Enzyme-catalyzed Synthesis of Vitamin E Succinate Using aChemically Modified Novozym-435*
2011-05-15YINChunhua尹春华ZHANGCong张聪andGAOMing高明
YIN Chunhua (尹春华), ZHANG Cong (张聪) and GAO Ming (高明)
Enzyme-catalyzed Synthesis of Vitamin E Succinate Using aChemically Modified Novozym-435*
YIN Chunhua (尹春华)**, ZHANG Cong (张聪) and GAO Ming (高明)
Department of Biological Science and Technology, School of Applied Science, University of Science and Technology Beijing, Beijing 100083, China
Vitamin E succinate was synthesized in organic solvents using a modified Novozym-435 as catalyst. In order to improve the catalytic performance of Novozym-435, the enzyme was modified using acetic anhydride, propionic anhydride and succinic anhydride separately. We found that both the hydrolytic activity and the thermal stability of the modified Novozym-435 were enhanced compared with the unmodified enzyme. The modified Novozym-435 catalysts were used to synthesize the succinate derivative of vitamin E. Compared with the native Novozym-435, the catalytic activity of the modified novozym-435 in promoting the synthesis of vitamin E succinate was dramatically increased, with the novozym-435 modified with succinic anhydride (N435-S) as the most active catalyst. Conditions for the synthesis of vitamin E succinate were also optimized. A mixture of-butanol and DMSO (volume ratio of 2︰3) was the most suitable medium for the reaction, whereas the appropriate molar ratio of vitamin E to succinic anhydride and reaction temperature were 1︰5 and 40°C, respectively. Under these reaction conditions, the yield of vitamin E succinate reached 94.4%. N435-S could be reused for five batches.
lipase, vitamin E succinate, vitamin E, modification
1 introduction
Vitamin E, a major natural antioxidant, comprises a group of eight naturally occurring tocopherols and tocotrienols of subtypes α, β, γ and δ. α-tocopherol has the highest vitamin E activity among all tocopherol isoforms [1, 2]. Vitamin E has many health benefits, such as preventing cardiovascular diseases, cancers and cataracts [3-8], and finds extensive applications in cosmetics and pharmaceutical industries. However, vitamin E is readily oxidized by light, air, oxidizing agents or heat, reducing its antioxidant value. To increase its stability and/or to adjust solubility and miscibility, vitamin E is generally administered as prodrug in the form of all-rac-α-tocopheryl acetate (vitamin E acetate) or all-α-tocopheryl succinate (vitamin E succinate). The vitamin E succinate derivative (VES) of α-tocopherol has received increasing attention for its potential use against cancer [9-11]. The vitamin E esters are synthesized by chemical acylation of all-rac- α-tocopherol with carboxylic acids or acid anhydrides as acyl donors, in which metal or toxic chemicals such as tertiary amine or pyridine are used as catalysts [12, 13]. As a green alternative, the use of lipases to catalyze synthesis of vitamin E ester may be a promising method. Lipase-catalyzed reactions are superior to conventional chemical methods, owing to their mild reaction conditions, high catalytic efficiency and the inherent selectivity of natural catalysts that have been used in the synthesis of a variety of esters [14-16]. Torres. [17] described for the first time the enzymatic acylation of the phenolic group of tocopherols (vitamin E) by transesterification with vinyl acetate in 2-methyl-2-butanol, in which Novozym-435 was chosen as catalyst, reaching a yield of vitamin E acetate of about 65% after 18 days, illustrating that Novozym- 435 lipase presents low synthetic activity for vitamin E esters. Chemical modification of enzymes is widely used for improving their catalytic performance such as overall catalytic activity, stability, specificity and enantioselectivity [18-22]. Novozym-435 is the isoform B of the lipase from(CAL-B), immobilized onto a macroporous acrylic polymer resin, and is one of the most sensitive enzymes to chemical modification [18, 19, 23].
In the present work, Novozym-435 is modified using a variety of organic acid anhydrides to improve its catalytic performance. Using such modified Novozym-435 as catalyst, we determine the efficiency of the enzymatic synthesis of vitamin E succinate by lipase-catalyzed acylation of vitamin E with succinate anhydride.
2 Materials and methods
2.1 Materials
Novozym-435 (lipase from, immobilized on a macroporous acrylic resin) was purchased from Novozymes (China). Vitamin E (all-rac- α-tocopherol, 97%) was obtained from Alfa Aesar (USA). Vitamin E succinate (99.9%) was obtained from Sigma-Aldrich. HPLC-grade methanol was from Dima Technology Inc. All other chemicals were of analytical grade and obtained from Beijing Chemicals Factory (China).
2.2 General procedure for modification of Novozym-435
Novozym-435 was modified with 0.15-0.20 mol·L-1acetic, propionic or succinic anhydride dissolved in dimethyl sulfoxide [20]. 3 ml of anhydride solution was slowly added dropwise over a period of 5 min to 10 ml 0.1 mol·L-1phosphate buffer at pH 8.0 containing 1.0 g Novozym-435, under rapid stirring at 35°C. The pH was kept at a constant by adding 0.1 mol·L-1sodium hydroxide. When the addition of the organic anhydride was complete, the mixture was allowed to react for an additional 25 min. After that, the modified Novozym-435 was filtered and washed with an excess of 0.1 mol·L-1phosphate buffer, and then freeze-dried to constant mass.
2.3 Enzyme hydrolytic activity assay
The lipase hydrolytic activity was determined by the olive oil emulsion method [24]. One unit of activity is defined as the amount of enzyme that liberates 1μmol of free fatty acid from olive oil per minute at 40°C.
2.4 Thermal stability tests
The stability tests of modified enzymes were performed in 100 mmol·L-1phosphate buffer (pH 8.0) at 70°C. 5 mg of enzyme was added to 5 ml of 0.1 mol·L-1phosphate buffer at pH 8 and incubated at 70°C for a defined period of time and then the residual activity of the enzyme was determined by using the olive oil emulsion method described above.
2.5 Succinylation of vitamin E
The succinylation reaction was conducted in 50 ml glass vials with plastic screw caps. 100 mg of the native Novozym-435 or of modified Novozym-435 was added to 5 ml of DMSO/-butanol containing 1.0 mmol (0.43 g) of vitamin E and 1.0 mmol (0.10 g) of succinic anhydride. The reaction was carried out in a shaker (200 r·min-1) at 37°C in the dark. These conditions were used unless stated otherwise. At 1-4 h intervals, samples were withdrawn for HPLC analysis to determine the yield.
2.6 HPLC analysis

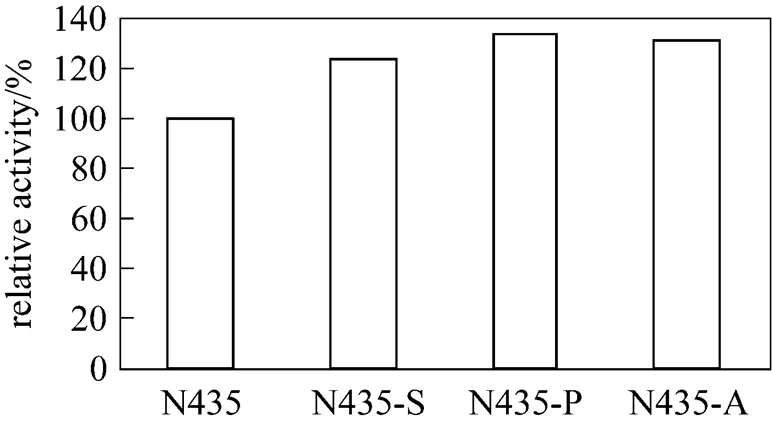
Figure 1 Comparison of activities of acetyl Novozym-435 (N435-A), propionyl Novozym-435 (N435-P), succinyl Novozym-435 (N435-S) and unmodified Novozym-435 (N435)
Figure 2 Thermal stability of N435-A, N435-S, N435-P and N435

3 Results and discussion
3.1 Hydrolytic activity and thermal stability of modified lipases
The reactions of organic acid anhydrides with the primary amino groups of enzymes can enhance catalytic activity and stability [19-22]. In the present study, Novozym-435 was modified using acetic, propionic, and succinic anhydride separately as chemical modifier. As indicated in Fig. 1, treatment of Novozym-435 with organic acid anhydrides results in a noticeable enhancement of its catalytic activity. In comparison with unmodified Novozym-435, the hydrolytic activity of the modified enzymes increases 24%-34% in phosphate buffer (pH 8.0). Among these modified Novozym-435 forms, propionyl Novozym-435 (N 435-P) exhibits the highest catalytic activity. Fig. 2 illustrates that the thermal stability of the modified Novozym-435 forms is distinctly better compared to unmodified Novozym-435. The half life of all of the modified Novozym-435 forms exceeds 13 h at 70°C, while that of the native Novozym-435 is only 5 h. These results show that the chemical modification of Novozym-435 increases the enzyme hydrolytic activity for olive oil and its thermal stability. One explanation may be the change of the net charge of the enzyme by the modifying groups. Monocarboxylic acid anhydrides (acetic and propionic anhydrides) neutralize some of the positive charges of the amino groups, whereas succinic anhydride introduces negative charges into the enzyme structure, changing the ionic interactions of the enzyme and producing positive effects on its catalytic characteristics.
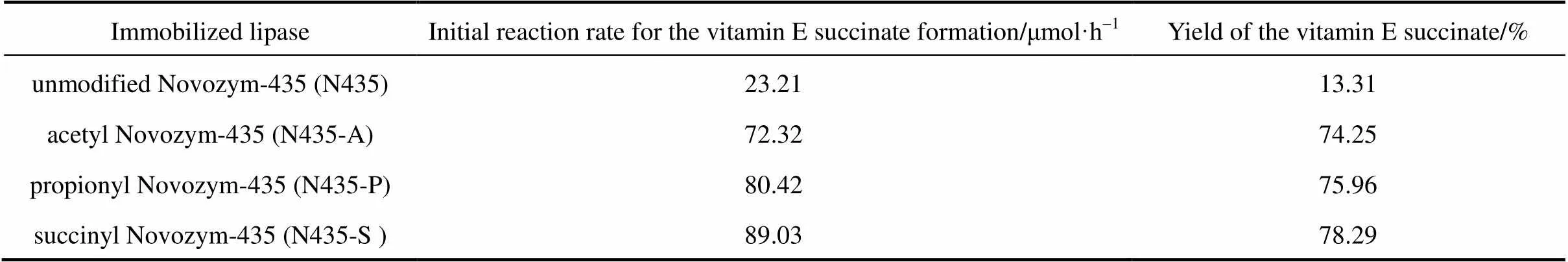
Table 1 Catalytic activity of the modified novozym-435 forms in promoting vitamin E succinate synthesis
3.2 Synthesis of vitamin E succinate using native and modified Novozym-435 forms
The reaction mixture consisted of vitamin E, 0.43 g; succinic anhydride, 0.10 g; DMSO, 5 ml and native or modified Novozym-435, 100 mg. This reaction mixture was placed in 50 ml screw-caped glass vials and incubated at 37°C under constant agitation in an orbital shaking air-bath at 200 r·min-1in the dark for 48 h. At the end of the incubation, the yield of vitamin E succinate was determined by HPLC. As shown in Table 1, all modified Novozym-435 forms exhibit good synthesis activity for vitamin E succinate, whereas native Novozym-435 is much less efficient. The modifications of novozym-435 neutralize or reverse the positive charges on the primary amino groups of the enzyme molecule owing to the negative charges of the modifying groups, which may lead to the change of the tertiary structure of the enzyme in organic solvents, increasing the activity for the synthesis of vitamin E succinate from succinic anhydride and vitamin E in organic solvents. Szabo. [20] found that the papain modified with organic acid anhydrides exhibited higher stability and catalytic activity in aqueous organic solvents than the native papain. In view of the relatively high activity for the synthesis of vitamin E succinate, modified Novozym-435 with succinic anhydride (N435-S) was used for further studies.
3.3 Effect of reaction medium on the synthesis of vitamin E succinate using N435-S
Organic solvents have various physicochemical effects on enzymes, and in particular the nature and polarity of organic media affect the activity of lipases [25]. The organic solvents change the native conformation of the enzyme by disrupting hydrogen bonds and hydrophobic interactions, changing the activity and stability [26]. lg(the logarithm of the partition coefficient of a given solvent between water and 1-octanol) is commonly used to measure the polarity of solvents and to predict enzymatic activity. Organic solvents with lgvalues <2.0 are generally not considered suitable for biocatalysts [27]. Nonpolar solvents, such as hexane and isooctane, are unable to remove substantial amounts of water from the enzyme, and therefore have little effect on the enzymatic activity [28]. In this study, eight commonly used organic solvents were tested in the lipase-catalyzed esterification of vitamin E and succinic anhydride (Table 2). The yields of vitamin E succinate did not appear to relate to lgvalues of the different solvents used. Very low yield of vitamin E succinate was achieved using nonpolar solvents such as hexane and petroleum ether. This may be due to the poor solubility of succinic anhydride in such solvents in spite of a good solubility of vitamin E. On the other hand, the yield of vitamin E succinate is higher in DMF (,-dimethylformamide) and DMSO, possibly because of the relatively better solubility of both succinic anhydride and vitamin E in these solvents. However, DMF and DMSO have a strong polarity, which reportedly has negative effects on the activity and stability of most enzymes, even disrupting their tertiary and secondary structure and thus preventing them from acting as a catalyst [29].

Table 2 Synthesis of vitamin E succinate indifferent organic solvents
Note: Reaction conditions: 0.43 g vitamin E, 0.10 g succinic anhydride, 5 ml organic solvent, 100 mg N435-S, 37°C, 48 h.
An effective strategy to achieve an optimal balance between substrate solubility and enzyme efficiency is to use a weak polar solvent such as tertiary alcohol with DMSO so as to obtain a solvent mixture of medium polarity [30]. As shown in Fig. 3, a mixture of DMSO/-butanol (volume ratio of 3︰2) is found to be optimal, yielding an effective balance between substrate solubility and biocatalyst efficiency.

Figure 3 Effect of the composition of solvent mixture (DMSO/-butanol) on the reaction yield
[Reaction conditions: 0.43 g vitamin E, 0.10 g succinic anhydride,5 ml organic solvent (DMSO/-butanol), 100 mg N435-S, 37°C, 48 h]
3.4 Effect of molar ratio of substrates on the synthesis of vitamin E succinate
The synthesis reactions were run using various molar ratios of substrates in DMSO/-butanol at 37°C. The molar ratio of the reactants was found to be an important parameter affecting the reaction equilibrium. Theoretically, excess succinic anhydride shifts the equilibrium to vitamin E succinate synthesis. In our study, the vitamin E content was kept at 1 mmoles (0.43 g) while the succinic anhydride content varied from 1.0 to 6 mmol (Fig. 4). Increasing the succinic anhydride/vitamin E molar ratio increased yields of vitamin E succinate, with a maximum yield at a ratio of 5︰1. Further increase in succinic anhydride content did not produce higher yields.

Figure 4 Effect of molar ratio of vitamin E to succinic anhydride on the yield of vitamin E succinate
[Reaction conditions: 5 ml DMSO/-butanol(volume ratio of 3︰2), 100 mg N435-S, 37°C, 48 h]
3.5 Effect of reaction temperature on the synthesis of vitamin E succinate
Temperature is another important factor affecting enzymatic activity and vitamin E stability. In this study, the optimal temperature for synthesis was 40°C, with 94.4% of input vitamin E converted into vitamin E succinate (Fig. 5). When the temperature was kept at below 30°C, the enzyme activity limited the efficacy of the synthesis reaction, whereas at higher temperature the stability of vitamin E decreased, decreasing the yield of vitamin E succinate.

Figure 5 Effect of temperature on the yield of vitamin E succinate
[Reaction conditions: 0.43 g vitamin E, 0.50 g succinic anhydride, 5 ml DMSO/-butanol(volume ratio of 3︰2), 100 mg N435-S, 48 h)
3.6 Reuse stability of N435-S
N435-S was used repeatedly to synthesize vitamin E succinate since reuse stability was important for an immobilized enzyme (Fig. 6). The reaction of each batch was carried out at 40°C for 48 h. From the first batch to the fifth batch, the yields of vitamin E succinate were all around 90%. However, from the sixth batch, the yield of vitamin E decreased obviously. Thus, the reuse of immobilized lipase was five batches.

Figure 6 Reuse stability of N435-S
[Reaction conditions: 0.43 g vitamin E, 0.50 g succinic anhydride, 5 ml DMSO/-butanol(volume ratio of 3︰2), 100 mg N435-S, 40 °C, 48 h]
4 CONCLUSIONS
Our studies have shown that Novozym-435 can be modified by acetic anhydride, propionic anhydride and succinic anhydride, thus improving enzymatic performance. The hydrolytic activity and the thermal stability of such modified Novozym-435 forms were increased in comparison with unmodified enzyme. Propionic anhydride-modified Novozym-435 (N435-P) was found to possess the highest hydrolytic activity, whereas succinic anhydride-modified Novozym-435 (N435-S) was the most efficient catalyst for the succinylation reaction of vitamin E using succinic anhydride as succinyl donor. As compared with the native Novozym-435, the catalytic activity of the modified novozym-435 for the synthesis of vitamin E succinate was dramatically increased. The conditions for the synthesis of vitamin E succinate were also optimized. The mixture of-butanol and DMSO (volume ratio of 2︰3) was the most suitable medium for the reaction, with the most effective molar ratio of vitamin E to succinic anhydride and reaction temperature being 1︰5 and 40°C, respectively. In conclusion, our collective results indicate that vitamin E succinate can be successfully synthesized using a modified Novozym- 435 as a catalyst and vitamin E and succinic anhydride as substrates, which may be a green and more practicalalternative to conventional chemical synthesis methods.
1 Valentin, H.E., Qi, Q.G., “Biotechnological production and application of vitamin E: Current state and prospects”,..., 68 (4), 436-44 (2005).
2 Barros, L., Correia, D.M., Ferreira, I. C.F.R., Baptista, P., Santos-Buelga, C., “Optimization of the determination of tocopherols in. edible mushrooms by a normal phase liquid chromatographic method”,, 110 (4), 1046-1050 (2008).
3 Diplock, A.T., “Antioxidants and disease prevention”,34, 1013-1023 (1996).
4 Knekt, P., “Vitamin E and cancer prevention”, Natural Antioxidants in Human Health and Disease, Academic Press, New York, 199-228 (1994).
5 Morrissey, P.A., Sheehy, P.J.A., “Optimal nutrition: vitamin E”,..., 58 (2), 459-468 (1999).
6 Muller, D.P.R., “Vitamin E and other antioxidants in neurological function and disease”, Natural Antioxidants in Human Health and Disease, Academic Press, New York, 535-558(1994).
7 Richard, M.J., Roussel, A.M., “Micronutrients and ageing: intakes and requirements”,..., 58 (3), 573-578 (1999).
8 Xu, Z., “Comparison of extraction methods for quantifying vitamin E from animal tissues”,.., 99 (18), 8705-8709 (2008).
9 Turley, J.M., Fu, T., Ruscetti, F.W., “Vitamin E succinate induces fas-mediated apoptosis in estrogen receptor-negative human breast cancer cells”,., 57 (5), 881-890 (1997).
10 Mokenge, P, Neitzel, L.T., “Vitamin E succinate promotes breast cancer tumor dormancy”,..., 93 (1), 163-170 (2000).
11 Quin, J., Engle, D., Litwiller, A., Peralta, E., Grasch, A., Boley, T., Hazelrigg, S., “Vitamin E succinate decreases lung cancer tumor growth in mice”,..., 127 (2), 139-143 (2005).
12 Bonrath, W., Giraudi, L., “Process for the manufacture of tocyl and tocopheryl acylates”, WO Pat., 096791 (2004).
13 Werner, B., Fabio, C., “Process for the preparation of tocol acylates and tocopherol acylates”, U.S. Pat., 6444098 (2002).
14 Yin, C., Liu, T., Tan, T., “Synthesis of vitamin A esters by immobilized. lipase in organic media”,...., 14, 81-86 (2006).
15 Adnani, A., Basri, M., Malek, E.A., Salleh, A.B., Rahman, M.B.A., Chaibakhsh, N., Rahman, R.N.Z., “Optimization of lipase-catalyzed synthesis of xylitol ester by Taguchi robust design method”,..., 31 (2), 350-356 (2010).
16 Dhake, K.P., Qureshi, Z.S., Singhal, R.S., Bhanage, B.M., “lipase B-catalyzed synthesis of acetamides using [BMIm(PF6)] as a reaction medium”,, 50 (23), 2811-2814 (2009).
17 Torres, P., Reyes-Duarte, D., Lopez-Cortes, N., Ferrer, M., Ballesteros, A., Plou, F., “Acetylation of vitamin E bylipase B immobilized on different carriers”,., 43 (2), 145-153 (2008).
18 Palomo, J.M., Fernandez-Lorente, G., Guisan, J.M., Fernandez-Lafuente, R., “Modulation of immobilized lipase enantioselectivitychemical amination”,..., 349, 1119-1127 (2007).
19 Cabrera, Z., Fernandez-Lorente, G., Fernandez-Lafuente, R., Palomo, J.M., Guisan, J.M., “Enhancement of Novozym-435 catalytic properties by physical or chemical modification”,., 44 (2), 226-231 (2009).
20 Szabo, A., Kotorman, M., Laczko, I., Simon, L.M., “Improved stability and catalytic activity of chemically modified papain in aqueous organic solvents”,., 44 (2), 199-204 (2009).
21 Khajeh, K., Naderi-Manesh, H., Ranjbarb, B., Moosavi-Movahedia, A., Nemat-Gorgania, M., “Chemical modification of lysine residues inα-amylases: Effect on activity and stability”,.., 28 (6), 543-549 (2001).
22 Sangeetha, K., Abraham, T.E., “Chemical modification of papain for use in alkaline medium”,..., 38, 171-177 (2006).
23 Palomo, J.M., Fernandez-Lorente, G., Mateo, C., Fuentes M., Fernandez-Lafuente, R., Guisan, J.M., “Modulation of the enantioselectivity ofB lipaseconformational engineering: Kinetic resolution of hydroxy-phenylacetic acid derivatives”,:, 13 (12), 1337-1345 (2002).
24 Gargouri, M., Legoy, M.D., “Bienzymatic reaction for hydroperoxide production in a multiphasic system”,.., 21 (2), 79-84 (1997).
25 Lanne, C., Boeren, S., Vos, K., Veeger, C., “Rules for optimization of biocatalysis in organic solvents”,.., 30 (1), 81-87 (1987).
26 Cremonesi, P., Carrea, P.J., Ferrara, L., Antonini, E., “Enzymatic dehydrogenation of testosterone coupled to pyruvate in a two-phase system”,..., 44, 401-407 (1974).
27 Claon, P.A., Akoh, C.C., “Enzymatic synthesis of geranyl acetate in-hexane withlipase”,....., 71 (6), 575-578 (1994).
28 Gorman, L.S., Dordick, J.S., “Organic solvents strip water of enzymes”,.., 38 (1), 75-81 (1992).
29 Knubovets, T., Osterhout, J.J., Klibanov, A.M., “Structure of lysozyme dissolved in neat organic solvents as assessed by NMR and CD spectroscopies”,.., 63 (2), 242-248 (1999).
30 Plou, F.J., Cruces, M.A., Ferrer, M., Fuentes, G., Pastor, E., Bernabe, M., “Enzymatic acylation of di- and trisaccharides with fatty acids: Choosing the appropriate enzyme, support and solvent”,.., 96 (1), 55-66 (2002).
** To whom correspondence should be addressed. E-mail: chyin@sina.com
2010-07-15,
2010-11-15.
the Fundamental Research Funds for the Central Universities and the State Key Development Program for Basic Research of China (2007CB714304).
猜你喜欢
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Effect of Boundary Layers on Polycrystalline Silicon Chemical Vapor Deposition in a Trichlorosilane and Hydrogen System*
- Experimental and CFD Study on the Role of Fluid Flow Pattern onMembrane Permeate Flux
- Separation of Eu3+ Using a Novel Dispersion Combined LiquidMembrane with P507 in Kerosene as the Carrier*
- Fabrication of SPES/Nano-TiO2 Composite Ultrafiltration Membrane and Its Anti-fouling Mechanism*
- Adsorption and Ozonation Kinetic Model for PhenolicWastewater Treatment*
- Properties of Bio-oil from Fast Pyrolysis of Rice Husk*
