Identification of zRAP55,a gene .preponderantly expressed in StagesⅠandⅡ oocytes of zebrafish
2010-12-25ZHAOCaiLianYANGQiWenHUJiaRuiYEDingGONGWuMingHaiYingXUZongYunZHANGXunPuSONGPing
ZHAO Cai-Lian, YANG Qi-Wen, HU Jia-Rui, YE Ding, GONG Wu-Ming, LÜ Hai-Ying XU Zong-Yun, ZHANG Xun-Pu*, SONG Ping,*
(1. Fisheries College, Huazhong Agricultural University, Wuhan 430070, China; 2. Laboratory of Molecular Genetics and Developmental Biology, College of Life Science, Wuhan University, Wuhan 430072, China; 3. Department of Gynaecology and Obstetrics, South Central Hospital, Wuhan University, Wuhan 430071, China)
Identification ofzRAP55,a gene .preponderantly expressed in StagesⅠandⅡ oocytes of zebrafish
ZHAO Cai-Lian1,2, YANG Qi-Wen2, HU Jia-Rui3, YE Ding2, GONG Wu-Ming2, LÜ Hai-Ying1, XU Zong-Yun2, ZHANG Xun-Pu1,*, SONG Ping2,*
(1.Fisheries College, Huazhong Agricultural University, Wuhan430070,China;2.Laboratory of Molecular Genetics and Developmental Biology, College of Life Science, Wuhan University, Wuhan430072, China;3.Department of Gynaecology and Obstetrics, South Central Hospital, Wuhan University, Wuhan430071, China)
In anin silicosearch for gonand specific expressed genes, we have identified zRAP55 which is enriched in the ovary of zebrafish . zRAP55 encodes a protein of 382 amino acids with a highly conserved Lsm domain. zRAP55 protein shares more than 56% identities with that of other vertebrate species. RT-PCR results show that it is predominantly expressed in the ovary.In situhybridization and immunohistochemistry studies reveal thatzRAP55is ubiquitously dispersed throughout the cytoplasm of stages Ⅰ and Ⅱ oocytes, whereas no expression is observed in stages Ⅲ andⅣ oocytes. As an RNA associated protein,zRAP55might function in the control of protein translation at the early stages of oogenesis in zebrafish.
Lsm domain;zRAP55; Oogenesis; Immunohistochemistry; Zebrafish
Specialized cells called germ cells are the only ones that can give rise to an entirely new organism (Cinalli et al, 2008). Both types of the egg cells and the sperm cells are created from germ cells undergoing a particular program of meiosis and differentiation (Matova & Cooley, 2001) (Matova & Ooley, 2001). We already know that oocyte-specific factors play essential roles during oogenesis, folliculogenesis, fertilization and early embryonic development (Au et al, 2008). In addition, the abnormal expression of these genes may lead to deviant oogenesis or early embryonic development, it therefore seems important to screen and investigate oocyte-speci-fied genes.
Several genes that have been cloned and characterized are specifically or preponderantly expressed in gonads and they may play crucial roles in oogenesis and spermatogenesis (Suzuki, 2000; Gu et al, 2005; Mo et al, 2005; Zhou et al, 2006; Draper et al, 2007; Li et al , 2007; Wang et al, 2007). We show here that zebrafishRAP55(zRAP55, RNA-Associated Protein with a Molecular Mass of 55 k) is specifically expressed at early stages ofⅠ and Ⅱ oocytes in the female adult zebrafish.
The zRAP55 protein contains an Lsm domain, which is conserved in the Sm-like proteins, Lsm domain associates with small nuclear RNA to form the core domain of the ribonucleoprotein particles responsible for RNA processing including pre-mRNA splicing, telomere replication and mRNA degradation (Bachellerie & Cavaille, 1997; Belfort & Weiner, 1997; Albrecht & Lengauer, 2004). InXenopus laevis, domain analyses reveal that the Lsm domain of RAP55 protein is essential for the localization to P-bodies and translational repression (Tanaka et al, 2006). InDrosophila, the N-terminal Lsm domain of EDC3 protein is important for mRNA decapping (Tritschler et al, 2007). Our results suggested thatzRAP55is predominantly expressed in the ovary, where it is specifically expressed in stages Ⅰand Ⅱ oocytes.zRAP55might contribute to the regulation of protein translation at the early stages (Ⅰand Ⅱ) of oogenesis.
1 Materials and Methods
1.1 Animals
Adult zebrafish (Danio rerio) were raised and maintained on a 14/10 h light/dark cycle at 28.5℃ and staged as previously described (Kimmel et al, 1995; Wang et al, 2005). Adult male New Zealand white rabbits were purchased from the Experimental Animal Center of Wuhan University.
1.2 In silico subtraction
A software was programmed by our lab to search some previously uncharacterized genes that were specifically or predominantly expressed in germ cells of zebrafish or mouse (Gu et al, 2005; Mo et al, 2005; Li et al, 2007; Wang et al, 2007). By the same way, a gene which is preponderantly expressed in ovary of zebrafish was identified in this paper.
1.3 RT-PCR
Total RNA was isolated from the various tissues of the adult zebrafish. Reverse transcription polymerase chain reaction (RT-PCR) was carried out to observe the expression ofzRAP55mRNA in these organizations by using the following primers: zRAP55RTf (5′-tgc tat tgt cca gtc ttc tgt cgg-3′) and zRAP55RTr (5′-ttc tgg tct tcg atc tct ttc tcc-3′) (Song et al, 1999).
1.4 Bioinformatics analysis of zRAP55
The nucleotide sequence and deduced amino acid sequence ofzRAP55were analyzed using the NCBI blast N server, ensembl blast X server, Tmpred program, Compute tools and EXPASY Proteomics respectively. Multiple alignments were carried out by using software Clustal W (Thompson et al, 1994).
1.5 Expression of zRAP55 fusion protein and preparation of its antiserum
Primers were designed based on the nucleotide sequence of zRAP55 (The GenBank accession number: NM_200171): zRAP55F (5′-agc gga act ccc tac atc-3′) and zRAP55R (5′-ctc gta ggc agc acc aaa ccc-3′), with EcoRI and XhoI sites on them, the PCR product was inserted into pGEX-6P-1 expression vector to generate fusion proteins. The bacterial culture was induced with IPTG (final concentration is 1 mmol/L), then the recombinant GST-fusion proteins were purified to raise antiserum by immunization of rabbit (Koyano et al, 1997; Li et al, 2007).
1.6 In situ hybridization and western blots
Whole mountin situhybridization (WISH) and western blots are conducted as standard protocol (Mo et al, 2005; Yang et al, 2010). Fluorescent doublein situhybridization was performed essentially as described (Jowett, 2001).
1.7 Immunohistochemistry
The ovary tissue was isolated from adult zebrafish and fixed in 4% PFA/PBS (pH 7.2) for 24 h at 4℃. Sections of 18 µm were prepared and were rinsed three times for 5 min with PBST (PBS with 1‰ Triton X-100 and 5% serum of newborn calf) and then subsequently blocked with PBS with 10% serum of newborn calf for 2 hr. The sections were incubated in the blocking solution containing the primary antibody (1:100) overnight at 4℃. Controls were incubated with similarly diluted preimmune serum. The sections were then washed with PBST for 4 times at least 10 min each. Thereafter, they were incubated with the FITC-labeled goat anti–rabbit IgG antibody (diluted 1:500; Proteintech Group, Inc. China. Product # 00003-2) overnight at 4℃. Finally, sections were rinsed again with PBS for four times mounted using vectashield (Vector Laboratories, Inc. U.S.A .Product # H-1000) and observed under a Leica fluorescence microscope.
2 Results
2.1 Genomic structure analysis of zRAP55
The cDNA ofzRAP55is 1699 nucleotides in length and contains an open reading frame of 1,149 nucleotides, with a 115 bp 5′untranslated region (5′UTR) and a 3′untranslated region (3′UTR) of 435 bp, and the 3′untranslated region contains a polyadenylation signal (AATAAA) (Fig. 1).ThezRAP55gene consists of nine exons and eight intervening introns, which span approximately 13.9 kb (997242-1011149 bp) on the zebrafish chromosome 23. The exon/ intron structure of the gene is determined by comparison of the cDNA to the genomic sequence at National Center for Biotechnology Information (NCBI), and the exon–intron boundaries strictly agree with the ‘‘GT-AG’’ rule (Tab. 1).The open reading frame initiates in exon 1 and stops in exon 8 (Fig. 1).

Fig. 1 Schematic representation of zRAP55 gene and its encoded protein
2.2 Predicted protein sequence analysis of zRAP55
The predicted zRAP55 protein contains 382 amino acids with a molecular weight of 41.55 k and the theoretical isoelectric point is 10.11. A transmembrane region is predited at amino acids 124 - 142.
Multiple sequence alignment reveals that the zRAP55 protein shares high sequence identities with several known protein, includingXenopus tropicalis(59.7%),Mus musculus(57.3%),Homo sapiens(57.2%) andRattus(56.7%). The Lsm domain is highly conserved (Fig. 2). Comparative analysis on protein sequence of Lsm domain from different species, we found that the Lsm domain from zRAP55 shares 92.5% identities with the Lsm domain fromMus musculus,Homo sapiensandRattus. Further, Lsm domain of zRAP55 shares the high homology (91% identity) with that ofXenopus tropicalis. In addition, the glutaminerich region was only identified in the zRAP55 protein but not in the proteins from other species (Mus musculus, Xenopus tropicalis, Homo sapiensandRattus).
2.3 The expression of zRAP55 mRNA in adult zebrafish tissues
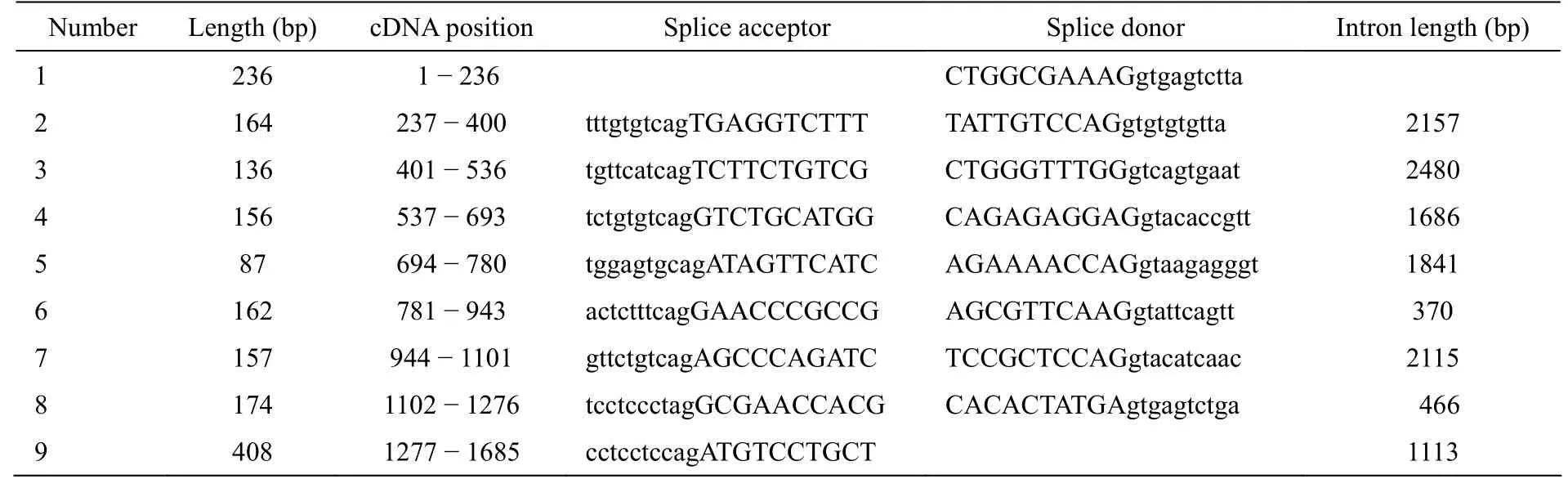
Tab. 1 The Exon-Intron analysis of zRAP55
By analyzing the tissue distribution ofzRAP55mRNA using RT-PCR, we identified that thezRAP55mRNA is abundantly present in ovary and very weak in kidney and liver, but not in brain, testis and heart (Fig. 3).
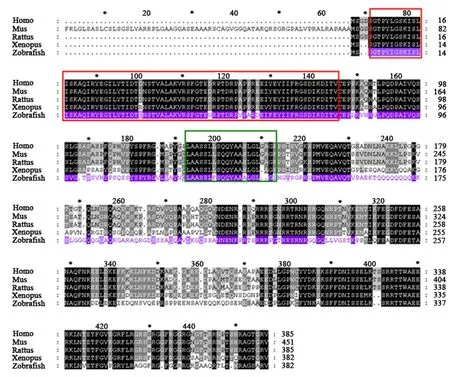
Fig. 2 Multiple protein sequence alignment in different species

Fig. 3 The RT-PCR analysis of zRAP55 expression in multiple tissues
To analysis thezRAP55expression level in the ovary, whole mount double fluorescencein situhybridization (FISH) was carried out , the signal was obviously appeared in early stages Ⅰ and Ⅱ oocytes. But there existed no detectable signals throughout the stages of Ⅲ and Ⅳ oocytes (Fig. 4A-C). Whole mountin situhybridization (WISH) with DIG-labeled RNA probes also denoted the significant expression ofzRAP55in stages of Ⅰ and Ⅱ and fade in older stages (Fig. 4D).
2.4 The expression of zRAP55 protein
For the investigation of zRAP55 protein expression in ovary of zebrafish, an antibody panel with epitopes spread over the zRAP55 protein (Fig. 2) was selected and the rabbit polyclonal antibody zRAP55 was raised against amino acids 3 - 244 of the zebrafish zRAP55.
Analysis of the distribution characteristic of zRAP55 protein in ovary by fluorescence immunohistochemistry revealed thatzRAP55is highly expressed in early stages Ⅰ and Ⅱ oocytes and the signal is evenly distributed throughout the cytoplasm (Fig. 5B, C and D). with the progression of oocyte growth, no signal could be detected in stages Ⅲ and Ⅳ oocytes, same result was got by whole mount immunohistochemistry using the intact ovary (Fig. 5E). In western blots, the band of 41.55 k was specifically recognized in ovary but absent from other tissues (Fig. 5F).
3 Discussion
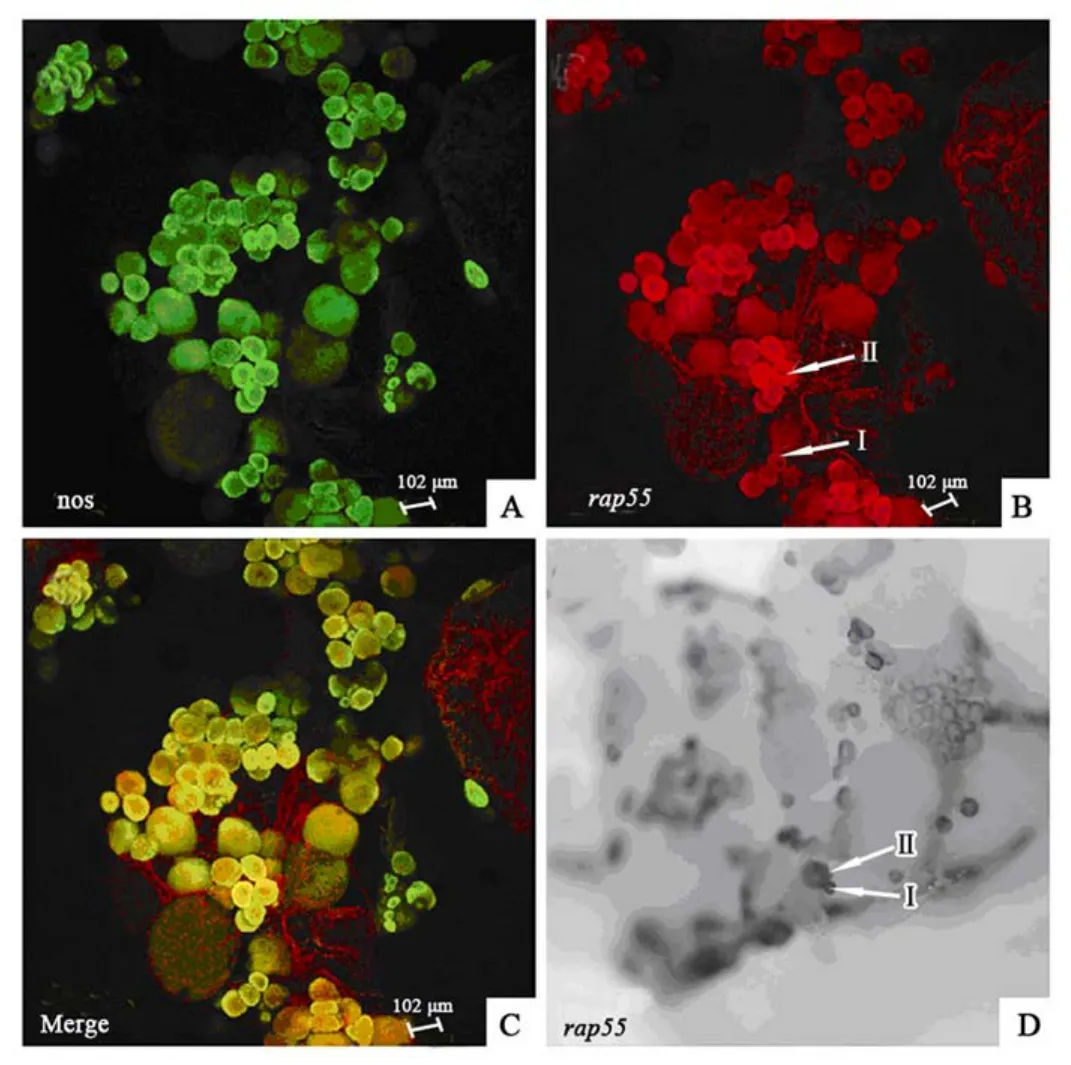
Fig. 4 The expression of zrap55 and zebrafish nanos mRNA in ovary of adult zebrafish

Fig. 5 The expression of ZRAP55 protein in ovary of adult zebrafish
Oocyte development in the ovary can be divided into four stages in zebrafish (Selman et al, 1993; Zeng & Gong, 2002). Stage Ⅰ is a stage of primary growth when the large nucleus (germinal vesicle) is surrounded by cytoplasm. Stage Ⅱ (cortical alveoli stage) is characterized by the appearance of cortical alveoli and the enrichment of vitelline envelope. In stage Ⅲ(vitellogenic stage), yolk proteins and yolk bodies with crystalline yolk appear in oocytes. In stage Ⅳ(maturation stage), the yolk becomes non-crystalline as they undergo final meiotic and the eggs are able to fertilize. At present, some oocyte-specific genes which expressed in early oocyte development have been cloned and they play essential roles during early oogenesis and embryonic development in the zebrafish (Koyano et al, 1997; Ramachandra et al, 2007). For example, Nanos1 is expressed in early stage oocytes (stage Ⅰ) and is required for the continued production of oocytes in zebrafish (Draper et al, 2007). XSox3 protein is specially expressed in Ⅰ and Ⅱ oocytes and may be concerned with early oogenesis as a transcription factor (Koyano et al, 1997). Our results indicated that thezRAP55is expressed at the early stages of oogenesis (stages I andⅡ )(Fig. 5) .Thus,zRAP55is likely to play a role at early stages of oogenesis in zebrafish.
Sm and Sm-like proteins of the Lsm (like Sm) domain family are suggested to function in RNA-processing tasks. Five novel groups of Lsm domain proteins (Lsm12 - 16) with long C-terminal tails were discovered and all of them were commonly involved in RNA metabolism. Lsm14 is part of mRNP particles and exclusively found in oocytes (Lieb et al, 1998). RAP55 mediates translational repression through its Lsm domain which recruits RAP55-bound mRNAs to specific sites for translational repression in the oocytes (Albrecht & Lengauer, 2004). The RNA-binding Sm and Sm-like proteins are characterized by mRNA decapping and pre-mRNA splicing activities (Bravo et al, 2005). Recently it is testified that RAP55 is oocyte-specific as a component of ribonucleoprotein particles.Xenopus RAP55is observed in the oocytes and early embryos and its N-terminal region including the Lsm domain plays an important role for the localization to P-bodies and translational repression (Tanaka et al, 2006). It is possible thatzRAP55is involved in regulation of protein translation in stages Ⅰ and Ⅱ oocytes.zRAP55gene can also be used as an ideal molecular marker at the early stages of oogenesis.
Albrecht M, Lengauer T. 2004. Novel Sm-like proteins with long C-terminal tails and associated methyltransferases[J].FEBS Lett, 569: 18-26.
Au PC, Whitley J, Vaux D, Selwood L, Familari M. 2008. Identification of novel and known ovary-specific genes including ZP2, in a marsupial, the stripe-faced dunnart[J].Mol Reprod Dev, 75: 318-325.
Bachellerie JP, Cavaille J. 1997. Guiding ribose methylation of rRNA[J].Trends Biochem Sci, 22: 257-261.
Belfort M, Weiner A. 1997. Another bridge between kingdoms: tRNA splicing in archaea and eukaryotes[J].Cell, 89: 1003-1006.
Bravo J, Aguilar-Henonin L, Olmedo G, Guzman P. 2005. Four distinct classes of proteins as interaction partners of the PABC domain ofArabidopsis thalianaPoly (A)-binding proteins[J].Mol Genet Genomics, 272: 651-665.
Cinalli RM, Rangan P, Lehmann R. 2008. Germ cells are forever[J].Cell, 132: 559-562.
Draper BW, McCallum CM, Moens CB. 2007. Nanos1 is required to maintain oocyte production in adult zebrafish[J].Dev Biol, 305: 589-598.
Gu S, Hu J, Song P, Gong W, Guo M. 2005. Identification of a new transcript specifically expressed in mouse spermatocytes: mmrp2[J].Mol Biol Rep, 32: 247-255.
Jowett T. 2001. Doublein situhybridization techniques in zebrafish[J].Methods, 23: 345-358.
Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. 1995. Stages of embryonic development of the zebrafish[J].Dev Dyn, 203: 253-310.
Koyano S, Ito M, Takamatsu N, Takiguchi S, Shiba T. 1997. TheXenopus Sox3gene expressed in oocytes of early stages[J].Gene, 188: 101-107.
Li B, Wu G, Hu J, Gong W, Yue M, Song P. 2007. Identification and characterization of TSAP, a novel gene specifically expressed in testis during spermatogenesis[J].Mol Reprod Dev, 74: 1141-1148.
Lieb B, Carl M, Hock R, Gebauer D, Scheer U. 1998. Identification of a novel mRNA-associated protein in oocytes of Pleurodeles waltl andXenopus laevis[J].Exp Cell Res, 245: 272-281.
Matova N, Cooley L. 2001. Comparative aspects of animal oogenesis[J].Dev Biol, 231: 291-320.
Mo S, Song P, Lv D, Chen Y, Zhou W, Gong W, Zhu Z. 2005. Zebrafish z-otu, a novel Otu and Tudor domain-containing gene, is expressed in early stages of oogenesis and embryogenesis[J].Biochim Biophys Acta,1732: 1-7.
Ramachandra RK, Lankford SE, Weber GM, Rexroad CE, 3rd, Yao J. 2007. Identification of OORP-T, a novel oocyte-specific gene encoding a protein with a conserved oxysterol binding protein domain in rainbow trout[J].Mol Reprod Dev, 74: 502-511.
Selman K, Wallace RA, Sarka A, Qi X. 1993. Stages of oocyte development in the zebrafish,Brachydanio rerio[J].J Morphol, 218: 203-224.
Song P, Malhotra P, Tuteja N, Chauhan VS. 1999. RNA helicase-related genes ofPlasmodium falciparumandPlasmodium cynomolgi[J].Biochem Biophys Res Commun255: 312-316.
Suzuki M MM, Hirabayashi K, Ogawara M, Nishiahara M,Takahashi M. 2000. Oocyte-specific expression of granulin precursor (acrogranin) in rat ovary[J].J Reprod Dev, 46: 271-277.
Tanaka KJ, Ogawa K, Takagi M, Imamoto N, Matsumoto K, Tsujimoto M. 2006. RAP55, a cytoplasmic mRNP component, represses translation inXenopusoocytes[J].J Biol Chem, 281: 40096-40106.
Thompson JD, Higgins DG, Gibson TJ. 1994. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice[J].Nucleic Acids Res, 22: 4673-4680.
Tritschler F, Eulalio A, Truffault V, Hartmann MD, Helms S, Schmidt S, Coles M, Izaurralde E, Weichenrieder O. 2007. A divergent Sm fold in EDC3 proteins mediates DCP1 binding and P-body targeting[J].Mol Cell Biol, 27: 8600-8611.
Wang F, Hu J, Song P, Gong W. 2007. Two novel transcripts encoding two Ankyrin repeat containing proteins have preponderant expression during the mouse spermatogenesis[J].Mol Biol Rep, 34: 249-260.
Wang PJ, Chien MS, Wu FJ, Chou HN, Lee SJ. 2005. Inhibition of embryonic development by microcystin-LR in zebrafish,Danio rerio[J].Toxicon, 45: 303-308.
Yang Q, Hu J, Ye D, Zhao C, Song S, Gong W, Tan Z, Song P. 2010. Identification and expression analysis of two zebrafishE2F5genes during oogenesis and development[J].Mol Biol Rep, 37: 1773-1780.
Zeng S, Gong Z. 2002. Expressed sequence tag analysis of expression profiles of zebrafish testis and ovary[J].Gene, 294: 45-53.
Zhou W, Song P. 2006. Molecular cloning of a novel gene ZAhi-1 and its expression analysis during zebrafish gametogenesis[J].Mol Biol Rep, 33: 111-116.
在斑马鱼Ⅰ和Ⅱ期卵母细胞中优势表达基因zRAP55的鉴定
赵彩莲1,2,杨启文2,胡珈瑞3,叶 鼎2,龚午鸣2,吕海英1,徐宗芸2,张训蒲1,*,宋 平2,*
(1. 华中农业大学 水产学院,武汉 430070;2. 武汉大学 生命科学学院分子遗传与发育实验室,武汉 430072;3. 武汉大学中南医院 妇产科,武汉 430071)
通过性腺特异性表达基因的筛选,作者发现了一个在斑马鱼卵巢中富集的基因zRAP55。zRAP55蛋白由382个氨基酸组成并含有一个高度保守的Lsm区。zRAP55蛋白与其他脊椎动物一致性在56%以上。RT-PCR结果表明,zRAP55优势表达于卵巢中。原位杂交和免疫组织化学结果表明:在Ⅰ期和Ⅱ期卵母细胞中,zRAP55的阳性信号强烈,均匀地分布于整个细胞质中,但是在Ⅲ期和Ⅳ期卵母细胞中均检测不到信号。作为一个RNA相关蛋白,zRAP55可能在早期卵母细胞中具有调节蛋白质翻译的重要作用。
Lsm区;zRAP55;卵母细胞;免疫组织化学;斑马鱼
Q959.468;Q133;Q786
A
0254-5853-(2010)05-0469-07
2010-03-15;接受日期:2010-08-11
国家基础研究项目(2004CB117400);国家自然科学基金(30150005;30270675)
赵彩莲,E-mail:cailian305@yahoo.com.cn
10.3724/SP.J.1141.2010.05469
date: 2010-03-15; Accepted date: 2010-08-11
s: This investigation was supported by grants from the National Basic Research Program of China (2004CB117400); the National Natural Science Foundation of China (30150005; 30270675)
*通讯作者(Corresponding authors),E-mail:pingsongs@gmail.com
猜你喜欢
杂志排行
Zoological Research的其它文章
- Rates and patterns of microsatellite mutations in common carp (Cyprinus carpio L.)
- Ethogram of Yangtze finless porpoise calves (Neophocaena phocaenoides asiaeorientalis)
- Embryonic development of the concave-eared torrent frog with its significance on taxonomy
- Seasonal variation and synchronization of sexual behaviors in free-ranging male Tibetan macaques (Macaca thibetana) at Huangshan, China
- Niche partitioning between sympatric rhesus macaques and Yunnan snub-nosed monkeys at Baimaxueshan Nature Reserve, China
- 小杜鹃对强脚树莺的巢寄生及其卵色模拟
