以无机硫为原料制备硫化铅量子点及其表征
2011-11-30张建文张敬波
岳 栋 张建文 张敬波 林 原
(1北京化工大学流体力学与传热研究室,北京100029;2中国科学院化学研究所光化学重点实验室,北京分子科学国家实验室,北京100190)
以无机硫为原料制备硫化铅量子点及其表征
岳 栋1,2张建文1,*张敬波2,*林 原2
(1北京化工大学流体力学与传热研究室,北京100029;2中国科学院化学研究所光化学重点实验室,北京分子科学国家实验室,北京100190)
根据高温下快速成核低温下慢速生长的量子点制备原理,采用胶体化学的方法成功制备了不同粒径的硫化铅半导体量子点.这种方法的特点是以无味和低毒的硫化钠作为制备硫化铅量子点硫的前驱物,因此这是一种量子点的绿色化学合成方法.油酸作为稳定剂控制硫化铅的粒径.采用X射线衍射和高分辨透射电镜表征了量子点的晶体结构、形貌和粒径,采用可见-近红外吸收光谱研究了硫化铅量子点的量子尺寸效应.通过降低油酸的添加量可以促进量子点的生长,得到较大粒径量子点.并探讨了量子点的生长机理.
量子点;硫化铅;硫化钠;绿色合成;粒径分布
1 Introduction
In recent years,scientists have followed awfully with interest of semiconductor quantum dots(QDs)due to their unique properties,such as the mechanical,chemical,optical,electrical,electro-optical,and magneto-optical properties,which are fully different from those of bulk semiconductors.In principle, electro-optical properties of quantum dots are intensively size and shape dependent.As the size of quantum dot is reduced to its exciton Bohr radius,its band structure begins to change, known as the quantum size effect.1
Lead sulfide(PbS)is an important IV-VI semiconductor material with a directly narrow bulk band gap of 0.41 eV at 300 K.Compared to other semiconductors,its exciton Bohr radius of 18 nm is relatively large,which results in a significant quantum confinement.Its optical absorption band is ease to be tuned from 0.4 to 1.5 eV.2Because PbS quantum dots can provide the luminescence over the whole visible and near-infrared (NIR)regions,this nanoscale materials can be potentially used for the universal optical applications,such as Pb2+ion-selective sensors,3photography,4IR detectors,5solar absorbers,6and optical switch.7Recently,efficient multiple exciton generation has been detected in PbS quantum dots,making it a promising candidate for highly efficient photovoltaic conversion devices.8
Various methods have been rapidly developed to fabricate PbS nanocrystals in recent years such as solution or interface route,9,10hydrothermal or solvothermal process,11-14sonochemical method,15and microemulsion technique.16,17Organometallic method has also been used to prepare PbS nanocrystals in an organic solution.18,19Hines and Scholes18reported the organometallic synthesis of PbS nanocrystals with size-tunable NIR emission.The best size distribution can be obtained by this method,and based on these samples,many novel properties of PbS quantum dots were studied further.20,21However,the formation of PbS nanocrystals synthesized by such methods is still challengeable,because these reported methods usually involve some dangerous and unstable chemicals such as(TMS)2S (bis(trimethylsilyl)sulfide)and trioctylphosphine.It is very meaningful to find a synthesis route of the narrow size distribution quantum dots based on environmentally benign precursors.
Here,we focused on the controlled synthesis of PbS quantum dots using relatively green inorganic sulfide,sodium sulfide,which is inodorous and less noxious than organic S.A successful PbS quantum dot synthesis depends on two key elements,a controlled nucleation event and subsequent particle growth.We gained a narrow size distribution of PbS quantum dots by adjusting the added amount of oleic acid(OA).As a stabilizing ligand in this system,OA influences the reactivity of the precursors and hence controls the nanoparticle growth.
2 Experimental
Oleic acid(Fluka)and phenylate(Aldrich)were used without further purification.Na2S·9H2O was purchased from Shanghai Mei Xing Chemical Co.,Ltd.,Pb(AC)2·5H2O,methanol,and carbon tetrachloride was obtained from Beijing Chemical Reagent Plant.All chemicals used in the synthesis were of analytical grade.
PbS quantum dots were synthesized using phenylate as a reaction solvent and OA as a stabilizing ligand.In a typical process,408.3 mg Na2S·9H2O,10 mL OA,and 10 mL phenylate were loaded into a 50 mL three-neck flask at room temperature.The mixture was purged by Ar to remove oxygen and then heated to 180°C to form the S precursor.Meanwhile,the Pb precursor was prepared by heating 644.9 mg Pb(AC)2·5H2O in 2 mL OA and 4 mL phenylate under Ar at 80°C for 30 min. Then,a solution of Pb precursor was injected quickly into the vigorously stirring sodium sulfide solution at 180°C with a 1:1 molar ratio of Pb to S.Upon injection,the mixed solution became black instantly meaning that PbS nucleation occurred quickly.The temperature of the reaction vessel was decreased to 150°C and maintained for the remaining growth time,then cooled to room temperature.Purification of PbS quantum dots was done by precipitation of quantum dots with ethanol.This precipitation was repeated for several times to completely remove the unreacted precursors and solvents.Finally,black products were dried in vacuum at 80°C.The synthesis process was also carried out at different temperatures and with different concentrations of OAto adjust the size of quantum dots.
Absorption spectra of quantum dots solution were acquired with NIR-900 spectro-photometer.The crystalline structure of the as-prepared powders was characterized by X-ray powder diffraction(XRD)on a Rigaku X-ray diffractometer with Cu Kαradiation(λ=0.15406 nm).High-resolution transmission electron microscope(HRTEM)images of PbS quantum dots were performed on a FEI-TecnaiG2 20 S-TWIN TEM(Fei Co., Ltd.)operated at 150 or 300 kV,and the sample was loaded on amorphous carbon-coated copper grids(Ernest F.Fullam Inc. No.14560)by drop casting a very dilute solution of QDs in 90%ethanol and allowing the film to assemble and dry in vacuum drying oven under room-temperature.
3 Results and discussion
The syntheses of sulfide semiconductor quantum dots using inorganic S in aqueous or organic solvent have been reported.22,23But it is difficult to control the size distribution of quantum dots prepared by these reported methods.In this experiment, we chose sodium sulfide as a sulfur source to combine with lead acetate in order to acquire best purity and granularity of PbS quantum dots.There are three reasons to use sodium sulfide.First,sodium sulfide is less noxious than organic S such as(TMS)2S,and it is more feasible to deal with than other inorganic S such as H2S.Second,sodium sulfide is apt to dissolve in mixture of oleic acid and phenyl ether to form transparent and viscous solution,which can release S2-ions to react with lead cation after injection of sodium sulfide precursor solution. Finally,this sulfur source mixes well with lead acetate and there are no precipitates or stable complexes formed at room temperature,and the residuum can be completely removed during purification process of quantum dots with organic reagents such as methanol or ethanol.The coordinating agent plays an important role in controlling the growth process,stabilizing the resulting colloidal dispersion,and electronically passivating the semiconductor surface.According to the reported synthetic route of PbS nanocrystals,24OAwas usually regarded as the stabilizing ligand to control the short burst of homogeneous nucleation with the injection of reagents into the hot reaction flask. Therefore,OA was used as a size-controlling agent to adjust the size of quantum dots and their size distribution.The reaction equation to synthesize the PbS nanoparticles could be described as following,
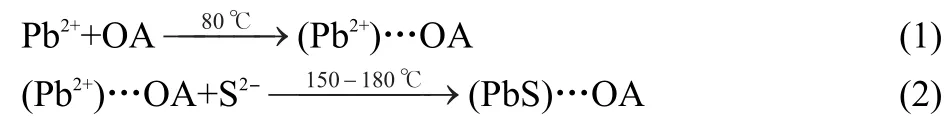
Vis-NIR absorption spectra of the as-prepared PbS nanocrystals were measured at room temperature and are shown in Fig.1.It can be seen that,the optical absorption spectra for two sizes of PbS nanocrystals samples prepared with different amounts of OA at 180°C show two clear exciton absorption peaks at 1720 and 1790 nm,respectively.The clear exciton absorption peak of PbS quantum dots reflects the narrow size distribution achieved without any post size-selective precipitation, which is usually used to optimize the size distribution of quantum dots according to the fact that larger particles are easier to precipitate than the smaller ones as the anti-solvent is added into the quantum dots solution.The blue shift of optical absorption edge shows low dimension of PbS nanoparticles obtained in present way is attributed to the size dependent band gap structure,which is reflected by the blue shifting toward short wavelength of the absorption edge with decreasing particle size.The effect of different OA amounts on the syntheses of PbS nanocrystals is obviously observed from Fig.1.The absorption edge shifts to blue in the NIR region with increasing addition amount of OA from 8 mL(a)to 12 mL(b).OA serves well in the capacity to influence the reactivity of the monomer species and to control the growth of nanocrystals.The higher concentration of OA will induce the lower precursor reactivity. Furthermore,the absorption spectrum of PbS quantum dots prepared with 8 mL OA displays a narrower size-distribution compared to that of the as-obtained samples prepared with 12 mL OA.Peng et al.24reported that the narrowly size-dispersed CdS nanocrystals can be successfully synthesized in high concentrations of OA.A rapid nucleation event occurs upon injection of lead acetate into the inorganic precursor S as evidenced by an immediate black color change in the reaction container.The rapid injection is critical to achieve a narrow size distribution. If the concentration of OA is too high,the rapid nucleation will be impressed and thus worsening size distribution.
Fig.1 Absorption spectra of PbS quantum dots prepared with addition of 12 mL(a)and 8 mL(b)OAat 180°C and with 3.5 mL OAat 150°C(c)
When the concentration of OA was decreased to 3.5 mL,the reaction of Pb2+and S2-ions becomes very fast,because the amount of OA is not enough to control the growth of nanocrystals.An alternate way to slow down the reaction is to decrease the reaction temperature.PbS quantum dots with smaller size and narrower size distribution were successfully synthesized with addition of 3.5 mL OA at 150°C and its absorption line (c)is showed in Fig.1.Therefore,the concentration of OA is not the only factor to control the growth of quantum dots.The monodisperse quantum dots with other particle sizes can be prepared by system optimization of synthesis conditions such as reaction temperature and time,added amount of OA,and ratio of S2-to Pb2+.
Fig.2 shows transmission electron microscope(TEM)images of PbS quantum dots prepared with addition of 12 mL OA. In low-resolution TEM(Fig.2(a)),regular circular spots were observed,which indicated that the tailoring of OA created to control the growth of PbS quantum dots with the mean size of about 5 nm.High-resolution TEM image,as shown in Fig.2(b), displays some well-defined crystal lattices.According to the distance of these lattices(0.34 nm),we can determine that PbS single crystals grow along the[111]direction.It is well known, nucleation is generally referred to the formation of seeds with a stable structure,and the shape of seeds is primarily determined by the minimization of surface energy,the growth rates on different facets are also dominated by the surface energy.25After nucleation process,the growth in the lower surface energy[111]direction is faster than others with higher surface energy.This favors the growth of the[111]facet leading to a spherical morphology with the lowest total surface energy.
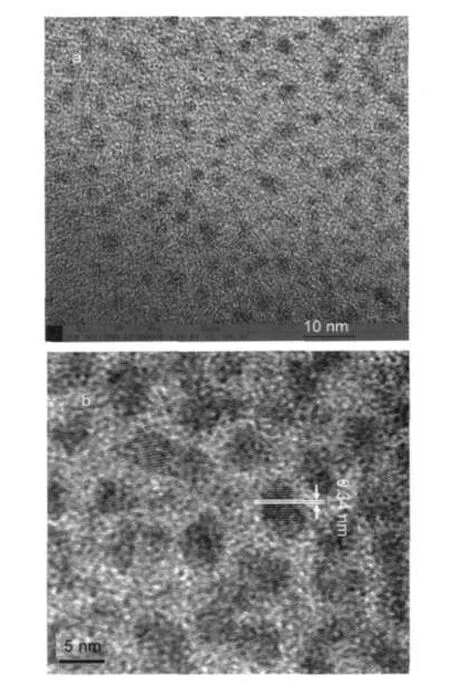
Fig.2 Low(a)and high(b)resolution TEM images of 5 nm PbS quantum dots synthesized with 12 mLOA
The crystalline structure of the synthesized PbS nanocrystals prepared with 12 mL OA is shown in Fig.3.It is obvious that all of the XRD peaks of the sample are consistent with the values in the standard card(JCPDS No.5-592).Its main diffraction peaks at 26.1°,29.9°,43.0°,50.7°,53.2°,62.9°,71.1°,and 79.1°are indexed as(111),(200),(220),(311),(222),(400), (420),and(422)planes of the cubic crystalline structure of PbS.Other as-prepared samples show same crystalline structure.It is well known,the average crystallite sizes D can be estimated from the half-width of the diffraction peaks according to Debye-Scherrer formula,26

where,D is the mean particle size,α is a geometric factor(here equals to 1.00),λ is the X-ray wavelength used in experiments (here equals to 0.154178 nm),β is the half-peak width of diffraction peak and can be measured from XRD pattern,and θ is the angle of the corresponding diffraction peak.The average crystallite size was estimated as 5.4 nm from the half-width of the diffraction peaks according to Debye-Scherrer formula. This estimated mean size is consistent with the result from the TEM observation.
PbS quantum dots have ability to extend their absorption range to near-IR region,emphasizing their application in solar cell.Recently,PbS quantum dots as photo sensitizer were intensively studied in different structural solar cells such as Schottky cell,depleted heterojuction cell,and quantum dots sensitized solar cell.27-30We fabricated PbS quantum dots sensitized TiO2porous thin film solid state solar cell with poly(3-hexylthiophene)(P3HT)as hole transport material(HTM).The light to electricity conversion efficiency of the device is not satisfied now.We wish to enhance its performance by optimizing the fabrication process,nanocrystalline film structure,layer thickness of HTM,and interfacial modification.
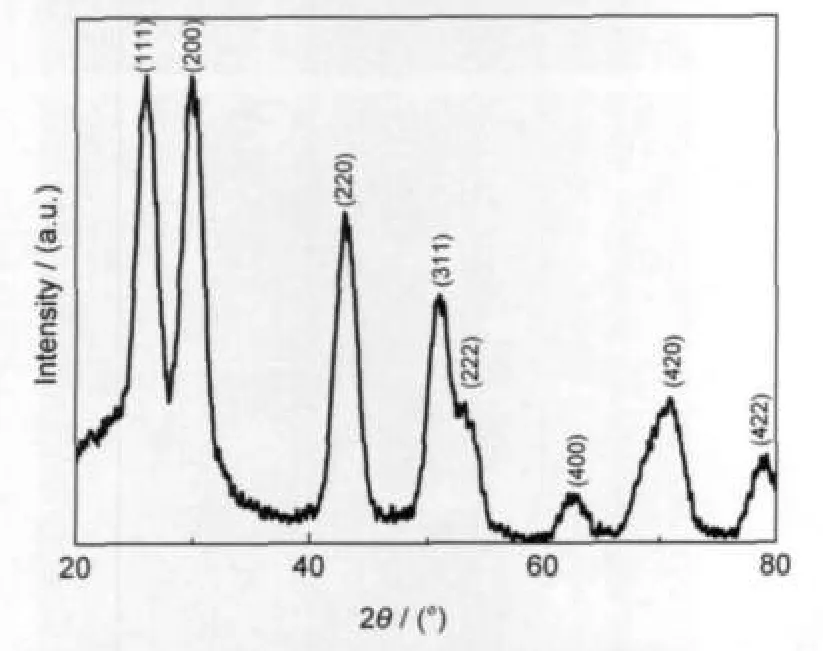
Fig.3 X-ray powder diffraction pattern of PbS quantum dots synthesized with 12 mLOA
4 Conclusions
A relatively novel route to synthesize macroscopic quantities of PbS quantum dots with uniform diameters was presented to use sodium sulfide as the ideal resource of S precursor due to its cost-effective,low toxicity,and stability.According to this synthetic way,the narrowly dispersed colloidal PbS nanocrystals with different sizes were successfully prepared by changing the concentration of oleic acid or temperature of nucleation and growth.It is expected that this approach may open new avenues for the green chemical synthesis of size-controlled semiconductor nanocrystallites,which would have potential applications in fabricating devices with special optical,electrical,and magnetic properties.
(1) Henglein,A.Chem.Rev.1989,89,1861.
(2) Dutta,A.K.;Ho,T.;Zhang,L.;Stroeve,P.Chem.Mater.2000, 12,1042.
(3)Wang,Y.;Suna,A.;Mahler,W.;Kasowski,R.J.Chem.Phys. 1987,87,7315.
(4) Hirata,H.;Higashiyama,K.Bull.Chem.Soc.Jpn.1971,44, 2420.
(5) Nair,P.K.;Gomezdaza,O.;Nair,M.T.S.Adv.Mater.Opt. Electron.1992,1,139.
(6) Gadenne,P.;Yagil,Y.;Deutscher,G.J.Appl.Phys.1989,66, 3019.
(7) Chaudhuri,T.K.;Chatterjes,S.Proc.Int.Conf.Thermoelectr. 1992,11,40.
(8) Kane,R.S.;Cohen,R.E.;Silbey,R.J.J.Phys.Chem.1996, 100,7928.
(9) Ellingson,R.J.;Beard,M.C.;Johnson,J.C.;Yu,P.;Micic,O. I.;Nozik,A.J.;Shabaev,A.;Efros,A.L.Nano Lett.2005,5, 865.
(10) Zeng,Z.;Wang,S.;Yang,S.Chem.Mater.1999,11,3365.
(11)Wang,S.;Yang,S.Langmuir 2000,16,389.
(12)Yu,D.;Wang,D.;Zhang,S.Liu,X.;Qian,Y.J.Cryst.Growth 2003,249,195.
(13)Trindade,T.;O′Brien,P.;Zhang,X.M.;Motevalli,M.J.Mater. Chem.1997,7,1011.
(14)Wang,D.;Yu,D.;Shao,M.S.;Liu,X.;Yu,W.;Qian,Y. J.Cryst.Growth 2003,257,384.
(15)Wang,S.F.;Gu,F.;Lu,M.K.Langmuir 2006,22,398.
(16) Ding,Y.H.;Liu,X.X.;Guo,R.J.Cryst.Growth 2007,307, 145.
(17) Ding,Y.H.;Liu,X.X.;Guo,R.Colloid.Surf.A-Physicochem. Eng.Asp.2007,296,8.
(18) Hines,M.A.;Scholes,G.D.Adv.Mater.2003,15,1844.
(19) Rogach,A.L.;Eychmüller,A.;Hickey,S.G.;Kershaw,S.V. Small 2007,3,536.
(20) Hyun,B.;Zhong,Y.;Bartnik,A.C.;Sun,L.;Abruna,H.D.; Wise,F.W.;Goodreau,J.D.;Matthews,J.R.;Leslie,T.M.; Borrelli,N.F.ACS Nano 2008,2,2206.
(21) Leventis,H.C.;O′Mahony,F.;Akhtar,J.;Afzaal,M.;O′Brien, P.;Haque,S.A.J.Am.Chem.Soc.2010,132,2743.
(22) Lee,H.;Wang,M.;Chen,P.;Gamelin,D.R.;Zakeeruddin,S. M.;Grätzel,M.;Nazeeruddin,M.K.Nano Lett.2009,9,4221.
(23)Wang,P.;Wang,L.;Ma,B.;Li,B.;Qiu,Y.J.Phys.Chem.B 2006,110,14406.
(24)Yu,W.W.;Peng,X.Angew.Chem.Int.Edit.2002,41,2368.
(25) Zhou,G.J.;Lu,M.K.;Xiu,Z.L.;Wang,S.F.;Zhang,H.P.; Zhou,Y.Y.;Wang,S.M.J.Phys.Chem.B 2006,110,6543.
(26) Wilson,A.J.C.Proc.Phys.Soc.London 1962,80,286.
(27) Zhao,N.;Osedach,T.P.;Chang,L.Y.;Geyer,S.M.;Wanger, D.;Binda,M.T.;Arango,A.C.;Bawendi,M.G.;Bulovic,V. ACS Nano 2010,4,3743.
(28) Pattantyus-Abraham,A.G.;Kramer,I.J.;Barkhouse,A.R.; Wang,X.;Konstantatos,G.;Debnath,R.;Levina,L.;Raabe,I.; Nazeeruddin,M.K.;Grätzel,M.;Sargent,E.H.ACS Nano 2010,4,3374.
(29) Ju,T.;Graham,R.L.;Zhai,G.;Rodriguez,Y.W.;Breeze,A.J.; Yang,L.;Alers,G.B.;Carter,S.A.Appl.Phys.Lett.2010,97, 043106.
(30) Luther,J.M.;Gao,J.;Lloyd,M.T.;Semonin,O.E.;Beard,M. C.;Nozik,A.J.Adv.Mater.2010,22,3704.
January 14,2011;Revised:March 8,2011;Published on Web:March 31,2011.
Preparation of PbS Quantum Dots Using Inorganic Sulfide as Precursor and Their Characterization
YUE Dong1,2ZHANG Jian-Wen1,*ZHANG Jing-Bo2,*LIN Yuan2
(1Laboratory of Computational Fluid Dynamics and Heat Transfer,Beijing University of Chemical Technology,Beijing 100029,P.R. China;2Beijing National Laboratory for Molecular Sciences,Key Laboratory of Photochemistry,Institute of Chemistry, Chinese Academy of Sciences,Beijing 100190,P.R.China)
PbS semiconductor quantum dots with different particle sizes were successfully prepared by the colloidal chemistry method according to the theory of fast nucleation at high temperature and slow growth at low temperature.Sodium sulfide was used as a sulfur precursor because it is odorless and is less noxious,which allows it to be classified as a green precursor.Oleic acid was used as a stabilizing agent to control the particle growth and it thus assisted in the formation of monodisperse PbS quantum dots.The crystalline structures,morphology,and particle size of the quantum dots were characterized by powder X-ray diffraction and high-resolution transmission electron microscopy.The quantum size effect of the PbS nanoparticles was analyzed by visible near-infrared(Vis-NIR)absorption spectroscopy.The mean size of the PbS quantum dots increased with a decrease in the concentration of oleic acid.A possible growth mechanism for the PbS nanoparticles was also discussed.
Quantum dots;Lead sulfide;Sodium sulfide;Green synthesis;Size distribution
O649
*Corresponding authors.ZHANG Jian-Wen,Email:zhangjw@mail.buct.edu.cn;Tel:+86-10-64436277.
ZHANG Jing-Bo,Email:jbzhang@iccas.ac.cn;Tel:+86-10-82615031.
The project was supported by the National Natural Science Foundation of China(20873162)and State Key Laboratory of Pollution Control and Resource Reuse Foundation of China(PCRRF09006).
国家自然科学基金(20873162)和污染控制与资源化研究国家重点实验室开放课题(PCRRF09006)资助项目
