Enhanced electrocatalytic activity of carbon cloth by synergetic effect of plasma and acid treatment
2021-02-27TingtingMEI梅婷婷MingGAO高明DanniLIU刘丹妮YuWANG王裕andYifanHUANG黄逸凡
Tingting MEI (梅婷婷), Ming GAO (高明), Danni LIU (刘丹妮),Yu WANG (王裕) and Yifan HUANG (黄逸凡)
1 Shenzhen Institute of Advanced Technology,Chinese Academy of Sciences,Shenzhen 518055,People’s Republic of China
2 University of Chinese Academy of Sciences, Beijing 100049, People’s Republic of China
Abstract Commercial carbon cloth (CC) is an ideal electrocatalysis material to produce oxygen evolution reaction(OER)due to its high conductive and 3D flexible structure,but the lacked active sites limit its application.For improving its OER performance, the present study proposed an effective method combining plasma and acid treatment to introduce oxygen-containing functional groups and produce more active sites on its surface.Compared to the pristine CC,the plasma and acid treated carbon cloth(PN-CC)delivers a reduced overpotential by 34.6%to achieve current density of 10 mA cm−2.The Tafel slope declines from 97.5 mV dec–1 (pristine CC) to 55.9 mV dec–1 (PN-CC), showing an increased OER kinetic.Additionally, PN-CC electrocatalyst shows outstanding stability after 5000 cycles or 25 000 s.The combination of plasma and acid treatment shows a significant potential in surface modification for electrocatalysts.
Keywords: carbon cloth, electrocatalytic activity, plasma treatment, acid oxidation
1.Introduction
Electrochemical water splitting, which consists of hydrogen evolution reaction (HER) in cathode and oxygen evolution reaction (OER) in anode, is widely acknowledged as an effective way to produce highly purified hydrogen[1,2].The OER related researches so far exhibit very sluggish kinetic due to the limitation of material performance, leading to an increasing demand of studies in the materials used as electrochemical catalysts [3–5].Precious metals, such as Ir, Rh etc, have excellent electrochemical performances, but are prone to excessive oxidation and corrosion under high potential, resulting in performance degradation [6, 7].The high cost also limits the development of precious metals in fuel cells [8].Therefore, more and more studies have been devoted to the improvement of electrochemical performance of non-precious metals.Although some non-precious metals with excellent performance have been reported, such as NiCo2O4and NiFe LDH, none of them has been industrialized [9, 10].Over the years, carbon-based materials,especially the commercial carbon cloth (CC), have attracted many researchers’attention[11,12].Owing to the advantages of 3D structures, ultra-low weights, outstanding mechanical flexibility, high porosities and accessibility, the commercial CC is very suitable for developing inexpensive and lightweight catalyst [13–15].But the lack of electrochemical active sites lowers its electrocatalytic performance.
Several methods have been developed to increase the electrochemical active sites of CC.For instance, Chenget alproved that acid oxidation of CC by concentrated nitric acid for 8 h could improve its electrocatalytic performance[16].Wuet alobtained carbon fiber with an overpotential of 224 mV at current density of 10 mA cm−2by thermal treatment in condition of 450 °C for 2 h [17].However, these treatment methods require complex and demanding conditions,such as high concentration acid, high temperature or pressure, and long treatment time.These conditions are difficult to control in industrial production and may cause structure damage.As an environmentally friendly and highly efficient surface modification technique, plasma is able to improve material surface performance without structure damage [18–20].The high-energy bombardment by plasma create cracks and defects to expose more edge sites,in terms of atomic vacancies, tensile strains or active functional groups,thereby increasing the catalytic activity of materials[21].Dixonet alimproved the electrocatalytic activity of the graphite felt electrode by O2plasma treatment.Nearly 20%functional groups including C–O, C=O and COOH were introduced to the material surface [22].Changet alimproved the electrochemical performance and wettability of CC by treating with atmospheric pressure plasma jet (APPJ) [23].As one figure of merit, APPJ does not require an expensive vacuum system and enables generation of different functional groups on material surfaces at low temperatures.It is a preferred method in areas such as coating and surface functionalization [22, 24–26].
In this paper,an effective method combining plasma and acid treatment was developed to improve the electrocatalytic performance of commercial CC.The cleaned CC was pretreated by APPJ with Ar flow, followed by acid treatment at room temperature.To study the mechanism of processes, two cleaned CCs were treated with APPJ and nitric acid separately.Several physical techniques were carried to characterize the surface properties and chemistry, and a typical three-electrode system was applied to measure the electrochemical performance of CCs,in which the CC was used as working electrode directly.After treatment,the overpotential required to produce a current density of 10 mA cm−2was reduced by 34.6%compared to the pristine CC.In this method,plasma treatment enhances the surface activity of material to facilitate the effect of acid and decrease the demands of processing time and conditions.It not only has a promised potential in surface activation and electrochemical functionalization of materials,but also has a strong universality since not closely involved with the morphology of material itself.
2.Experimental
2.1.Preparation of activated CC
The CC (W0S1002) was obtained from Ce Tech Co., Ltd Before treatment, four pieces of CC (2 cm×3 cm) were ultrasonic cleaned by ethanol and deionized water for 5 min.One of the cleaned CC was soaked into nitric acid (HNO3,65.0 wt%)at room temperature for 90 min(denoted as N-CC).Two of the cleaned CC were treated by Ar(3.1 l min−1)APPJ for 2 min, one of which was denoted as P-CC, and the other was further soaked into nitric acid(HNO3,65.0 wt%)at room temperature for 90 min (denoted as PN-CC).The remaining piece of cleaned cloth was served as control sample.
2.2.Characterizations
The morphologies of the prepared samples were analyzed by Zeiss Supra 55 field-emission scanning electron micro-scope(SEM, Carl Zeiss, Oberkochen, Germany).The surface chemical compositions were determined by x-ray photoelectron spectroscopy (XPS, Thermo Fisher ESCALAB 250 Xi,Waltham, MA, USA) and the binding energies were calibrated based on the standard C1s peak (284.5 eV).The deconvolution of XPS peak was performed by Shirley-type baseline and Gaussian line shape.The surface wettability was assessed by a water contact angle measurement (6 μl each droplets) based on the sessile drop technique on the Theta Lite instrument (WCA, Biolin Scientific, Gothenburg,Sweden).All measurements were repeated 5 times to calculate the averaged values and the standard deviation.
2.3.Catalytic activity evaluation
The electrocatalytic activity measurement was carried out on a CHI 760E electrochemical analyzer (CH Instruments, Inc.,Shanghai, China) in a typical three-electrode setup at room temperature.A platinum plate and a saturated calomel electrode (SCE) were applied as counter electrode and reference electrode respectively,while the prepared sample was directly applied as the working electrode.Linear sweep voltammetry was conducted in 1 M KOH solution with a scan rate of 5 mV s−1.All potentials measured versus the SCE were calibrated with respect to RHE scale through the equation:
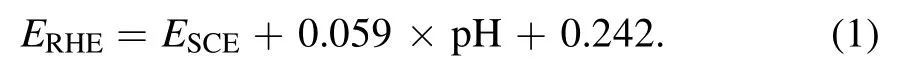
The cyclic voltammograms (CVs) were recorded at a non-Faradic voltage from −0.16 to 0.16 V at scanning rates of 25, 50, 80, 100, 150 and 200 mV s−1.The double-layer capacitance (Cdl) was calculated by equation:

where Δjwas the current density difference andrwas the scanning rate.The stability of the samples was tested by conducting CVs for 5000 cycles at a scanning rate of 100 mV s−1.Chronoamperometry was measured at the potential of 0.65 V for 50 000 s.
3.Results and discussion
3.1.Surface morphological and structural study of activated CC
The preparation process of PN-CC is shown in figure 1.In view of the insufficient active sites on the surface of CC, it cannot be directly used as an electrocatalyst.The introduction of oxygen-containing functional groups on the surface of CC is acknowledged as a good strategy to improve its OER performance,such as CO group,which has been proved to be able to increase the number of active site and enlarge the active surface area [27, 28].In our approach, we treated the commercial CC with Ar APPJ to simultaneously induce O atoms onto its surface.The nozzle of APPJ was set 10 mm away from the surface of CC, providing a processing area of 5×5 cm2at speed of 25 mm s−1,and plasma was generated under Ar gas flow (6 l min−1).The high-energy particles in plasma enhanced the surface energy and promoted the wetting properties of CC.The plasma-treated CC was then soaked in nitric acid to achieve surface oxidation and improve its compatibility with electroactive materials and electrolyte ions,thereby further improving its OER performance.In order to better understand the modification process of CC surface, we prepared plasma-treated samples (P-CC) and nitric acid-treated samples (N-CC) for comparison.
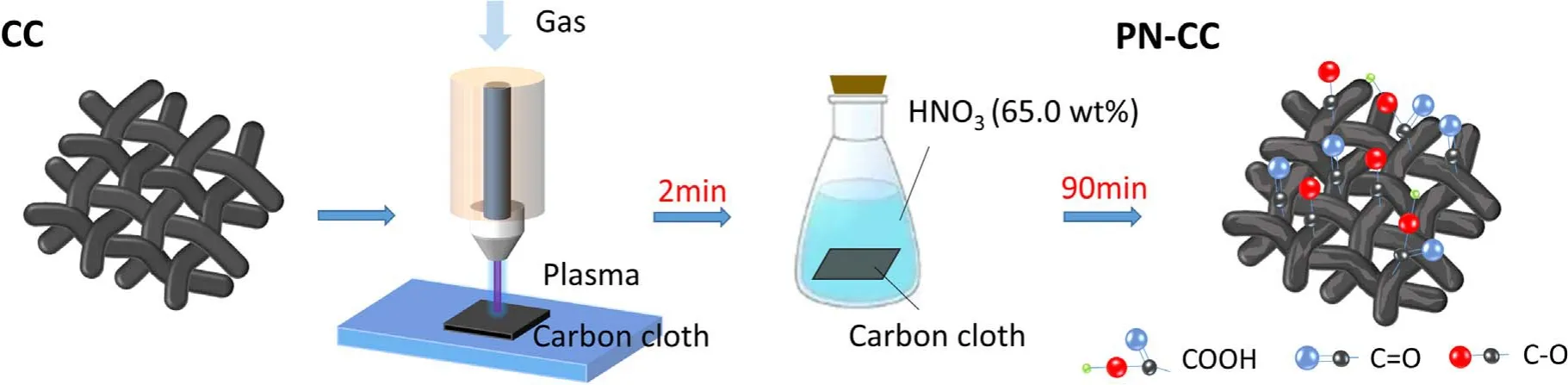
Figure 1.Schematic of the preparation of carbon cloth by plasma and acid process.
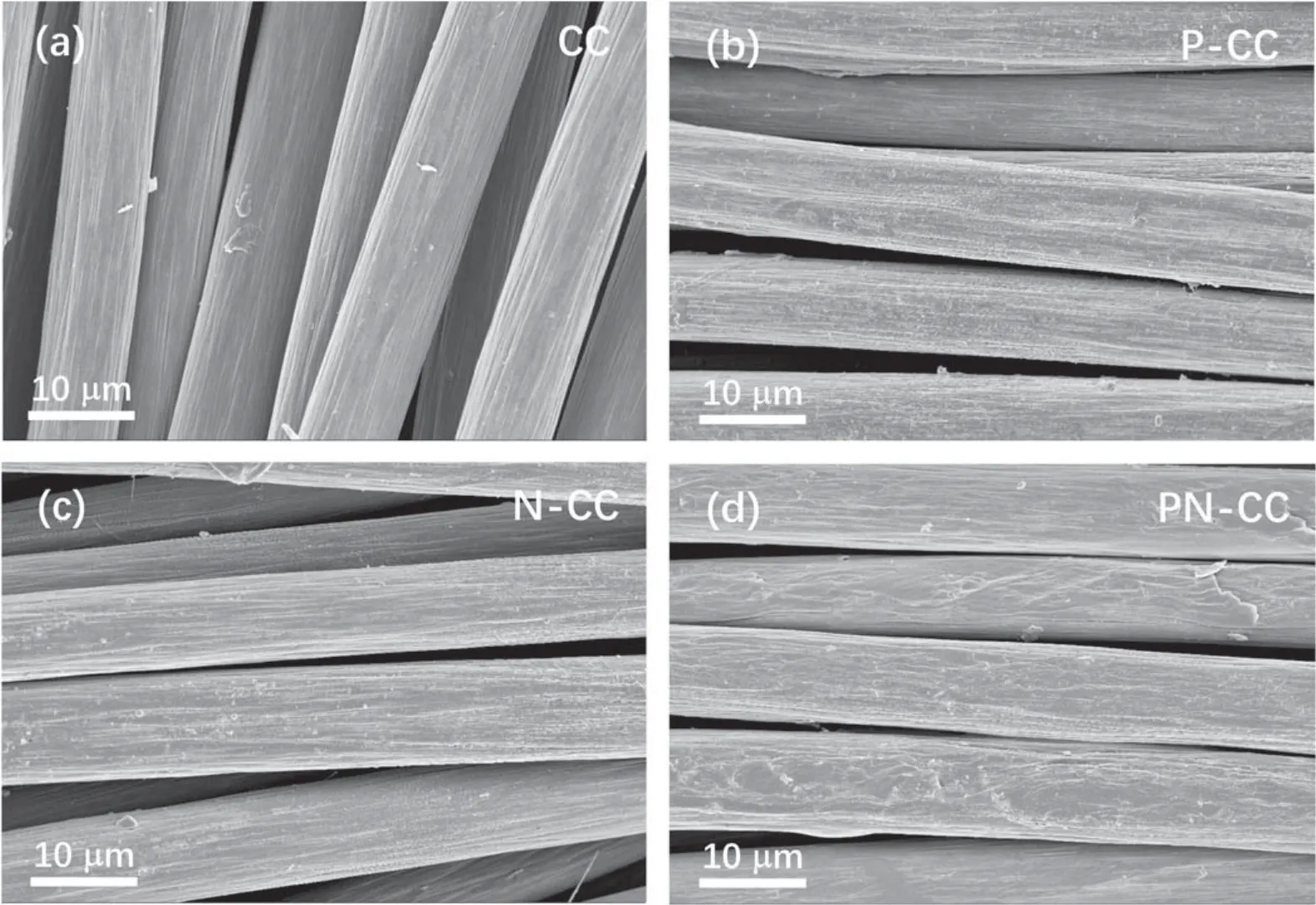
Figure 2.SEM images of (a) CC, (b) P-CC, (c) N-CC, (d) PN-CC.
SEM characterization was conducted to observe the changes of surface morphology of CC before and after treatment.As shown in figure 2, the pristine CC surface appears smooth and flat grooves, which shows the specific feature of wet-spun fibers[29].Compared to the pristine CC,the surface of CC treated by plasma (P-CC) shows a pitted etching morphology,which is caused by the bombardment of high-energy particles in plasma.After acid treatment(N-CC),the fiber surface appears concave shape.In particular, the fibers treated with plasma and acid (PN-CC) have a wavy rough surface, exhibiting the most significant surface change compared to P-CC and N-CC.This indicates that both plasma and nitric acid treatment are able to modify the surface morphology of CC and more obvious modification can be achieved when combining them.
XPS was used to analyze the surface modification of CC after plasma functionalization and acid activation.Figure 3 shows the high-resolution C1s spectra of CC, P-CC, N-CC and PN-CC, while the most prominent peaks of them, which are centered at 284.5 eV, represent C–C bonds.The C1s spectra can be deconvoluted into multiple distinct peaks,assigned to C–O (285.1 eV), C=O (287.3 eV) and COOH(288.9 eV) [30], respectively.The pristine CC contains a small amount of C–O group as shown in figure 3(a), with 1.97% oxygen atoms, which may be due to the absorption of air on the fiber surface.The C1s XPS spectrum of P-CC in figure 3(b) demonstrates that C–O and C=O groups are formed on the CC surface after plasma treatment.The high oxygen atom content of 33.5%can be ascribed to the reaction of C atoms of the CC with O2−during the plasma treatment process.The content percentage of oxygen atoms on the surface of P-CC increases dramatically from 1.97%to 33.5%.Figure 3(c) shows the C1s XPS spectrum of N-CC, confirming that acid treatment can oxidize the carbon surface.The C–O groups are generated on the CC surface after acid treatment while O atoms account for 8.45%.Moreover, the C1s XPS spectrum of PN-CC verifies that C–O–C,C=O and COOH groups are formed (figure 3(d)).The content of O atoms on the surface of PN-CC is 23.68%, which is between those of P-CC and N-CC.These results indicate that the further acid treatment can produce new carboxyl functional groups, and lose some O atoms at the same time.Overall,according to all the evidence provided, oxygen-containing functional groups have been successfully and densely introduced on the surface of CC through the combination of plasma functionalization and acid oxidation.
Figure 3.C1s XPS spectra for (a) CC, (b) P-CC, (c) N-CC, (d) PN-CC.
The surface wettability of the samples was evaluated by measuring the water contact angle (6 μl each droplet) on the surface of CC.As shown in figure 4(a), the pristine CC exhibits hydrophobic property, which has a water contact angle of around 129.84°.After Ar-APPJ treatment, the hydrophilicity of obtained P-CC has been considerably improved,as water droplets can quickly spread on the surface of P-CC, with a contact angle of only 32.15° (figure 4(b)).This may be due to the O atoms introduced on the surface of P-CC as mentioned above, which make the material more hydrophilic[31].The contact angle of N-CC is 113°,which is similar to the original, indicating that acid treatment does not have much effect on the surface hydrophilicity of the material.It is worth noting that the contact angle of the PN-CC increases from 32.15° to 92.46°, indicating a passivating effect of acid treatment on the surface of CC.The hydrophilicity of PN-CC is also between that of P-CC and N-CC,which is consistent with the O atom content on the surface of the material in XPS analysis.
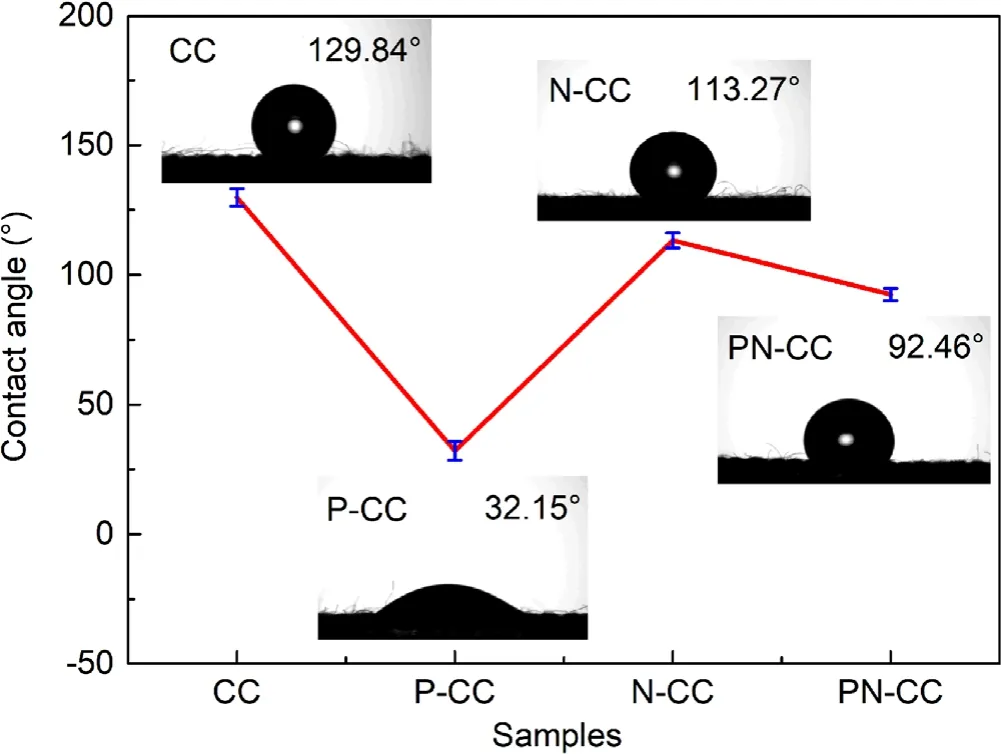
Figure 4.Contact angle results for CC, P-CC, N-CC, and PN-CC.
3.2.Electrocatalytic activity
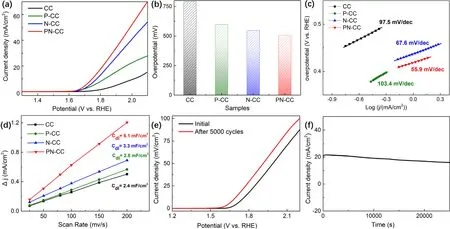
Figure 5.(a) LSV polarization curves of CC, P-CC, N-CC and PN-CC in 1 M KOH at the scan rate of 5 mV s−1, (b) the overpotential required to achieve current density of 10 mA cm−2, (c) Tafel plots of CC, P-CC, N-CC and PN-CC, (d) Δ j of as-above electrodes plotted against scan rates, the slopes represent double-layer capacitance (Cdl), (e) polarization curves for PN-CC before and after 5000 CV cycles between 0 and 1.0 V at a scan rate of 100 mV s−1, (f) the time-dependent current density curve for PN-CC at 0.6 V for 25 000 s.All the measurements were performed in 1 M KOH with iR correction.
Based on the study of surface morphology and structure,our attention is directly on the study of the OER activity of oxygen-containing functional groups on CC.It is believed that the mechanism of OER involves the adsorption of OH−and O species on the surface of working electrode (M) [32].In the alkaline electrolyte, OH−is firstly absorbed on the surface and then reacts with another OH−in electrolyte to form M‐O,which can be called secondary absorption as following:
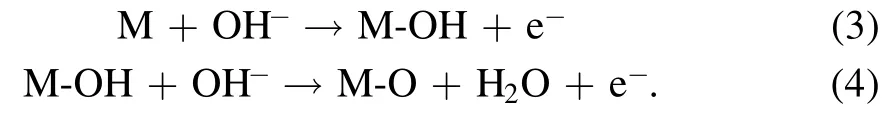
The secondary absorption has a high energy barrier and runs very slowly, which determines the overall rate of OER.Theoretically, the recombination of two M‐O intermediates can form O2directly as
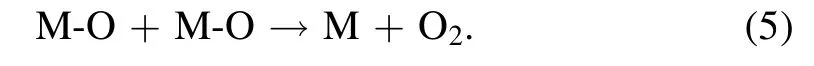
The high thermodynamic requirement of reaction(5)makes it hard to do, leading to another intermediate M–OOH formed by M–O and OH−in the solution.Finally,O2can be formed via the following two steps:
Therefore,the mechanical process of OER can almost be defined as scheme 3, 4, 6, and 7 at room temperature, in which scheme 4 is dominant [33].In order to investigate the changes in the catalytic performance of CC after treatment in terms of OER activity, the electrochemical analysis of CC was conducted under a three-electrode system in 1 M KOH solution with a scanning rate of 5 mV s−1.The prepared sample was directly applied as the working electrode,while a platinum plate and a SCE were applied as counter electrode and reference electrode respectively.After iR correction, the LSV curves of CC, P-CC, N-CC, PN-CC can be seen in figure 5(a).In the whole potential window, the pristine CC exhibits negligible current density.The onset potential of pristine CC is around 1.78 V,and it needs an overpotential of 792 mV to achieve a current density of 10 mA cm−2, which indicates a relatively poor intrinsic electrocatalytic activity.In comparison, due to the synergetic effect of plasma and nitric acid, PN-CC exhibits a significant improvement in activity after treatment, with an onset potential of ∼1.64 V.The overpotential required for PN-CC to drive a current density 10 mA cm−2is around 518 mV,which has a 34.6%reduction from the pristine CC(figure 5(b)).To analyze the contribution of plasma and acid individually, the overpotential results of P-CC and N-CC are compared.N-CC (548 mV) shows a higher activity in OER than P-CC (599 mV).
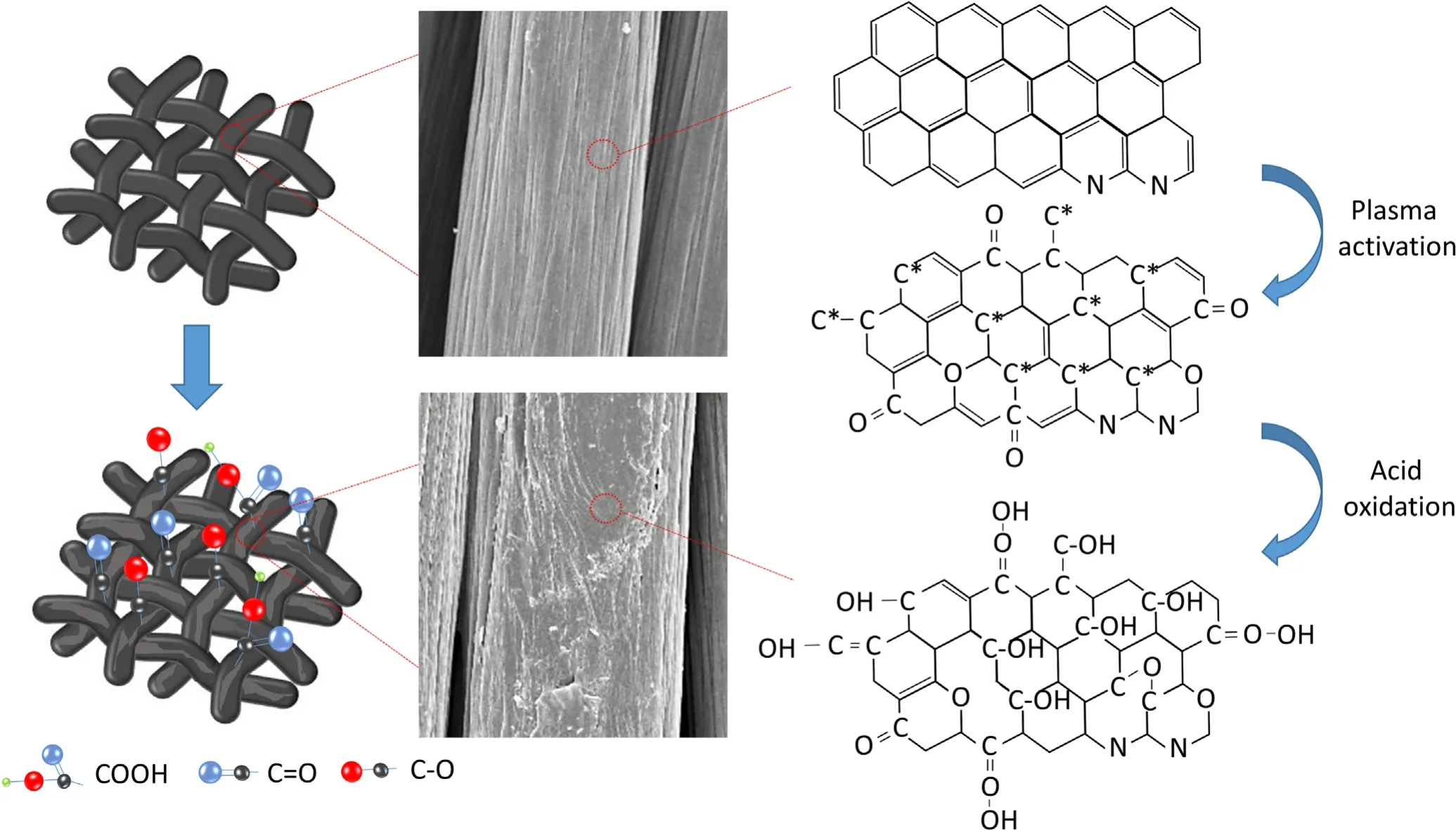
Figure 6.Schematic diagram of mechanism for improving the OER activity of CC by synergetic effect of plasma and acid treatment [40].
The superior improvement of OER activity is also reflected by the much smaller Tafel slope of PN-CC(55.9 mV dec–1)compared to that of CC (97.5 mV dec–1), indicating more favorable OER kinetics and superior catalytic activity of PNCC(figure 5(c))[34].Due to the increase of oxygen-containing functional groups on the surface of treated CC, OH−in the alkaline electrolyte is more likely to be adsorbed on the surface.The increased adsorption improves the formation rate of M‐O intermediates, thus increasing the overall OER response rate[32].However,it is interesting to notice that the Tafel slope of P-CC (103.4 mV dec–1) is slightly higher than that of pristine CC, which suggests a decrease in OER kinetics.This may be attributed to the excessive oxidation of the fiber surface, on which many O=O groups are found in C1 spectrum.In addition, the Tafel slope of N-CC (67.6 mV dec–1) is also smaller than that of P-CC (103.4 mV dec–1), suggesting that acid treatment provides a better OER kinetics than plasma treatment.Since the previous characterization results show that N-CC contains less O atoms than P-CC,there is another reason for the increase in OER kinetics.As we know, in spite of the increase of surface ion adsorption rate, the enlargement of active surface can also contribute to the improvement of electrochemical performance [35, 36].To prove this, the CV is recorded at a non-Faradic voltage ranging from −0.16 to 0.16 V at scanning rates of 25, 50, 80, 100, 150 and 200 mV s−1.The electrochemical double-layer capacitance(Cdl)is calculated by equation(2).The electrochemically active surface area(ECSA)of the working electrode can be calculated by equation:

whereCSis the specific capacitance of the sample [37].The higherCdlvalue indicates larger ECSA.Apparently,as shown in figure 5(d), PN-CC exhibits a higher current density compared with other samples at the same scanning rates.The value ofCdlfor PN-CC(6.1 mF cm−2)is 2.5 times larger than that of pristine CC (2.4 mF cm−2), and also approximately 2 times larger than those of N-CC (3.3 mF cm−2) and P-CC (2.8 mF cm−2),indicating a significant enlargement of ECSA.It is also noticed that the active surface area of N-CC is larger than that of P-CC,which can confirm the above findings that N-CC has a better electrocatalytic activity and OER kinetics.Therefore, we deduce that the effect of acid treatment is to increase the number of active surface sites.After co-processing of plasma and acid,not only a large number of oxygen-containing groups are introduced on the surface of PN-CC, but also lots of active sites are added.
The electrochemical stability is also a significant property in practical application.In this study, the stability of PN-CC was tested by CV test of 5000 cycles at a scanning rate of 100 mV s−1.Comparing the polarization curve of PN-CC after 5000 cycles of CVs with the initial PN-CC as shown in figure 5(e), it can be seen that the onset overpotential of PNCC after 5000 cycles CVs is similar,even slightly lower than that of initial PN-CC.This result indicates an excellent stability of PN-CC.Furthermore, the electrocatalytic durability of PN-CC is measured at 0.6 V as figure 5(f)shows,the timedependent current density exhibits a good stability of PN-CC,which can preserve its catalytic activity for at least 25 000 s.
It can be seen from the results that the CC treated with plasma and acid has the best electrochemical performance improvement, as a result of the synergistic effect of the two processes.The mechanism for improving the OER activity of CC is shown in figure 6.Basically,the physical and chemical interaction between plasma and material adjusts the inherent characteristics of carbon fibers by rapidly and uniformly changing the surface energy and morphology [38].This generates a large number of activated carbon atoms(C*)with unpaired electrons, which have relatively high activity and react with O2−ionized from the air quickly.Thus, many O atoms are introduced onto the surface of materials,in forms of C–O–C, C=O groups.Many recent studies have shown that oxygen-containing functional groups,such as C=O,can alter the surface electronic structure of CC.The strong electronabsorbing ability can induce the charge distribution of its adjacent carbon atoms to be positively charged, thereby increasing the electron transfer rate on the surface and the adsorption energy of intermediate products at the active site[27, 39].However, only the presence of oxygen-containing groups without enough active sites cannot meet the requirements for electrochemical performance improvement.Acid treatment can oxidize the surface of materials and provide more active sites.Cheng’s study shows that concentrated nitric acid treated CC exhibits excellent electrochemical performance, but the treatment takes long time [16].Plasma treatment actives the surface and increases its hydrophilicity, thus facilitating the effect of acid.The treatment time of acid decreases from 8 to 1.5 h with a better improved electrochemical performance.After acid treatment for 1.5 h, the PN-CC not only retains a large number of oxygen content, but also adds lots of active sites.Those oxygen-containing functional groups and active sites promote the oxygen adsorption intermediate during the electrochemical process, thus improving the electrocatalytic performance of treated CC.In addition,the surface roughness of CC is improved by the etching effect of plasma pretreatment.On one hand, the increased hydrophilicity of rough surface facilitates the processing efficiency of acid oxidation.On the other hand,the structural defects formed by plasma pretreatment can be regarded as catalytic active sites, which have been proven to directly improve its electrochemical performance [25, 41].Furthermore, the plasma treatment itself can introduce some oxygen-containing groups on the surface, contributing to not only the improvement of electrochemical performance, but also the enhanced hydrophilicity of the carbon fiber, which facilitates the oxidation efficiency of the acid treatment.From the above results, it can be inferred that the combining method of plasma and acid treatment is highly efficient and feasible in surface activation and electrocatalytic performance enhancement.
4.Conclusion
In summary, a simple and effective method combining plasma treatment and wet chemistry process has been developed to improve the electrocatalytic performance of CC.Commercial CC was treated with APPJ and nitric acid.Compared with the pristine CC, the overpotential required for PN-CC to produce a current density of 10 mA cm−2is 34.6%lower.The Tafel slope of PN-CC is reduced from 97.5 mV dec–1to 55.9 mV dec–1(CC), while it also exhibits a high catalytic activity after 5000 cycles or 25 000 s, indicating its high stability.The synergetic effect of plasma and acid treatment generates numerous oxygencontaining groups and active surface sites, contributing to the OER performance improvement.This work has provided an effective method in the improvement of electrochemical water splitting performance of commercial CC.It greatly reduces the conditions required for conventional wet chemical processing,which lays the foundation for mass production.Limited by the size and processing efficiency of the existing APPJ in the laboratory, only small-scale CC processing can be achieved temporarily, large scale processing may need to be further studied.
Acknowledgments
This work was funded by Shenzhen Science and Technology Innovation Committee (No.JCYJ20180507182200750).
杂志排行
Plasma Science and Technology的其它文章
- Effect of edge turbulent transport on scrapeoff layer width on HL-2A tokamak
- An investigation on improving the homogeneity of plasma generated by linear microwave plasma source with a length of 1550 mm
- Spatio-temporal evolution characteristics and pattern formation of a gas–liquid interfacial AC current argon discharge plasma with a deionized water electrode
- Turbulent boundary layer control with a spanwise array of DBD plasma actuators
- Plasma activation towards oxidized nanocarbons for efficient electrochemical synthesis of hydrogen peroxide
- Spatio-temporal evaluation of Zr plasma parameters in a single-beam-splitting double-pulse laser-induced plasma
