (10): 4679-4683.
Wang CX, Huang ZY, Du Y, Cheng Y, Chen SH, Guo FF. 2010. ATF4 regulates lipid metabolism and thermogenesis [J].Cell Res, 20 (2): 174-184.
Yang XL, Matsuda K, Bialek P, Jacquot S, Masuoka HC, Schinke T, Li LZ, Brancorsini T, Sassone-Corsi P, Townes TM, Hanauer A, Karsenty G. 2004. ATF4 is a substrate of RSK2 and an essential regulator of osteoblast biology: implication for Coffin–Lowry Syndrome [J].Cell, 117 (3): 387-398.
Zhou X, Hollemann T, Pieler T, Gruss P. 2000. Cloning and expression of xSix3, the Xenopus homologue of murine Six3 [J].Mech Dev, 91 (1-2): 327-330.
在非洲爪蛙胚胎中过表达atf4影响早期神经发育及眼睛的形成
刘建桃1,2,#, 杨 勇3,#, 郭晓刚3, 陈 淼3, 丁焕中1, 陈永龙3,*, 王敏儒2,*
(1.华南农业大学 兽医学院兽医药理实验室,广东 广州510642; 2.佛山科学技术学院 动物医学系,广东 佛山528000; 3.中国科学院广州生物医药与健康研究院 再生生物学重点实验室,广东 广州510663)
越来越多的证据表明转录激活因子4(atf4)是一个与胚胎发育相关的基因。该文研究了非洲爪蛙atf4在胚胎发育过程中的表达和功能。atf4特异性地表达在非洲爪蛙胚胎的脑部、眼睛、血岛、原肾、肝脏、胰腺以及胃和十二指肠的部分细胞。在非洲爪蛙胚胎的动物极半球过表达适量(不影响胚胎整体形态发生的剂量)的atf4, 对神经上皮细胞中sox3的表达无明显影响, 也不引起细胞凋亡; 但是对原始神经元的标记基因以及预定形成前脑、中脑、视网膜和晶状体的前体细胞的标记基因表达都有不同程度的抑制, 最终导致无晶状体小眼的表型。该研究结果首次提示对正常的早期神经发育及眼睛形成而言, atf4的活性需受到严格的调控。
非洲爪蛙;atf4基因; 眼睛; 神经发育; 细胞凋亡
Q959.53; Q786
A
0254-5853-(2011)05-0485-07
2011-06-23;接受日期:2011-08-04
#共同第一作者(Authors contributed equally to the work)
10.3724/SP.J.1141.2011.05485
date: 2011-06-23; Accepted date: 2011-08-04
s: This work was supported in part by funds from the Key Project of Knowledge Innovation Program of the Chinese Academy of Sciences (KSCX2-YW-R-083) and the National Basic Research Program of China (2009CB941202)
*Corresponding authors (通信作者), E-mail: fswangmr@sina.com; chen_yonglong@gibh.ac.cn
book=490,ebook=264
