Na2O掺杂C12A7材料的结构及其抗菌性能
2011-09-15李全新
沈 静 宫 璐 李全新
(中国科学技术大学化学物理系,合肥 230026)
Na2O掺杂C12A7材料的结构及其抗菌性能
沈 静 宫 璐 李全新*
(中国科学技术大学化学物理系,合肥 230026)
本文利用溶胶-凝胶方法制备了Na2O掺杂的C12A7(12CaO·7Al2O3-Na2O)材料,通过X-射线衍射(XRD)、电子顺磁共振(EPR)、电感等离子体耦合-原子发射光谱(ICP-AES)和飞行时间质谱(TOF-MS)研究了材料结构与性能。选用金黄色葡萄球菌和大肠杆菌为受试菌种,研究了C12A7-Na2O的抗菌性能。结果表明,Na2O掺杂对C12A7的晶体结构以及材料中的氧负离子的浓度没有显著的影响,且C12A7-Na2O材料具有较高的抗菌性能。利用扫描电镜(SEM)对杀菌前后的金黄色葡萄球菌和大肠杆菌的菌体形态进行了观测,初步探讨了C12A7-Na2O的抗菌机理。
溶胶-凝胶;Na2O掺杂;C12A7;抗菌性能
0 Introduction
Itis wellknown thatdiseases,caused by microorganism such as bacteria and viruses,frequently broke out since time immemorial.In order to prevent human beings from infection of pathogenic bacteria,several of antibacterial materials have been explored,containing organic and inorganic substances such as the N-chloramine[1],metal(e.g.,silver;copper)[2-4],metallic ion (e.g.,silver ions)[5-6],metallic oxide (e.g.,titanium dioxide)[7-9].Inorganic antibacterialmaterials are attractive for prevention of bacteria in many fields due to less harm to the organs of human beings.Recently,the antibacterial activity of metallic oxides attracts researchers as the promising inorganic antibacterial materials that can substitute for conventional organicagents[10].Titanium dioxide(TiO2)functioned by photo catalysis is a significant alternative to conventional chemical disinfectors[11].However,this technology should perform with ultraviolet light and require a relatively complex device[12].It has been reported that the powders of zinc oxide and magnesium oxide have a well antibacterial activity without the presence of light.The application of these materials has the advantages including strong antibacterial activity without the light irradiation and some mineral elements being beneficial to the human body[13].
The crystal 12CaO·7Al2O3(C12A7)has a cubic structure,which contains two molecules per unit cell,is characterized by a positively charged lattice framework[Ca24Al28O64]4+including 12 sub-nanometer sized cages with a free space about 0.4 nm in diameter[14].The remaining two oxide ions(O2-),referred as free oxygen ions,are clathrated in the cages,and can be partially or completely replaced by other oxygen containing anions such as O-[15-16],O2-[15-17]as well as extrinsic species,e.g.,H-[18],OH-[19],F-[14],Cl-[14,20]and Au-[21].It has been revealed that the nanoporous crystal of C12A7 was very useful in the wide fields.For example,we developed an approach to selectively generate anions of O-[15,17],OH-[22-23],H-[24-25]and F-[26-27]in the gas-phase via the synthesized anionic storageemission materials of C12A7-X(X=O,OH,H,and F).The nanoporous material of C12A7-O-can selectively store and emit O-,which was prepared by the solid-state reaction of CaCO3and γ-Al2O3in the dry oxygen environment[14-15].The emitted species from the C12A7-O-surface were dominating with O (about 90%).More recently,we have synthesized various derivatives such as C12A7-OH-,C12A7-H-and C12A7-F-and found that these materials or the modified ones would be potentially used in semiconductor-etching[27],polymer surface-modification[28-29],one-step synthesis of phenol from benzene[30],reduction of NO[31],fast microorganisms inactivation[32-33]and the dissociation and oxidation of oxygenated organic compounds[34-35].Hayashi et al.successfully substituted H-for O-and formed the C12A7-H-crystal,which can be converted into an electrical conductor,yielding electrical conductivity up to 0.3 S·cm-1with UV irradiation[18].In 2003,Matsushi et al.first synthesized a unique inorganic electrode by exchanging free oxygen ions in the C12A7 cages with electrons[36].The synthesized material,named as C12A7:e-,was the first room-temperature-stable electrode and exhibited unique physical properties such as can be used as excellent electronic conductors and electrodes[36-39].
Present work aims to synthesize the Na2O doped C12A7 nano-crystal material by the sol-gel method,and to test the antibacterial effects of the resulting nanoparticles.The structure of the nano-crystal material was investigated through X-ray diffraction (XRD),electron paramagnetic resonance (EPR),inductively coupled plasma and atomic emission spectroscopy(ICP-AES),and time of flight mass spectrometer(TOF-MS).The antibacterial effects of the resulting material against Staphylococcus aureus(S.aureus)and Escherichia coli(E.coli)were examined by the attachment method.The excellentantibacterialperformance ofthe nanoparticles against S.aureus and E.coli may be partly due to direct interactions between the particles and bacteria membrane surface and partly due to the presence of active oxygen species(e.g.,O-and O2-)as proved by EP R and TOF.
1 Experimental
1.1 Preparation of C12A7-Na2O
The material of Na2O doped C12A7 was prepared by the sol-gel method.In the first step,11.34 g Ca(NO3)2·4H2O(Analytical reagent(A.R.),Sinopharm Chemical Reagent Co.Ltd (SCRC),Shanghai,China),21.00 g Al(NO3)3·9H2O (A.R.,Zhenxin Chemical Reagent Factory,Shanghai,China)and 0.35 g NaNO3(A.R.,SCRC,Shanghai,China)were added into 100 mL distilled water.The solution was mixed and stirred at room temperature for 3 h.In the second step,12.48 g urea(A.R.,SCRC,Shanghai,China)was added into the above solution,leading to the sol by stirring at room temperature for another 3 h.The resulting sol was aged at 90℃for 24 h and formed the gel.In the third step,the thermal treatment was carried out in air(350℃)for 2 h.Then the resulting powder was grinded and pressedto pellets with diameter of 15 mm and thickness of 1.5 mm.To obtain the final Na2O doped C12A7 sample,the pellets were sintered at 1 200℃for 10 h under flowing oxygen atmosphere (50 mL·min-1)and then quenched to the room temperature.
1.2 Characterization
X-ray diffraction analysis (X′pert Pro Philips diffractometer usingCu Kα radiation (λ=0.154 059 8 nm)was carried out to determine the crystal structure of the final resulting nano-material.The measurement conditions were in the 2θ range of 10°~80°,step counting time of 5 s,and step size of 0.017°at 298 K.The measurement of the lattice constant was made from diffractometer traces using Si as the internal standard.The Brunauer-Emmett-Teller(BET)surface area and pore volume was determined by the N2physisorption at-196 ℃ using a COULTER SA 3100 analyzer.The content of Na2O doped in the resulting sample was measured by inductively coupled plasma and atomic emission spectroscopy(ICP-AES,USA).The results of BET surface area,pore volume and ICP-AES analyses were shown in the Table 1.EPR measurements were performed to investigate the anionic species (e.g.,O-and O2-)in the C12A7-Na2O bulk.The experiments were conducted at ~9.1 GHz (X-band)using a JESFA200 spectrometer at 77 K.Spin concentrations were determined from the second integral of the spectrum using CuSO4·5H2O as a standard with an error of about 20%[40].The emitted anions from the C12A7-Na2O surface were mass-analyzed by time of flight(TOF),and the detailed conditions are the same as the previous study[4].
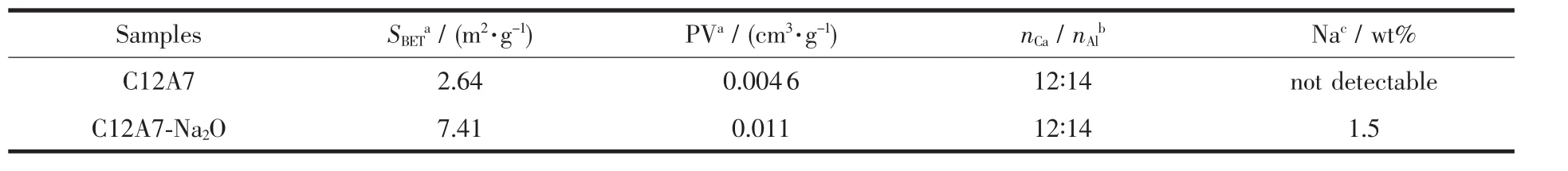
Table 1 Chemical and physical properties of C12A7 and Na2O doped C12A7 material
1.3 Antibacterial test
Staphylococcus aureus (S.aureus)strain ATCC 6538 was obtained from the China General Microbiological Culture Collection Center.Escherichia Coli(E.coli)strain DH5α was donated by Professor Yang,School of Life Science in University of Science and Technology of China.S.aureus and E.coli were grown on Luria-Bertani(LB)agar plate for 24 h at 37℃before test.
Antibacterial tests were conducted via the conventionalattachmentmethod[41].The sample matrixes prepared by medical cotton were uniformly covered with a given amount of the resulting nanoparticles (loading amount:5 mg·cm-2)and then the matrixes were first sterilized at 120℃for 5 h before used.A bacterial suspension of S.aureus or E.coli was diluted to 103cuf·mL-1(cuf=colony forming units).Then,0.1 mL bacteria suspensions were deposited on the surface ofdisinfected matrixes,respectively.The matrixes loading with the bacteria were dried at ambient conditions for 10 min and incubated at 37℃for 1 h.Then each matrix was transferred into a sterile Erlenmeyerflask containing 10mL ofphosphatebuffered saline(PBS)solution with 1wt%Na2S2O3.The flask with the matrix was shaken for 20 s and left undisturbed for 15 min.Finally,0.1 mL of appropriate dilution in PBS solution was poured on nutrient agar plates and incubated at 37℃for 24 h to count the clones.All the trials were repeated three times.The reported data is the mean values of three trials±standard deviation with origin software release 7.0(Origin Lab Corporation,MA01060,USA).
SEM analyses for the S.aureus and E.coli cells were performed to investigate the morphological changes before and after the treatment via the resulting nanoparticles.The treatment steps were same as those for the antibacterial tests.The treated bacteria cells were rinsed by the 0.2 mol·L-1PBS solution then wereimmersed in 3%glutaraldehyde in 0.2 mol·L-1PBS for over night at 4℃.For the SEM measurements,the SEM specimen was rinsed for three times in 0.2 mol·L-1PBS and was dehydrated by successive soakings in a gradient series of ethanol solution (40,70,90 and 100%,V/V).The specimen was dried in air,coating with gold,and then was viewed by FE-SEM(FEI,Sirion-200,American)at 5 kV accelerating voltage.
2 Results and discussion
2.1 XRD analysis
The XRD analysis was carried out to investigate the structure of C12A7-Na2O,which was prepared by the sol-gel method.Fig.1 shows the XRD patterns obtained from C12A7 and C12A7-Na2O,which were sintered at a temperature of 1 200℃in the flowing oxygen ambience for 10 h respectively.The mainly positions of the XRD diffraction peaks observed were 2θ=18.09°,33.38°,36.69°and 41.05°.By comparing the measured positions and intensities of the XRD patterns with the standard data in the PDF-09-0413 card(International Centre for Diffraction Data(ICDD),2002),the X-ray diffraction structure for the C12A7-Na2O completely accords with that of C12A7.This means that the material structure of C12A7-Na2O prepared by the sol-gel method has a cubic structure with a unit-cell content of Ca24Al28O68and belongs to I43d space group.No Na2O phase was observed.Thus,the other phase in the resulting material was negligible beside the Ca24Al28O68phase when the dried gels were sintered at the temperature of 1 200℃.The unit cell constant for the C12A7-Na2O,derived from the stronger diffraction peaks,is about(1.199±0.003)nm.
The mean particle sizes of both C12A7-Na2O and C12A7 were estimated by analyzing the broadening of the diffraction peaks.The mean particle sizes were calculated by the Scherrer equation[42-43]:

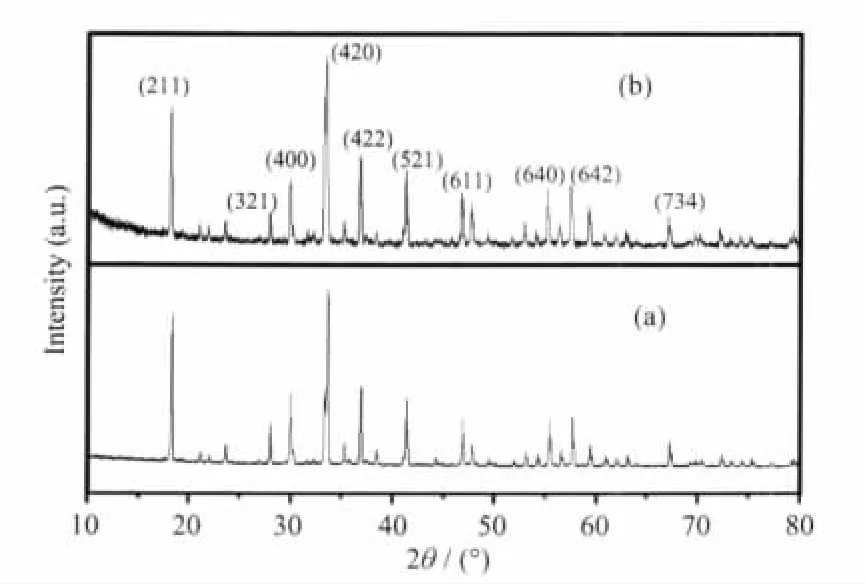
Fig.1 XRD patterns of the samples(a)C12A7 and(b)Na2O doped C12A7
where λ is the wavelength of X-ray source(Cu Kα radiation,λ=0.154 056 nm),β is the full-width at halfmaximum(FWHM),and θ is the diffraction angle of the X-ray diffraction.The mean diameter of the C12A7-Na2O is 59 and 86 nm for C12A7.
2.2 EPR analysis
TheEPR measurementswere performed to investigate the oxygen-containing species such as O-and O2-in the resulting material.Fig.2 shows the EPR spectra from the samples of C12A7-Na2O and C12A7.As shown in Fig.2,the EPR spectra of the samples can bedecomposed intotwocomponents,which are attributed to the anionic oxygen species of O-(gxx=gyy=2.036 and gzz=1.994)and O2(gxx=2.001,gyy=2.008,and gzz=2.074).By simulating the EPR spectra and using CuSO4·5H2O as a spin concentrations standard[40],the oxygen species concentrations were estimated in the resulting material.For the sample C12A7-Na2O,the concentration of O-and O2-is calculated by simulating the measured EPR spectra,being about(1.2±0.3)×1020cm-3.On the other hand,the remained specie of O2-was estimated by the charge balance,total positive charge concentration (2.33×1021cm-3)[44]as well as all anionic species estimated by EPR.Accordingly,the anionicspecies in the synthesized C12A7-Na2O material was dominated by the anions of O-and O2-,accompanied by other oxygen-containing anions of O2-.
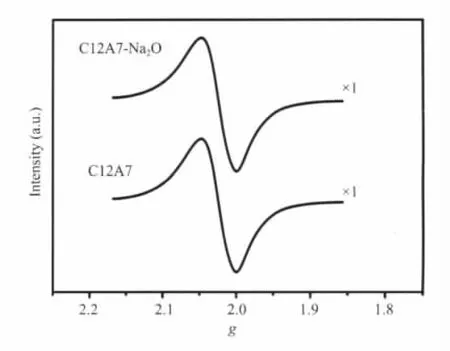
Fig.2 EPR spectra from the samples of C12A7 and C12A7-Na2O
2.3 TOF analysis
The anions emitted from the C12A7-Na2O surface were identified by TOF-MS.Fig.3 shows a typical anionic TOF mass spectrum from the C12A7-Na2O sample at the emission temperature of 700℃and an extraction field of 800 V·cm-1.An intense peak at the mass number of 16 corresponds to the O-anions emitted from C12A7-Na2O.The weak but detectable peak at m/z=0 was synchronously observed,attributed to weak emission of the electrons.It was found that the anionic species emitted from the C12A7-Na2O material surface were dominating with the O-anions together with a small amount of electrons in our investigated range,which agreed with the EPR results.It is also noticed that anions emitted from the C12A7-Na2O material surface were quite same from C12A7 surface.
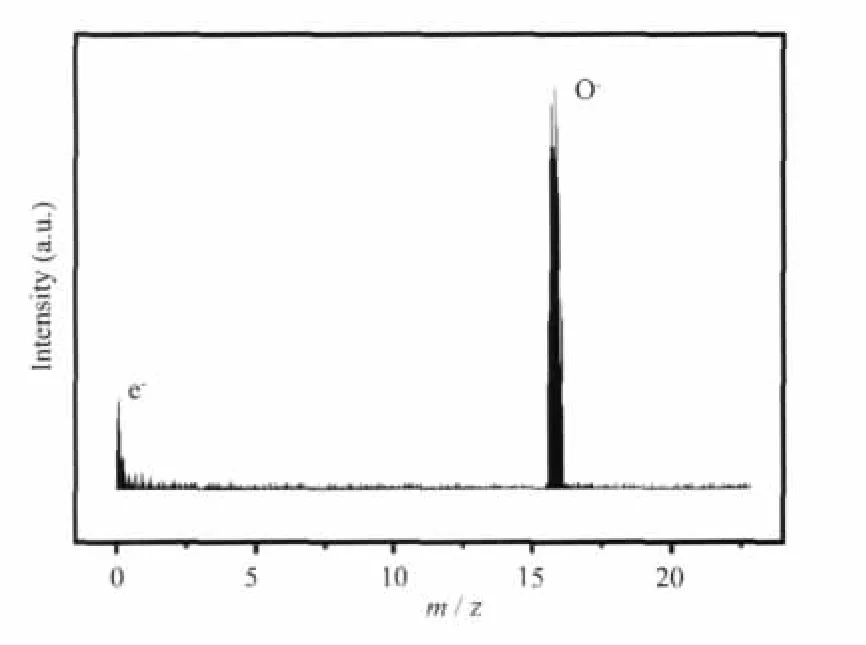
Fig.3 Typical TOF mass spectrum measured from the C12A7-Na2O surface.Conditions:700 ℃ and the extraction field of 800 V·cm-1
2.4 Antibacterial activity
The tests of the bactericidal activity of the C12A7-Na2O particles were performed for both the grampositive (S.aureus)and the gram-negative (E.coli)bacteria using a three-step protocol.In the first step,the living cells were deposited onto the surface of properly cultures uniformly covered with C12A7-Na2O particles(loading amount:5 mg·cm-2)and were let in contact with the resulting particles for 1 h.The same procedure was performed on specimens prepared for the “blank”experiments in order to exclude that any bactericidal effect had to be ascribed to the bare matrixes.As a comparison,in the third step,the living cells were let in contact with the cultures containing the Al2O3,C12A7 particles(loading amout:5 mg·cm-2)and commercial benzalkonium chloride patches(BAND-AID)for 1 h.
Table 2 depicts the bactericidal efficiency for both the gram-positive (S.aureus)and gram-negative(E.coli)bacteria in the presence of different antibacterial material particles with same loading amounts.According to standard reduction of bacteria criterion,less than ten reductions indicates no bactericidal effect;between 102~103reduction indicates an expressive bactericide;greater than 103reductions is considered a powerful bactericidal effect[45].Present results(Table 2)show that the C12A7-Na2O particles of 5 mg·cm-2support an powerful bactericide effect for both S.aureus and E.coli,which is a little higher than that of C12A7,but a little lower than commercial benzalkonium chloride patches(BAND-AID).
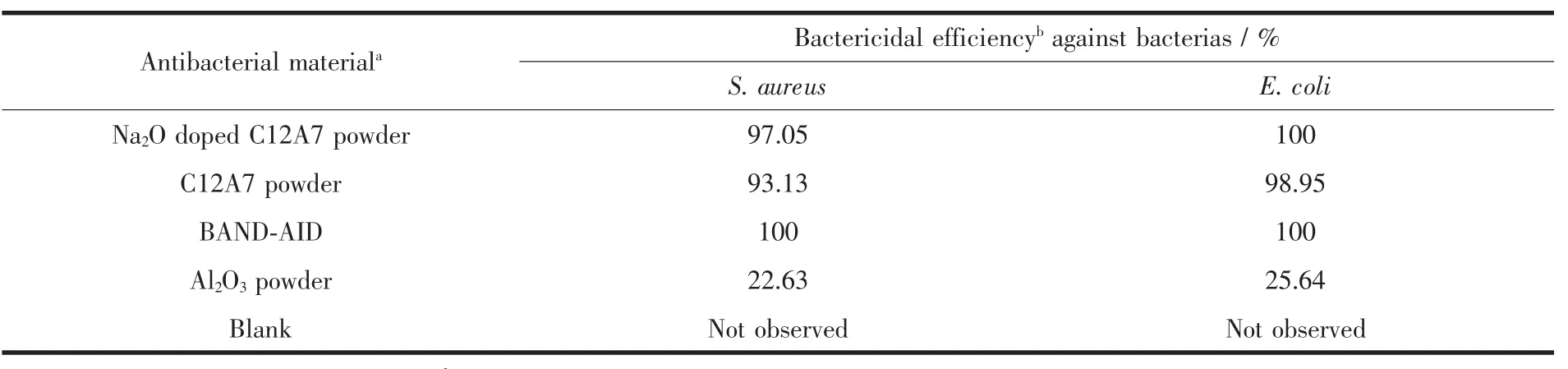
Table 2 Bactericidal efficiency in the preference of different antibacterial material particles with same loading amounts
The tests of the bactericidal activity of the C12A7-Na2O particles were carried out for the gram-positive(S.aureus)and the gram-negative (E.coil)bacteria in different time.Fig.4 shows the bactericidal efficiency of the C12A7-Na2O particles against S.aureus and E.coil(loading amount:5 mg·cm-2)in different time.The initial population of bacteria was about 103cuf·mL-1.After the treatments via the C12A7-Na2O particles(loading amounts:5 mg·cm-2)for 6 h,the averaged bactericidal efficiency against S.aureus were 97%and 99%for E.coil.The results indicated that the C12A7-Na2O particles(loading amount:5 mg·cm-2)denoted a powerful bactericidal effect.
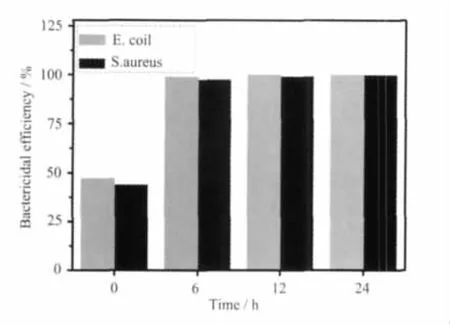
Fig.4 Bactericidal efficiency of C12A7-Na2O against S.aureus and E.coil in different time
Morphological changes of the S.aureus and E.coil cells before and after the treatments via the C12A7-Na2O particles were investigated by the FE-SEM observation.Fig.5 shows the FE-SEM images of the S.aureus cells for(a)without the particle,(b)contacting with the Al2O3particles of 5 mg·cm-2for 1 h,(c)the treated onebycontactingwith theC12A7-Na2O particles of 5 mg·cm-2for 1 h,respectively.A tidy appearance kept after the treatment via the C12A7-Na2O particles (Fig.5c).In addition,small crack was also observed for the treated S.aureus cells,as shown in the inserted part in Fig.5c.Fig.6 shows the FE-SEM images of the E.coil cells for(a)without the particle,(b)contacting with the Al2O3particles of 5 mg·cm-2for 1 h,(c)the treated one by contacting with the C12A7-Na2O particles of 5 mg·cm-2for 1 h,respectively.No obvious morphological change was observed for the cells treated by contacting with the Al2O3particles of 5 mg·cm-2for 1 h as compared with the original cells.However,the E.colicells treated by the C12A7-Na2O particles appeared rough and dramatically collapsed(Fig.6c),leading to the release of intracellular constituents.The morphological results clearly show that the cell walls were seriously damaged by the treatment via C12A7-Na2O.
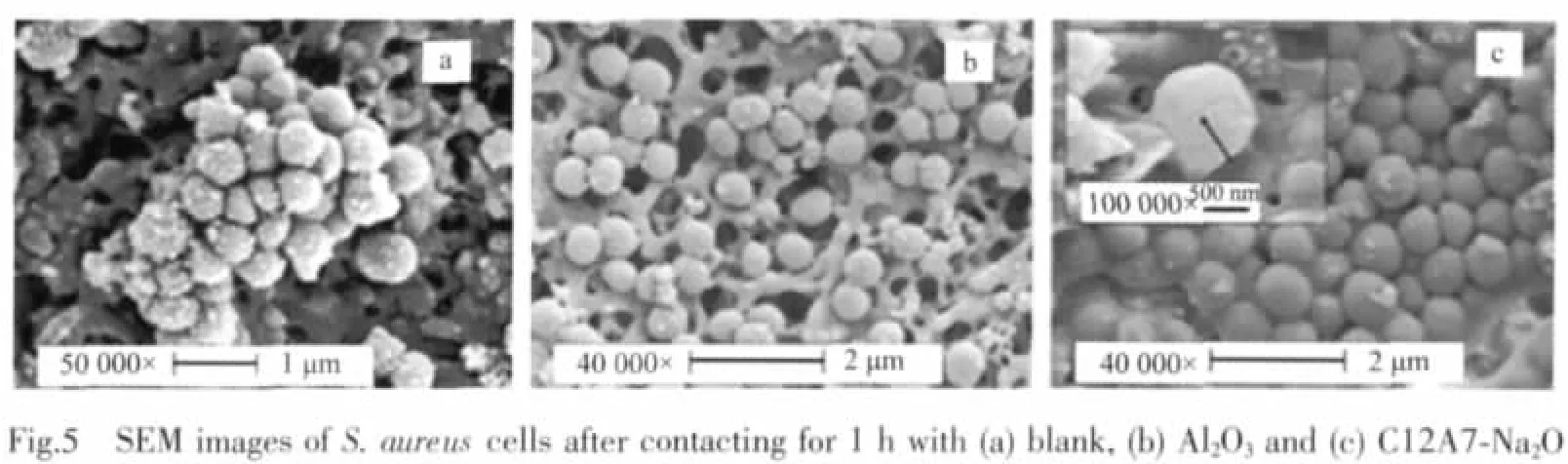
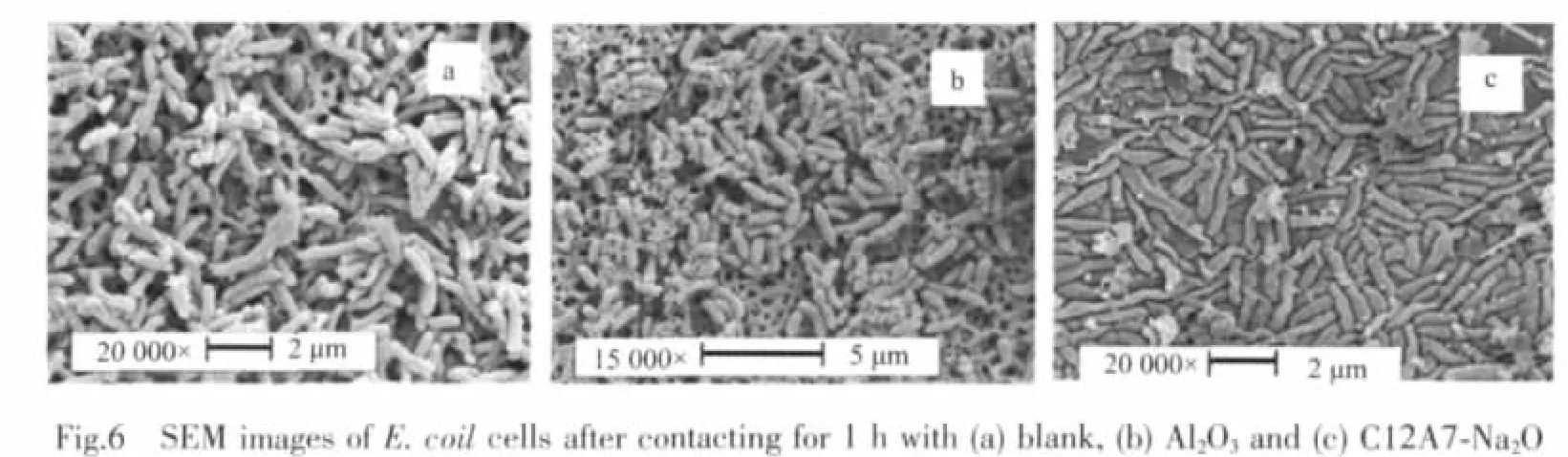
2.5 Antibacterial mechanism
According to the composition of the antimicrobial material,the antimicrobials can be divided into natural,organic and inorganic antimicrobial material.The antibacterialmechanisms were differentbetween different antimicrobials.The following four factors maymostly affect the antibacterial activity of inorganic antimicrobial:(1)The cations eluted from powder,such as Ag+、Cu2+、Zn2+.It is generally considered that heavy metals react with proteins by combing the SH groups of enzymes,which leads to the inactivation of the proteins.In addition metal ions show strong oxidation abilities,which can destroy the metabolism of the bacterial[47].(2)Active oxygen generated from powder.Makhluf et al.investigated the antibacterial behavior of MgO and partly attributed the behavior to the production of active oxygen species due to the presence of MgO[45].It has been reported that H2O2was produced in ZnO slurry[48].(3)The pH value affection.Under the high pH,the protein of the cell membrane will be dissolved,and the lipid can be saponified,finally the cell membrane is ruptured,leading the bacterial to death[49]. (4)The electrostatic interactions between the bacteria surface and nanoparticles[42].The analysis exhibited above suggested that the interaction between nano-particle and cell membrane wall may be one of the antibacterial mechanisms for C12A7-Na2O nanoparticles.Additionally it seems that the presence of active oxygen species(e.g.,O-and O2-)in the C12A7-Na2O particles could be another possible impact to explain the antibacterial behavior observed in this study.However,it is less clear how the active oxygen species interact with the bacteria surface and how to damage to the cells.Further work is underway aiming at answering these questions,which will be reported in the future.
3 Conclusions
The nanocrystal material of C12A7-Na2O was successfully prepared by the sol-gel method and applied for antibacterial material.According to the EPR measurements,main anions encaged in the material of C12A7-Na2O are O-with the order of 1020cm-3.Moreover,the excellent antibacterial performance of the C12A7-Na2O against S.aureus and E.coli were observed,which may be partly due to direct interactions between the particles and bacteria membrane surface and partly due to the presence of active oxygen species(e.g.,O-and O2-).Potentially,this material could be used as a good bactericidal material due to its good properties such as environmentally benign,good safety.
Acknowledgements:The authors are grateful to General Program of the National Natural Science Foundation of China(No.50772107),the National Basic Research Program (973 Program No.2007CB210206)of Ministry of Science and Technology of China,and the National High Tech Research and Development Program(863 Program No.2009AA05Z435).
[1]Elder E D,Worley S D,Williams D E,et al.Appl.Microbiol.,1987,62:457-464
[2]Panáck A,Kvítek L,Prucek R,et al.J.Phys.Chem.B,2006,110:16248-16253
[3]Kim Y H,Lee D K,Cha H G,et al.J.Phys.Chem.B,2006,110:24923-24928
[4]Kim Y H,Lee D K,Cha H G,et al.J.Phys.Chem.C,2007,111:3629-3635
[5]Jeon H J,Yi S C,Oh S G.Biomaterials,2003,24:4921-4928
[6]Xu K,Wang J X,Kang X L,et al.Mater.Lett.,2009,63:31-33
[7]WANG Hong(王 虹),WANG Peng(王 鹏).Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2009,25(11):1928-1934
[8]ZHANG Yun(张 云),ZHAO Lang(赵 逸 浪),YIN Guang-Fu(尹光福)et al.Chinese.J.Inorg.Chem.(Wuji Huaxue Xuebao),2004,20(8):991-995
[9]YAN Li-Li(闫丽丽),WANG Yan(王艳),XIONG Lang-Bin(熊良斌),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2009,25(11):1960-1964
[10]Yamamoto O,Sawai J,Sasamoto T.Int.J.Inorg.Mater.,2000,2:451-454
[11]Rincon A G,Pulgarin C.Appl.Catal.B,2004,49:99-112
[12]Sokmen M,Candan F,Sumer Z J.Photochem.Photobiol.,2001,143:241-244
[13]Yamamoto O,Sawai J,Kojima H,et al.J.Mater.Sci.-Mater.Med.,2002,13:789-792
[14]Jeevaratnam J,Glasser F P,Glasser D,et al.J.Am.Ceram.Soc.,1964,47:105-106
[15]Li Q X,Nishioka M,Kashiwagi H,et al.Surf.Sci.,2003,527:100-112
[16]Li Q X,Hayashi K,Nishioka M,et al.Jap.J.Appl.Phys.,2002,41:238-240
[17]Li Q X,Hayashi K,Nishioka M,et al.Appl.Phys.Lett.,2002,80:4259-4261
[18]Hayashi K,Matsuishi S,Kamiya T,et al.Nature,2002,419:462-465
[19]Hayashi K,Hirano M,Hosono H.J.Phys.Chem.B,2005,109:11900-11906
[20]Fujita S,Suzuki K,Ohkawa M,et al.Chem.Mater.,2003,15:255-263
[21]Miyakawa A,Kamioka H,Hirano A,et al.Phys.Rev.B,2005,73:205108-205115
[22]Li J,Huang F,Wang L,et al.Chem.Mater.,2005,17:2771-2774
[23]Li J,Huang F,Wang L,et al.J.Phys.Chem.B,2005,109:14599-14603
[24]Huang F,Li J,Wang L,et al.J.Phys.Chem.B,2005,109:12032-12037
[25]Huang F,Li J,Xian H,et al.Appl.Phys.Lett.,2005,86:3-6
[26]Song C F,Sun J Q,Li J,et al.J.Phys.Chem.C,2008,112:19061-19068
[27]Song C F,Sun J Q,Qiu S B,et al.Chem.Mater.,2008,20:3473-3479
[28]Wang L,Yan L F,Zhao P T,et al.Appl.Surf.Sci.,2008,254:4191-4200
[29]Zhao E,Wang L,Yan L F,et al.J.Appl.Polym.Sci.,2008,110:39-48
[30]Dong T,Li J,Huang F,et al.Chem.Commun,2005,21:2724-2726
[31]Gao A M,Zhu X F,Wang H J,et al.J.Phys.Chem.B,2006,110:11854-11862
[32]Wang L,Gong L,Zhao E,et al.Lett.Appl.Microbiol.,2007,45:200-205
[33]Li L C,Wang L,Yu Z,et al.Plasma Sci.Technol.,2007,9:119-126
[34]Wang Z X,Dong T,Yuan L X,et al.Energy Fuels,2007,21:2421-2432
[35]Dong T,Wang Z X,Yuan L X,et al.Catal.Lett.,2007,119:29-39
[36]Matsuishi S,Toda Y,Miyakawa M,et al.Science,2003,301:626-629
[37]Kamiya T,Aiba S,Miyakawa M,et al.Chem.Mater.,2005,17:6311-6216
[38]Kim S W,Matsuishi S,Nomura T,et al.Nano Lett.,2007,7:1138-1143
[39]Miyakawa M,Kim S W,Hirano M,et al.J.Am.Chem.Soc.,2007,129:7270-7271
[40]Hayashi K,Matsuishi S,Ueda N,et al.Chem.Mater.,2003,15:1851-1854
[41]Stoimenov P K,Klinger R L,Marchin G L,et al.Langmuir,2002,18:6679-6686
[42]Sánchez-Bajo F,Cumbrera F L,Guiberteau F,et al.Mater.Lett.,1998,33:283-297
[43]Zawrah M F,Khalil N M.Ceram.Int.,2007,33:1419-1423
[44]Hayashi K,Hirano M,Matsuishi S,et al.J.Am.Chem.Soc.,2002,124:738-739
[45]Tatar P,Kiraz N,Asiltürk M,et al.J.Inorg.Organomet.Polym.Mater.,2007,17:525-523
[46]Makhluf S,Dror R,Nitzan Y,et al.A.Adv.Funct.Mater.,2005,15:1708-1715
[47]LI Ji-Dong(李吉东),LI Yu-Bao(李玉宝),WANG Xue-Jiang(王学江),et al.J.Inorg.Mater.(Wuji Cailiao Xuebao),2006,21(1):162-168
[48]Sawai J,Shoji S,Igarashi H,et al.J.Ferment.Bioeng.,1998,86:521-522
[49]Mendonca A F,Amoroso T L,Knabel S J.Appl.Environ.Microbiol.,1994,60:4009-4014
Structure and Antibacterial Property of Na2O Doped C12A7
SHEN Jing GONG Lu LI Quan-Xin*
(Department of Chemical Physics,University of Science and Technology of China,Hefei 230026,China)
Present study provides an approach to produce the nano-crystal material of Na2O doped 12CaO·7Al2O3(C12A7-Na2O)by the sol-gel method,which was successfully applied for the antibacterial material.The nanocrystal material was investigated via X-ray diffraction(XRD),electron paramagnetic resonance(EPR),inductively coupled plasma and atomic emission spectroscopy(ICP-AES),and time of flight mass spectrometer(TOF-MS).It was found that the structure of C12A7 was not changed by doping a small quantity of Na2O.The antibacterial activities of C12A7-Na2O against Staphylococcus aureus and Escherichia coli were examined by the attachment method.The resulting particles showed an excellent antibacterial performance.The morphology change of the bacteria was observed by field emission-scanning electron microscope (FE-SEM).The antibacterial mechanism of the C12A7-Na2O material was also discussed.
sol-gel;Na2O doped;C12A7;antibacterial property
TQ455
A
1001-4861(2011)02-0353-08
2010-08-24。收修改稿日期:2010-09-21。
国家自然科学基金(No.50772107);国家“863”(No.2009AA05Z435);国家“973”(No.2007CB210206)项目资助。
*通讯联系人。E-mail:liqx@ustc.edu.cn;Tel:0551-3601118
