A mutation in the type II hair keratin KRT86 gene in a Han family with monilethrix☆
2011-11-02JinWuYongliLinWenrongXuZhongmingLiWeixinFan
Jin Wu, Yongli Lin, Wenrong Xu, Zhongming Li, Weixin Fan
Department of Dermatology, the First Affiliated Hospital of Nanjing Medical University, Nanjing, Jiangsu 210029, China
INTRODUCTION
Monilethrix is a congenital defect of hair, with a high penetrance and variable expression[1,2]. The disease was first reported by Smith in 1829[3]. Affected individuals may have normal hair at birth, but within the first few months of life, they develop fragile, brittle hair, which then tends to fracture and produce varying degrees of dystrophic alopecia. In mild cases, only the occipital region of the scalp is involved; however, in severe cases, the secondary sexual hair, the eyebrows,and the eyelashes may also be involved[4]. Follicular hyperkeratosis has a tendency to occur on the scalp,the nape of the neck and the extensor surfaces of the upper arm and thigh. Light microscopic and scanning electron microscopic (SEM)examination can distinguish and display elliptical nodes of normal thickness and intermittent narrowing and internodes. The hair is easily disrupted at internodes. Ultrastructurally,vacuolation and alterations in the fibrillar structures of lower cortex cells have been described[5]. Occasionally, regrowth of apparently normal hair may occur during puberty or pregnancy, but the lesion never disappears completely[6].
Generally, monilethrix is inherited in an autosomal dominant fashion and associated with mutations in three keratin genes (KRT81, KRT83, and KRT86)located on chromosome 12q13[7-10]. In this study, we have identified a Chinese family with monilethrix,and sequenced three keratin genes (KRT81, KRT83,and KRT86)of the subjects to elucidate the underlying molecular basis of this disorder.
MATERIALS AND METHODS
Clinical features of patients
The proband (Ⅲ-3)was an 8-year-old boy. He visited our department with a complaint of increasing hair loss and inability to grow long hair. At birth, the hair shaft appeared normal but soon thereafter, nodes began to form along the shafts at regular intervals of 0.5-0.7 mm and the hair grew slowly, and became thin and brittle. The hair loss was concentrated in the forehead and both temples, and was exacerbated by mechanical stress such as cap wearing. Hairs could fall off at the end. In general, hairs were short and brittle, but some reached a length of up to 20 cm.There were no other subjective symptoms. Upon examination, we found that the hairs were sparse and withered, with a pronounced follicular hyperkeratosis in the forehead region (Fig. 1A)and also on the neck and the extensor aspect of four limbs. The eyebrows and eyelashes were uncompleted (not shown). His father (Ⅱ-3), who was 40 years, was also affected, with sparse and fragile hair since birth. His scalp hairs were short, dry, brittle, lusterless and were broken easily,and most of them emerged from keratotic follicular papules. His eyebrows were normal but axillary hairs were absent and pubic hairs were sparse. His nails and teeth were normal and had no other complications.He never received any treatment. In middle age, his hair symptoms gradually improved. Long terminal hairs increasingly appeared, but short and thin hairs and perifollicular papules also remained (Fig. 1B).His symptoms were reported to be serious in winter but mild in summer. To be noted, one uncle (Ⅱ-7)of the proband had normal hairs but loss in hearing and visual senses. The pedigree of the three-generation monilethrix family is shown in Fig. 2.
Scanning electron microscopy
Hair samples from the patients were examined by SEM (PHILIPS XL30 ESEM TMP), which was performed at Nanjing Agricultural University.
Source of DNA
The study was approved by the Ethics Committee of Nanjing Medical University and informed consent was obtained from all the study participants. Peripheral blood samples were obtained from the 8 affected patients and 10 unaffected members of this family and from 100 unrelated healthy Chinese individuals.Genomic DNA was extracted using a puregene kit(RN0601/02)from Galen BeiJin Inc., China.
Analysis of theKRT81, KRT83andKRT86gene
The exons of the KRT81, KRT83 and KRT86 gene along with the adjacent sequences of the exon–intron borders were amplified by PCR. The sequences of the primers for the KRT81, KRT83 and KRT86 genes were reported previously[5], and are listed in Table 1. PCR was performed using AdvantageTM2 DNA polymerase(Clontech, Japan).
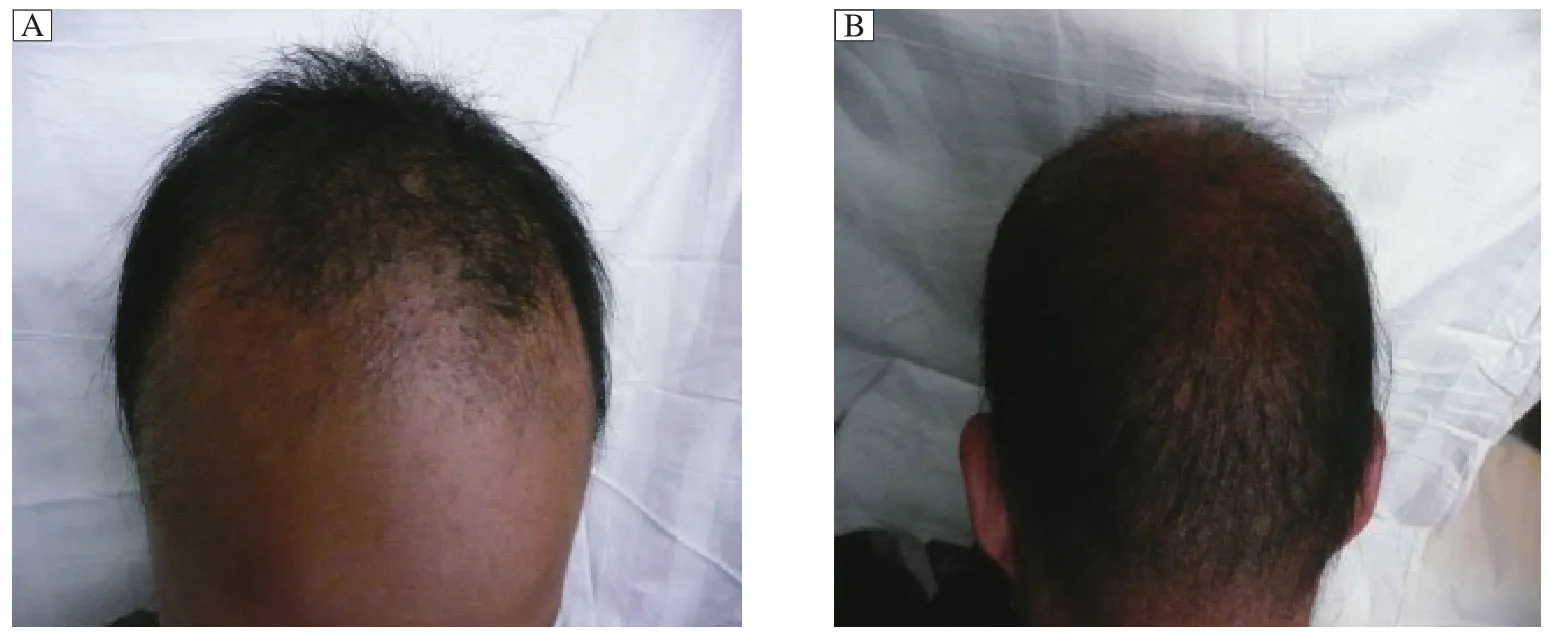
Fig.1 Scalp hair dystrophy in two monilethrix patients. A: The hair status of the proband (Ⅲ-3)patient. The hair shaft showed nodes at regular intervals of 0.5-0.7 mm and was thin and brittle. Hair loss was concentrated in the forehead region and both temples, which was exacerbated by mechanical stress such as wearing a cap. Hairs would fall off at their ends. Hairs in general were short and brittle, but some hairs reached a length of up to 20 cm. There was pronounced follicular hyperkeratosis on the forehead. B:The hair picture of the patient’s father (Ⅱ -3), whose hairs were also sparse and fragile. The scalp hairs were short, dry, brittle and lusterless, and most of them emerged from keratotic follicular papules and the hairs broke easily, but the eyebrows were normal.
After an initial denaturation of 3 min at 96°C, 30 cycles were performed (96°C for 30 s, 56°C for 45 s,and 72°C for 30 s), followed by a final extension of 7 min at 72°C. The amplified PCR fragments were analyzed on 1.5% agarose gels. After gel extraction of the fragments, direct fluorescent chain-termination DNA cycle sequencing was performed (Big Dye DNA Sequencing kit, Applied Biosystems, USA). The DNA sequences were analyzed on an ABI 310 DNA sequencer(Applied Biosystems). The mutations were identified by visual inspection and comparison with control sequences from unrelated, unaffected individuals.
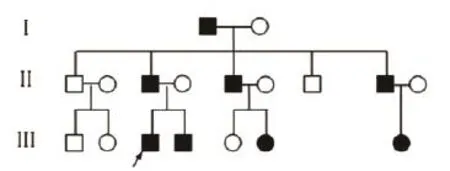
Fig. 2 Summary of the family tree. The proband patient was indicated by the arrow.
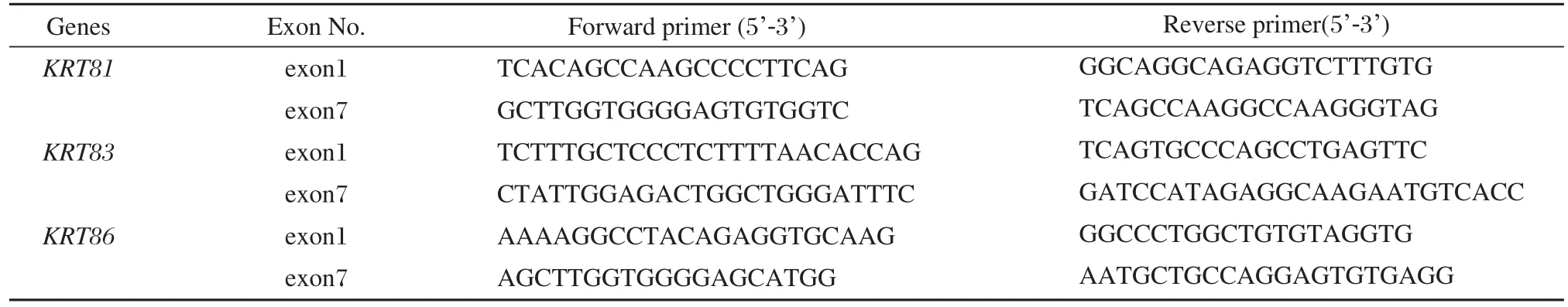
Table 1 The primer sequences for KRT81, KRT83 and KKT86
RESULTS
Light microscopy and SEM observation
Light microscopic examination of plucked hairs showed typical monilethrix of the patients. The beading could appear gross subtle, and the breaks in the hair shafts always occurred at internodes (Fig. 3A and Fig. 3B). SEM revealed that the thickness of the hair shaft was slightly, but obviously, inconsistent in some parts, resulting in nodes and internodes. The nodes seemed to be of normal thickness, but the internodes were abnormally thin (Fig. 3C and Fig. 3D), and the latter did not show a constant periodicity. The formation of fissure or cavities were evident in the transverse section of the hair shafts of the patients (Fig. 3E and Fig. 3F). This phenomenon was more serious in the transverse section of the hair shafts of the grandfather(Fig. 3E)than that of the proband (Fig. 3F). Fig. 3G shows the transverse sections of the hair shafts lacking a medulla. The normal transverse sections of the hair shafts had a cortex and medulla, and were well distributed (Fig. 3H). The hair cuticle exhibited a jagged shape and was fragmentated or lost (Fig. 3I)compared to the hair cuticle in a normal person, which was slick and well arranged (Fig. 3J).
Identification of a new mutation in theKRT86gene
The light microscopy and SEM findings suggested that our patients represented lesions of monilethrix.We then analyzed the genomic DNA for typeⅡ hair keratin KRT81, KRT83, and KRT86 genes. Mutation analysis was carried out of the gene sequences coding for the helix initiation motifs (HIMs)and helix termination motifs (HTMs)of the three typeⅡ hair keratins in which mutations associated with monilethrix have been reported. Direct sequencing of the PCR-amplified gene regions encoding the HIMs of the various keratins did not reveal deviations from the reported sequences in both affected and unaffected members of the family. However, analyses of the HTM-encoding regions of the KRT86, KRT83 and KRT81 gene led to the detection of a heterozygous G to A point mutation in the KRT86 gene in the family (Fig. 4A). The KRT86 gene mutation was found in all eight affected family members with clinical manifestations (Fig. 2). The mutation identified in all patients was a G-to-A transition mutation at nucleotide 1,289 in exon 7, (CGA to CAA), resulting in the substitution of arginine at amino acid 267 by glutamine (R430Q)(Fig. 4A).The mutation R430Q was not found in 10 unaffected members of this family (Fig. 4B)and 100 unrelated healthy Chinese individuals (Fig. 4C). The results showed that the patients had the R430Q mutation heterogeneously, whereas 10 unaffected members of this family and 100 control individuals were homozygotes for the wild-type sequence. We found that there were no novel mutations or recurrent mutations in the entire sequence of exons 1 and 7 of KRT81 and KRT83 and exon 1 of KRT86 among the patients.
Mutations in theKRT86gene confirmed by RFLP analysis
In order to confirm this mutation, the PCR products were digested with BseR1 and resolved by agarosegel electrophoresis. As shown in Fig. 5, the wildtype 387-bp fragments containing a BseR1 restriction site were completely cleaved into an 80- and a 307-bp fragment in unaffected members of this family (lane 2 in Fig. 5)and in normal control (lane 5 and 6 in Fig. 5). On the other hand, the mutant allele lacked the BseR1 restriction site and was not digested by the enzyme (lane 1,3 and 4 in Fig. 5). These findings confirmed the presence of a G→A substitution in exon 7 of KRT86 in the patients.
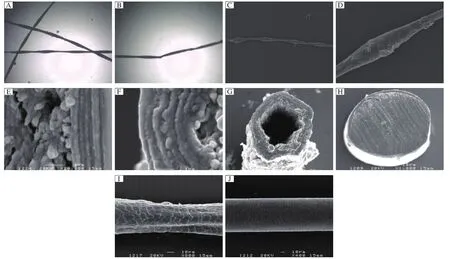
Fig. 3 Light microscopy and SEM observation. Light microscopic examination of plucked hairs showed typical features of monilethrix. The beading could be gross or subtle. The breaks in the hair shafts always occurred at internodes (A, B). SEM revealed that the thickness of the hair shaft was slightly, but obviously, inconsistent in some parts, resulting in nodes and internodes. The nodes seemed to be of normal thickness, but the internodes were abnormally thin (C, D). The internodes did not show a constant periodicity. The formation of fissure or cavities were evident in the transverse section of the hair shafts of the patients (E, F). This phenomenon was more serious in the transverse section of the hair shafts of the grandfather (E)than that of the proband (F). G shows that the transverse sections of the hair shafts lack a medulla. The normal transverse sections of the hair shafts had a cortex and medulla and the transverse section was well distributed (H). The hair cuticle exhibited a jagged shape and were fragmentated or lost (I)compared to the hair cuticle in a normal person, which was slick and well arranged (J).
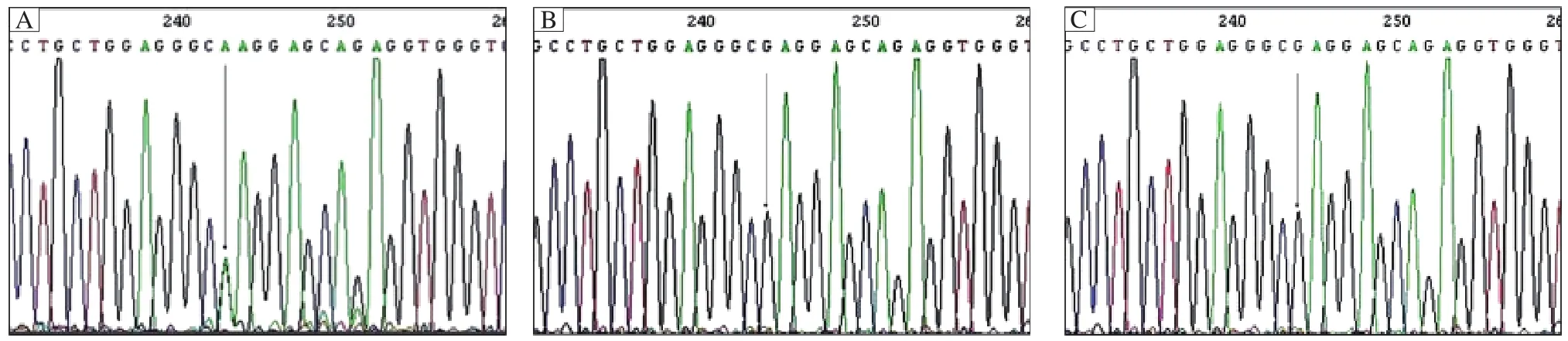
Fig. 4 Mutation in the KRT86 gene. A: The mutation in all patients is a G-to-A transition at nucleotide position 1,289 in exon 7,and the mutation occurs in the second position of an arginine residue (CGA to CAA), resulting in the substitution of arginine at amino acid 267 by glutamine (R430Q). The mutation R430Q was not identified in 10 unaffected controls (B)and 100 normal controls (C).
DISCUSSION
Monilethrix is a rare developmental disease that is characterized by small elliptical node-like deformities with increased hair fragility, resulting in partial or diffuse alopecia. This disorder has been reported to be caused by mutations in the HTMs or HIMs of three typeⅡ cortex keratin genes (KRT81, KRT83 and KRT86). In this study, we described a novel Arg→Gln mutation caused by a G→A point mutation in the second position of the CGA codon of Arg430 in the type Ⅱcortex KRT86 gene of a monilethrix patient in a Chinese family of Han ethnicity. The same type of mutation has not been found previously in the type Ⅱcortex keratin KRT86 gene of affected members. To our knowledge, because of the linkage of monilethrix to the type Ⅱ keratin locus[11,12], the gene regions coding for the HIM and HTM of typeⅡ hair keratins have been analyzed for possible mutations. In different families, different types of mutations in the HTMs of hair keratin KRT86, as well as an HTM E413K mutation in hair keratin KRT81, have been reported[8,13]. A KRT83 mutation was reported in a single monilethrix family in 2005[10]. The reason for the low mutation rate in KRT83 is unknown. Among the KRT86 mutations,the non-conservative E413K substitution was by far the most frequent mutation, followed by the conservative E413D and the non-conservative E402K substitutions[14]. However, we have not found these frequent mutations in KRT86 and the less frequent mutation in KRT83 in our patients.
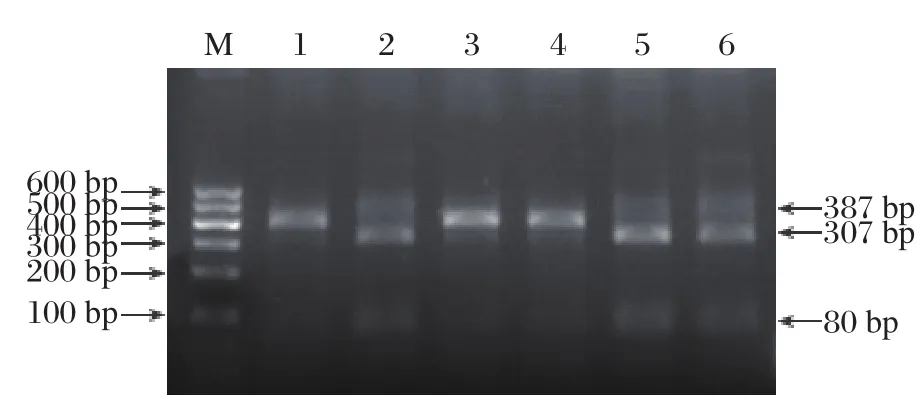
Fig. 5 RFLP analysis. Wildtype 387-bp fragments that containing BseR1 restriction site were completely digested into 80-and 307-bp fragments in normal control samples (lanes 2, 5 and 6). On the other hand, the mutant allele lacked the BseR1 restriction site and was not digested by the enzyme in the patients(lanes 1, 3 and 4).
Most mutations described to date have been located in the KRT81, KRT83 or KRT86 gene and are subject to speculation. Table 2 summarizes the mutations reported in monilethrix, including most of the data of monilethrix in the world and all the data of the mutations of monilethrix in China. Table 2 lists mutations associated with monilethrix including 9 types of mutations in KRT86 and 1 type of mutation in KRT83, as well as two kinds of mutations in KRT81.Among these, 2 out of 9 mutations at codon 413 in KRT86 and 1 of 2 mutations at codon 413 in KRT81 have been reported. Thus, codon 413 of KRT86 and KRT81 is thought to be a possible mutation hot spot in monilethrix. Most mutations reported show amino acid substitutions at the end of the 2B domain in the HTM of both hair keratin genes. These two mutations,E402K and E413K in KRT86, have been previously documented in monilethrix patients. The number of patients with E413K in KRT86 is greater than that of patients with other mutations. This outcome implies that this site, the HTM of KRT86, may be a ‘hot spot’ for mutagenesis in monilethrix. However, these two recurrent mutations, E402K and E413K, are the most common mutations known in monilethrix. In the present study, this tendency was not confirmed in the monilethrix family. Until now, pathogenic mutations in monilethrix seem to have been restricted to type Ⅱcortex keratins.
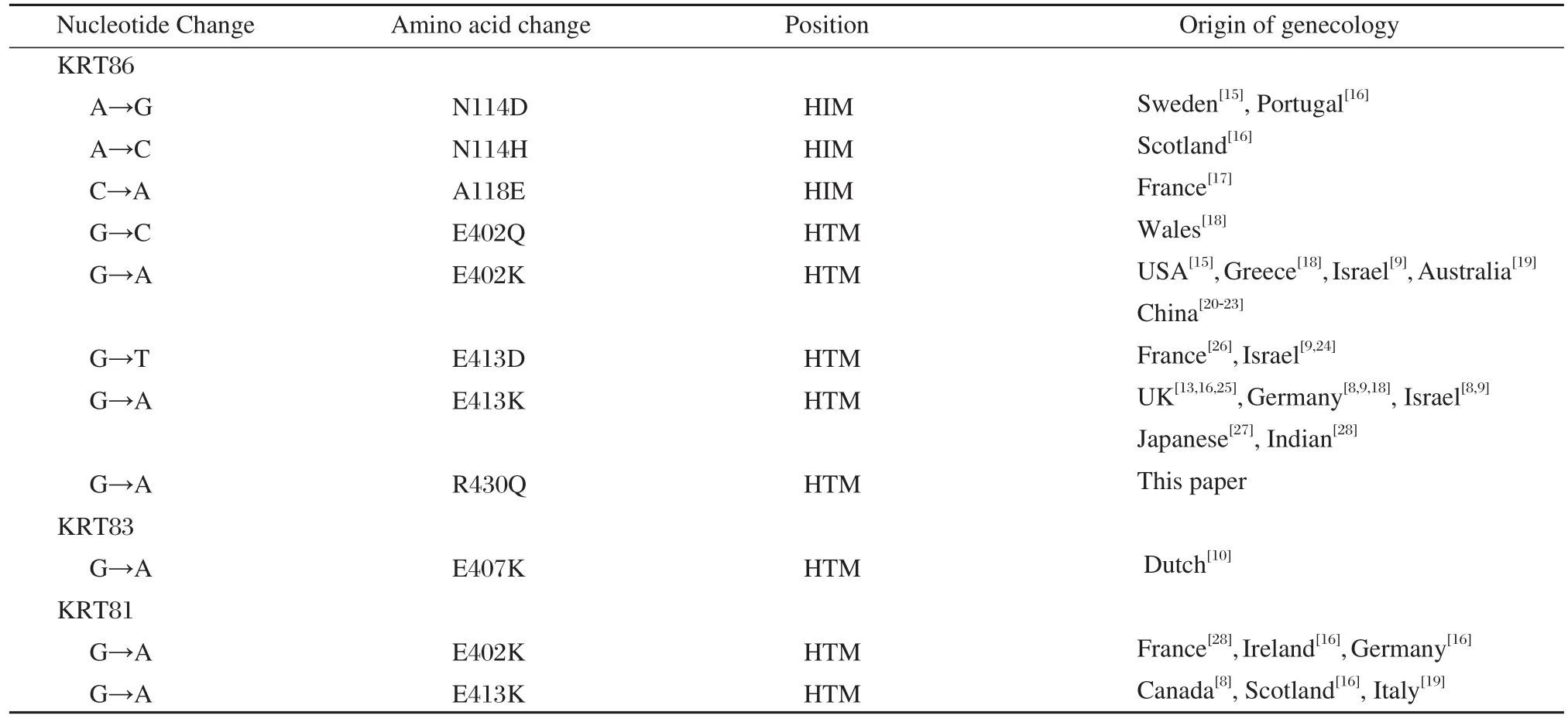
Table 2 Pathogenetic mutations in three hair keratin genes KR81, KR83 and KR86
In this family, we identified a glutamine substitution of the corresponding arginine acid residue, Arg430,in the typeⅡ hair keratin KRT86 gene in all eight affected individuals, suggesting that this site represents a new mutational hot spot in these highly related typeⅡ hair keratins. The KRT86 gene is mainly expressed in cortical trichocytes of the hair shaft. This indicates that monilethrix is a disease of the hair cortex.Arginine is a basic amino acid that carries a positive charge; however, glutamine is a neutral amino acid that does not carry a charge. The change in charge may result in an abnormality of the formation of the intermediate filament proteins, causing a defect in the hair keratins.
On the other hand, Oshimura et al.[29]measured the protective effects of arginine in an oxidative coloring or bleaching process through the methods of contact angle measurement, tensile measurement and amino acid analysis. Their results suggested that arginine prevents an undesirable attack by hydrogen peroxide on hair proteins and hair surface lipids. Furthermore,it has also been suggested from amino acid analysis that a considerable amount of arginine is deposited on or in hair fibers with coloring agents.
The variable clinical expressions of monilethrix is evident. Previous studies have indicated that there is no clear correlation between the phenotype and genotype of monilethrix and the clinical phenomenon varies among patients. The clinical phenomenon of the mutant gene may involve serious alopecia and also hair follicle keratinization, even with only carrier status. In our study, intrafamilial variation was evident.Family members had mild to moderate scalp involvement with moniliform hairs. In the mildest form, the disease involved only the occiput and nape of the neck, but in other family members with severe forms,most areas of the scalp, eyebrows and hairs on the legs were also involved. The differences in clinical phenomena also support the conclusion that a correlation between the phenotype and genotype of monilethrix has not been established.
This study emphasizes the key role of hair keratin K86 in the pathogenesis of monilethrix, which also demonstrates the heterogeneity of this disease and its potential for new mutations.
[1]Richard G, Itin P, Lin JP, Bon A, Bale SJ. Evidence for genetic heterogeneity in monilethrix. J Invest Dermatol 1996;107:812-4.
[2]Schweizer J. More than one gene involved in monilethrix:intracellular but also extracellular players. J Invest Dermatol 2006;126:1216-9.
[3]Smith WG. A rare nodose condition of the hair. Brit Med J 1879;2:291-6.
[4]Heydt GE. Zur Kenntnis der Monilethrix. Arch Klin Exp Derm 1963;217:15-29.
[5]Langbein L, Schweizer J. Keratins of the human hair follicle. Int Rev Cytol 2005;243:1-78.
[6]Baker H. An investigation of monilethrix. Br J Dermatol 1962;74:24-30.
[7]Rogers MA, Winter H, Langbein L, Wolf C, Schweizer J. Characterization of a 300 kbp region of human DNA containing the type II hair keratin gene domain. J Invest Dermatol 2000;114:464-72.
[8]Winter H, Rogers MA, Gebhardt M, Wollina U, Boxall L,Chitayat D et al. A new mutation in the type II hair cortex keratin hHb1 involved in the inherited hair disorder monilethrix. Hum Genet 1997;101:165-9.
[9]Horev L, Glaser B, Metzker A, Ben-Amitai D, Vardy D,Zlotogorski A. Monilethrix: mutational hotspot in the helix termination motif of the human hair basic keratin 6.Hum Hered 2000;50:325-30.
[10]van Steensel MA, Steijlen PM, Bladergroen RS, Vermeer M, van Geel M. A missense mutation in the type II hair keratin hHb3 is associated with monilethrix. J Med Genet 2005;42:e19.
[11]Healy E, Holmes SC, Belgaid CE, Stephenson AM,McLean WHI, Rees J et al. A gene for monilethrix is closely linked to the type II keratin gene cluster at 12q13. Hum Mol Genet 1995;4:2399-402.
[12]Stevens HP, Keisell DP, Bryant SP, Bishop DT, Dawber RPR, Spurr NK et al. Linkage of monilethrix to the trichocyte and epithelial keratin gene cluster on 12q11–13.J Invest Dermatol 1996;106:795-805.
[13]Winter H, Rogers MA, Langbein L, Stevens HP, Leigh IM, Labreze C et al. Mutations in the hair cortex keratin hHb6 cause the inherited hair disease monilethrix. Nat Genet 1997;16:372-4.
[14]Smith FJD. The molecular genetics of keratin disorders.Am. J Clin Dermatol 2003;4:347-64.
[15]Winter H, Clark RD, Tarras-Wahlberg C,Rogers MA,Schweizer J. Monilethrix: a novel mutation (Glu-402Lys)in the helix termination motif and the first causative mutation (Asn114Asp)in the helix termination motif of the typeⅡ hair keratin hHb6. J Invest Dermatol 1999;113:263-6.
[16]Korge BP, Hamm H, Jury CS, Traupe H, Irvine AD,Healy E et al. Identification of novel mutations in basic hair keratins hHb1 and hHb6 in monilethrix: implications for protein structure and clinical phenotype. J Invest Dermatol 1999;113:607-12.
[17]Winter H, Vabres P, Larregue M, Rogers MA, Schweizer J. A novel missense mutation, A118E, in the helix initiation motif of the type Ⅱ hair cortex keratin hHb6,causing monilethrix. Hum Hered 2000;50:322-4.
[18]Pearce EG, Smith SK, Lanigan SW, Bowden PE. Two different mutations in the same codon of a type Ⅱ hair keratin (hHb6)in patients with monilethrix. J Invest Dermatol 1999;113:1123-7.
[19]Horev L, Djabali K, Green J, Sinclair R, Martinez-Mir A,Ingber A, et al. De novo mutations in monilethrix. Exp Dermatol 2003;12:882-5.
[20]Zhang SD, Meng J, Zhao JJ, Tian W. Mutation E402K of the hHb6 in a Chinese Han family with monilethrix. Eur J Dermatol 2009;19:508-9.
[21]Deng YH, Shu KL, Li H. Detection of Mutation of TypeⅡ Hair Keratin Gene in a Han Family of Monilethrix.Acta Med Univ Sci Technol Huazhong 2007;3:392
[22]Feng AP, Liu P, Yang T. Analysis of human hair basic keratin 6 gene mutation in a Chinese Han family with monilethrix. Zhonghua Yi Xue Yi Chuan Xue Za Zhi (in Chinese)2008;25:141-4.
[23]Li JG, Li ZL, Wang YP. Detection of gene mutation in a pedigree with monilethrix. Chin J Dermatol (in Chinese)2006;7:374-6.
[24]Zlotogorski A, Horev L, Glaser B. Monilethrix: a keratin hHb6 mutation is co dominant with variable expression.Exp Dermatol 1998;7: 268-72.
[25]Korge BP, Healy E, Munro CS, Pünter C, Birch-Machin M, Holmes SC, et al. A mutational hotspot in the 2B domain of human hair basic keratin 6 in monilethrix patients. J Invest Dermatol 1998;111:896-9.
[26]Muramatsu S, Kimura T, Ueki R, Tsuboi R, Ikeda S,Ogawa H. Recurrent E413K Mutation of hHb6 in a Japanese Family with Monilethrix. Dermatology 2003;206:338-40.
[27]Khandpur S, Bairwa NK, Reddy BS, Bamezai R. A study of phenotypic correlation with the genotypic status of HTM regions of KRTHB6 and KRTHB1 genes in monilethrix families of Indian origin. Annales de Génétique 2004;47:77-84.
[28]Winter H, Labrèze C, Chapalain V, Surlève-Bazeille JE,Mercier M, Rogers MA, et al. A variable monilethrix phenotype associated with a novel mutation,Glu402Lys,in the helix termination motif of the type 2 hair keratin hHb1. J Invest Dermatol 1998;111:169-72.
[29]Oshimura E, Ino M. Effects of arginine on hair damage via oxidative coloring process. J Cosmet Sci 2004;55(Suppl):S155-S70.
杂志排行
THE JOURNAL OF BIOMEDICAL RESEARCH的其它文章
- Single-dose and multiple-dose pharmacokinetics of zaltoprofen after oral administration in healthy Chinese volunteers
- Development and optimization of an antibody array method for potential cancer biomarker detection☆
- The role of microRNAs during the genesis of medulloblastomas induced by the hedgehog pathway
- 《生物医学研究杂志(Journal of Biomedical Research)》宣布与汤森路透合作——采用国际一流的在线投审稿系统ScholarOne Manuscripts
- Instructions for Authors
- Cryptosporidiosis-an overview
