Seasonal variation and synchronization of sexual behaviors in free-ranging male Tibetan macaques (Macaca thibetana) at Huangshan, China
2010-12-25XIADongPoLIJinHuaZHUYongSUNBingHuaLoriSHEERANMeganMATHESON
XIA Dong-Po, LI Jin-Hua,*, ZHU Yong, SUN Bing-Hua, Lori K SHEERAN, Megan D MATHESON
(1. School of Life Science, Anhui University, Hefei 230039; 2. Anhui Key Laboratory for Eco-engineering and Bio-techniques, Hefei 230039; 3. Anhui Research Center of Ecological Economy, Hefei 230039; 4. Department of Anthropology, Central Washington University, Ellensburg, WA 98926; 5. Department of Psychology, Central Washington University, Ellensburg, WA 98926)
Seasonal variation and synchronization of sexual behaviors in free-ranging male Tibetan macaques (Macaca thibetana) at Huangshan, China
XIA Dong-Po1,2,3, LI Jin-Hua1,2,3,*, ZHU Yong1,2,3, SUN Bing-Hua1,2,3, Lori K SHEERAN4, Megan D MATHESON5
(1.School of Life Science, Anhui University, Hefei230039; 2.Anhui Key Laboratory for Eco-engineering and Bio-techniques, Hefei230039; 3.Anhui Research Center of Ecological Economy, Hefei230039; 4.Department of Anthropology, Central Washington University, Ellensburg, WA98926; 5. Department of Psychology, Central Washington University, Ellensburg, WA98926)
Although seasonal breeding has been documented in many non-human primates, it is not clear whether sexual behaviors show seasonal variation among male individuals. To test this hypothesis, the focal animal sampling method and continuous recording were used to investigate seasonal variation and synchronization of sexual behaviors in five male Tibetan macaques (Macaca thibetana) at Mt. Huangshan from Oct 2005 to Sept 2006. Both copulatory and sexually motivated behaviors (i.e., sexual chase, grimace, and sexual-inspection), which were significantly higher in the mating season than non-mating season. Furthermore, seasonal variations of sexual behaviors, including copulatory and sexually motivated behaviors, were synchronized among males. The results shed light on sexual competition and tactics for reproductive success of maleM. thibetanaand other non-human primates with seasonal breeding.
Tibetan macaques (Macaca thibetana); Males; Sexual behavior; Seasonal variation; Synchrony
Seasonal breeding (reviewed in Prendergast et al, 2002) and reproductive synchrony (Connor et al, 2006; MacFarlane & Coulson, 2005) have been well documented in female mammals, including non-human primates (Setchell & Wickings, 2004; Li et al, 2005; Sharma et al, 2006). For these species female distribution and reproductive status are the key factors for males to respond to ensure reproductive success (Bateman, 1948;Strier, 2003; Herndon, 1983). Theoretical and empirical studies have shown that males should attempt to fertilize as many females as possible, in part because sperm is arguably the less costly gamete (Bateman, 1948; Roughgarden et al, 2006). However, high energetic and reproductive costs may be imposed on males through sperm production (Nakatsuru & Kramer, 1982), ejaculations (Thomsen et al, 2006) and intermale competition. Males might maximize mating opportunities by coordinating their behaviors with females’ physiological conditions (Trivers, 1972). In species exhibiting multi-male grouping patterns and strong seasonal breeding cycles, the reproductive behaviors of males and females should be strongly coordinated. There are much more sexually receptive females in the mating season than the non-mating season (Li, 1999; Bercovitch, 1987a; Li et al, 2005), which hypothetically allows the males to concentrate their reproductive efforts during the mating season (also see in time budget dilemma; Bercovitch, 1987b). It is important for males to determine the time when sexually receptive females are available and to concentrate mating efforts at those times (Bercovitch, 1987b; Gordon & Bernstein, 1973); sexual behaviors in males are expected to show synchronously seasonal variation with females’ seasonal strategies (Bateman, 1948; Strier, 2003). However, this hypothesis has not been yet been tested in free-ranging male non-human primates.
Tibetan macaques (Macaca thibetana) live in multi-male, multi-female groups with strictly linear dominance hierarchies (Li, 1999). Although seasonal breeding has been the focus of considerable research in Tibetan macaques, a number of criticisms of these researchers have been raised. Firstly, the occurrence of seasonal mating in Tibetan macaques is still debated. Wada & Xiong (1996) demonstrated that Tibetan macaques are non-seasonal mating, but further studies manifested the seasonal mating in Tibetan macaques (Xiong, 1998; Li et al, 2005). Secondly, previous studies pointed out that the mating season in Tibetan macaques is between July and December (Xiong, 1998; Li et al, 2005), and the non-mating season is from February to June (Li, 1999) in female Tibetan macaques, however, comparable data were not collected on males. Thirdly, male sexual behavior includes not only copulatory behavior, but also sexual effort (Zhang et al, 2010) or motivation (Wallen, 2001). Few studies have focused on the latter aspect of male sexual behavior (Gordon & Bernstein, 1973), and no studies of sexually motivated behaviors in Tibetan macaques. Free-ranging Tibetan macaques provide an opportunity to study seasonal variation of sexual behaviors in male non-human primates. This study also provides insight into the character of sexual behaviors of similar species.
In the present study, we hypothesized that sexual behaviors in males show seasonal variation due to males’adaption to seasonal changes in females’ sexual behavior (Bateman, 1948; Strier, 2003; Herndon, 1983). The following predictions should be valid: (1) copulatory behaviors show seasonal variation in male Tibetan macaques; (2) sexually motivated behaviors should vary with season; (3) in order to maximize mating opportunities and reproductive success (Lack, 1968), males should exhibit synchronized sexual behaviors, which mean all the males show sexual behaviors at the same period. We collected behavioral data from five target males during the period from October 2005 to September 2006, to verify these predictions.
1 Material and Methods
1.1 Study site and subjects
This study was conducted over a one-year period from October 2005 to September 2006 at Mt. Huangshan National Reserve located in Anhui Province, China. The reserve is a World Culture and Nature Heritage site that is well-known as a tourist destination and research site for the study of Tibetan macaques (Macaca thibetana). The reserve (Mt. Huangshan) reaches an altitude of 1,841 m above sea level, and covers an area of 154 km2(Li, 1999; Li et al, 2005). Two non-human primate species,Macaca mulattaandM. thibetana, are found in this mountain reserve. TheM. mulattaranges below 600 m andM.thibetanaabove 600 m (Wada et al, 1987). Like many other species in the genusMacaca, Tibetan macaques live in multi-male/ multi-female groups. There are several groups of Tibetan macaques found throughout the reserve (Berman & Li, 2002; Xia et al, 2008b).
The Tibetan macaque group of focus in the present study was the Yulinkeng 2 (YA2) group, which fissioned from the Yulinkeng 1(YA1) group in 1996 (Li et al, 1996). These two groups are located in an area within the reserve known as the “Valley of the Monkeys” (N30° 04′25.1′′/E118°08′59.3′′) characterized by the extremely steep, mountainous terrain. Both groups are habituated to people and receive the modest daily provisioning of corn by the reserve staff to maintain their presence at the designated tourists viewing sites. A detailed account of the groups’ history and demography can be found in Li (1999).
Behavioral data was collected from five adult males in the YA2 group throughout the one-year study. During the study period, the group grew from 37 to 42 animals and was composition of 7 adult males, 7 adult females, 22 subadults/juveniles, and 2 - 7 infants (5 were born during the study period). The five subject males (GBZ, CT, YX, JT, and BL) selected for study were thoroughly habituated to humans and readily distinguishable by facial/body characteristics. Two other adult males were not included as subjects as one was not well habituated to humans and so difficult to sample, the other had lost one testicle during a fight several years earlier, this likely impacted his sexual behavior in unknown ways.
1.2 Behavioral data collection
All behavioral data were collected by a single observer (Xia), during an intensive study period totally 303 days from October 2005 to September 2006 (mean =25 days/month, range=15 - 31). Behavioral observation began at approximately 08:00 and concluded at 17:30 each day. The observer maintained an observation distance of 5 – 10 m from monkeys. Focal animal sampling method and continuous recording (using a digital voice recorder) were used to score copulatory and sexually motivated behaviors (Altmann, 1974). The order of observation for focal animals was determined daily via random selection. Focal sample duration was set at 20 min (Li et al, 2005; 2007). If a given focal monkey could not be found at its sampling time, or was lost from view during sampling, the observer chose the next monkey in order and returned to the previous monkey when he reappeared (Li et al, 2005; 2007). Focal sampling yielded a total of 452 h of data and was evenly distributed between the mating {226 h [monthly Mean ±SD= (45.2 ±2.43) h,n=5]} and non-mating seasons{226 h [monthly Mean ±SD= (45.2±3.73) hn=5]}Data collected were approximately equivalent among the five males (range: 80-94.3 total h per male).
According to the definition of the mating season in female Tibetan macaques (Xiong, 1998; Li, 1999), the mating season and non-mating season are defined from July to January next year and between February and June, respectively. And sexual behavior is defined as copulatory behavior and sexually motivated behaviors, including sexual chase, grimace, and sexual-inspection (Li, 1999; Tab. 1).

Tab. 1 Behavioral definitions
1.3 Data analysis
We report data as mean (±SE) frequencies of behaviors per hour. When the distribution of data differed significantly from the normal distribution (one-sample Kolmogorov-Smirnov test,P<0.05), the Kruskal-Wallis H-test was used to check for the difference among months in sexual behaviors (i.e., copulatory behavior, sexual chase, grimace, sexual-inspection). A Wilcoxon test was used to analyze differences between the mating season and non-mating season. Linear regression was used to test for sexual synchronization in the sexual behaviors of the five males. All analyses were two-tailed and were carried out using the SPSS 13.0 software (Norusis, 2005), with the significance level set at 0.05.
2 Results
2.1 Seasonal variation of copulatory behavior
A monthly difference was found in copulatory behavior of each male (Kruskal-Wallis H-test: GBZ:χ2=79.412,df=11,P<0.01; CT:χ2=59.656,df=11,P<0.01; YX:χ2=45.634,df=11,P<0.01; JT:χ2=57.398,df=11,P<0.01; BL:χ2=33.185,df=11,P<0.01). And the difference was significant between the mating and non-mating season for each male (Wiloxon test: GBZ:Z=-6.235,P<0.01; CT:Z=-5.584,P<0.01; YX:Z=-4.707,P<0.01; JT:Z=-6.078,P<0.01; BL:Z=-3.710,P<0.01) (Fig.1).
2.2 Seasonal variation of sexually motivated behaviors
2.2.1 Seasonal variation in sexual chase A significant monthly difference was also found for the sexually motivated behavior of sexual chase for four of the adult males (Kruskal-Wallis H-test: GBZ:χ2=54.825,df=11,P<0.01; CT:χ2=20.313,df=11,P<0.05; YX:χ2=28.854,df=11,P<0.01; JT:χ2=45.797,df=11,P<0.01), but not for BL (Kruskal-Wallis H-test:χ2=19.218,df=11,P<0.01). Sexual chase differed significantly between mating and non-mating seasons in GBZ, CT, and JT (Wilcoxon test: GBZ:Z=-5.838,P<0.01; CT:Z=-3.541,P<0.01; JT:Z=-4.416,P<0.01), whereas no seasonal difference for this behavior was found for either YX or BL (YX:Z=-1.439,P>0.05; BL:Z=-1.720,P>0.05) (Fig.2).

Fig. 1 Seasonal variation of copulatory behaviors (M±SE) in adult male Macaca thibetana (** P<0.01; * P<0.05)

Fig. 2 Seasonal variation of sexual chases (M±SE) in adult male Macaca thibetana (** P<0.01; * P<0.05)
2.2.2 Seasonal variation in grimace Grimaces differed by month for all the five adult males (Kruskal-Wallis H-test: GBZ:χ2=100.081,df=11,P<0.01; CT:χ2=23.073,df=11,P<0.05; YX:χ2=43.746,df=11,P<0.01; JT:χ2=51.088,df=11,P<0.01; BL:χ2=42.649,df=11,P<0.01). Differences were found between the mating and non-mating season for all five males (Wilcoxon test: GBZ:Z=-7.358,P<0.01; CT:Z=-4.725,P<0.01; YX:Z=-3.439,P<0.01; JT:Z=-4/717,P<0.01; BL:Z=-2.540,P<0.05) (Fig.3).
2.2.3 Seasonal variation in sexual-inspection Sexualinspections were found to be significantly different by month in the five target monkeys (Kruskal-Wallis H-test: GBZ:χ2=44.168,df=11,P<0.01; CT:χ2=22.579,df=11,P<0.05; YX:χ2=28.367,df=11,P<0.01; JT:χ2=33.096,df=11,P<0.01; BL:χ2=28.784,df=11,P<0.01). A difference was found between mating and non-mating seasons for four of the males (Wilcoxon test: GBZ:Z=-4.625,P<0.01; CT:Z=-2.140,P<0.05; YX:Z=-2.008,P<0.05; JT:Z=-4.083,P<0.01), but not for BL (Wilcoxon test:Z=-1.293,P>0.05) (Fig.4).
2.3 Synchronously seasonal variation in male Tibetan macaques
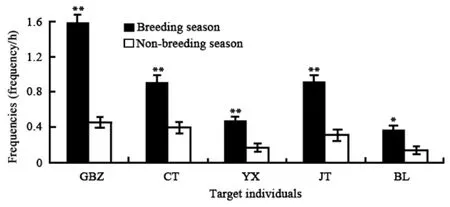
Fig. 3 Seasonal variation of grimace (M±SE) in adult male Macaca thibetana (** P<0.01; * P<0.05)
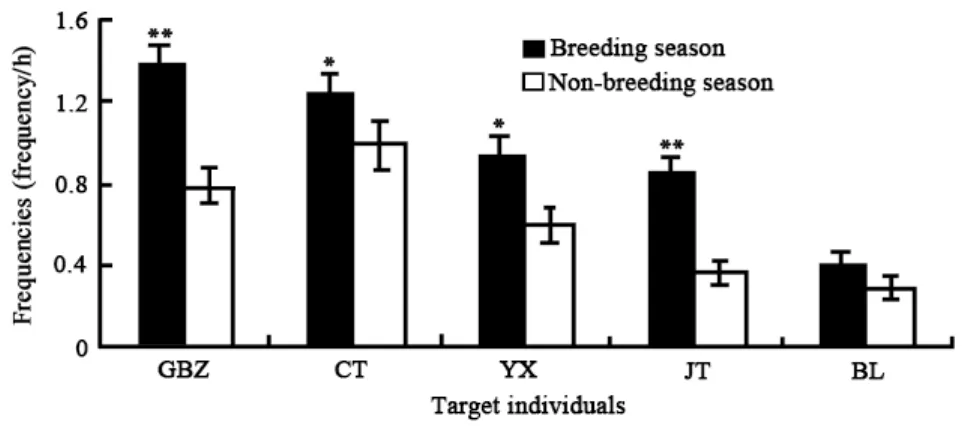
Fig. 4 Seasonal variation of sexual-inspection (M±SE) in adult male Macaca thibetana (** P<0.01; * P<0.05)
Sexual behaviors showed quadratic trends from October 2005 to September 2006, including copulation (P<0.01) and sexually motivated behaviors (P<0.01) (sexual chase:P<0.01; grimace:P<0.01; sexual-inspection:P<0.01). Furthermore, linear regression analysis showed that copulatory behaviors, which had higher frequencies in the mating season and lower frequencies in the non-mating season, showed synchronous change with the seasons in the five target monkeys (adjustedR2=0.926,N=12,F=35.627,P<0.01), as did sexually motivated behaviors (sexual chase: adjustedR2=0.848,N=12,F=16.289,P<0.01; grimace: adjustedR2=0.873,N=12,F=19.823,P<0.01; sexual- inspection: adjustedR2=0.622,N=12,F=8.241,P<0.05), that is to say, the target males showed sexual behaviors at the same period (Fig.5).
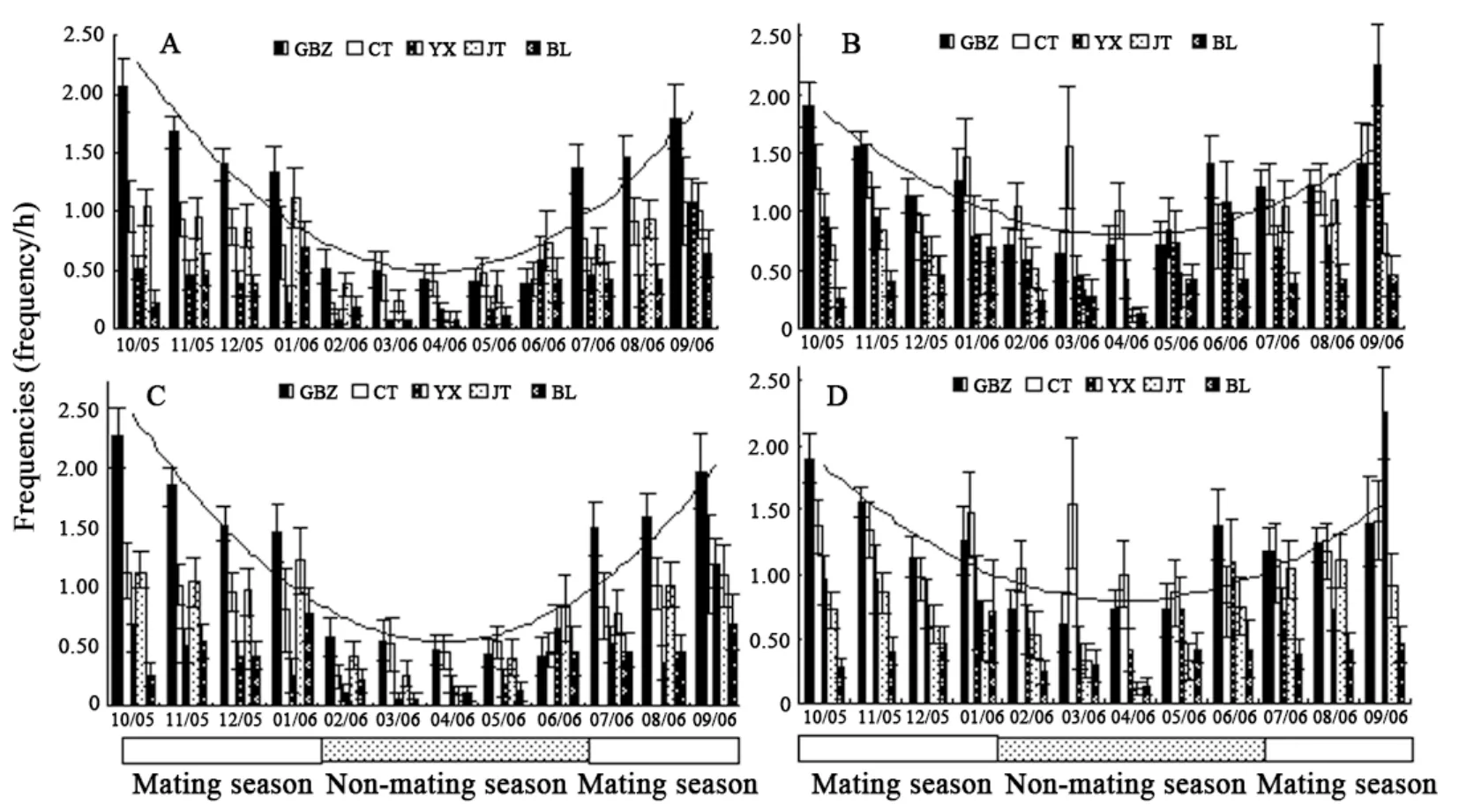
Fig. 5 Synchronization of seasonal variation in adult male Macaca thibetana, including copulatory behavior (P<0.01) (A), sexual chase (P<0.01) (B), grimace (P<0.01) (C), and sexual-inspection (P<0.05) (D)
3 Discussion
3.1 Seasonal variation of sexual behaviors in male Tibetan macaques
Mechanisms of seasonal breeding in female non-human primates have been intensely studied (Setchell & Wickings, 2004; Li et al, 2005; Sharma et al, 2006). However, it is yet to be known whether sexual behaviors also show seasonal variation among male individuals. In current study, seasonal variations of copulatory and sexually motivated behaviors were found in male Tibetan macaques. Frequencies of copulatory behaviors were significantly higher during the mating season. The same is true with sexually motivated behaviors (i.e., sexual chase, grimace, and sexual-inspection), which are the primary coordinators between sexual behavior and fertility (Wallen, 2001; Wallen & Zehr, 2004). Our prediction that male Tibetan macaques should show seasonal variation in sexual behavior is well supported. In non-human primate groups, male behaviors are distributed with females’ behaviors (Strier, 2003; Bateman, 1948), through direct and indirect olfactory investigation (Palagi et al, 2004), and the most conspicuous feature of the reproductive cycle of many male cercopithecine monkeys is seasonality (Bercovitch, 1999), show parallel seasonal distributions with females’ reproductive status. As opposed to the abundant studies of seasonal variation of sexual behaviors in females, the studies in males are scarce. However, many viewpoints in different fields can still be used to illustrate seasonal variation of sexual behaviors in male non-human primates. Firstly, the achievement of peak testis size in mating season is the most important factor in the morphological changes (Bercovitch, 1999). Secondly, some studies emphasize the fundamental role of chemical signals in the regulation of seasonal behavior in primates (Kappeler, 1998; Pereira, 1991). Thirdly, physiological changes (i.e. circulating the testosterone level), which are probably the honest messages about the male’s reproductive state (Kappeler, 1998), facilitate the sexual behaviors (Adkins-Regan, 2005) and sexual motivation (Wallen & Zehr, 2004). Previous studies demonstrate that testosterone levels are higher in the mating season than non-mating season, and they show seasonal variation (Xia et al, 2008a) and positive correlations with copulatory behavior (Xia et al, unpublished data). Our study showed seasonal variation of sexual behaviors in free-ranging maleM. thibetana, and the function of testosterone is probably the most direct illustration to data.
3.2 Sexual synchrony in male Tibetan macaques
Synchronization in sexual behaviors is universal among invertebrates (Danley et al, 2007) and vertebrates (Moore et al, 2005), including non-human primates (Sharma et al, 2006). Synchronous reproduction is universal in female consorts (Connor et al, 2006), however, it is not clear if males show synchronization of sexual behaviors. Furthermore, as synchronization reproduction can also be associated with cultural function (Moore et al, 2005), it is significant to discussion the synchronization of sexual behaviors in free-ranging social group. In accordance with our expectation that male sexual behaviors should be synchronized in both the breeding and non-mating season, copulatory behavior and sexually motivated behaviors showed significantly higher frequencies in the mating season than non-mating season were found in the target males. That is to say, all males showed sexual behaviors at the same period. The prediction that males exhibit synchronous variation by season was strongly supported. Our results support the previous conclusion about the synchronization of sexual behaviors in non-human primates (Sharma et al, 2006).
Historically, the study of reproductive strategies in male macaques and other primates has focused on competition of accessing to females (Altmann, 1962). Reproductive synchronization will increase the likelihood of mating because males have a specific window of time corresponding to female reproductive behaviors and status (Danley et al, 2007). Seasonal reproductive synchrony thus facilitates male reproductive success (Lack, 1968). Although some authors suggest that synchronization is genetically determined (Moore et al, 2005), others argued that synchronous reproduction is also associated with alliance membership and that synchronization between members of cooperating alliances is universal in sexual behavior (Connor et al, 2006). In some social species, males living in the same group show synchronous variation with the seasons within the same group. Our results confirmed this expectation. Conversely, synchronization leads to more intense male-male competition (Eberle & Kappeler, 2004), which plays a pivotal role for female choice in sexual selection theory (Lyengar & Sarks, 2008). Our study provides an insight into male sexual competition and strategies used to maximize reproductive success inM. thibetanaand other non-human primates.
Acknowledgements:We are very grateful to the Huangshan Monkey Center and Huangshan Garden and Forest Bureau. We are also very grateful to Mr. HB CHENG’s family for their permission and/or logistic support for our study. Special thanks also go to Prof. LX SUN in the Central Washington University for his valuable comments and English revision, which greatly improved the draft.
Adkins-Regan E. 2005. Hormones and Social Behavior [M]. Princeton and Oxford: Princeton University Press.
Altmann J. 1962. A field study of the sociobiology of rhesus monkeys,Macaca mulatta[J].Ann N Y Acad Sci, 102: 338-435.
Altmann J. 1974. Observational study of behavior: Sampling methods [J].Behaviour, 49: 227-267.
Bateman AJ. 1948. Intra-sexual selection inDrosophila[J].Heredity, 2: 349-368.
Bercovitch FB. 1987a. Female weight and reproductive condition in a population of olive baboons (Papio cynocephalus anubis) [J].Am J Primatol, 12: 189-195.
Bercovitch FB.1987b. Reproductive success in male savanna baboons [J].Behav Ecol Socialbiol, 21: 163-172.
Bercovitch FB. 1999. The physiology of male reproductive strategies [C]// Dolhinow P, Fuentes A. The Nonhuman Primates Mountain View. California: Mayfield Publishing Company, 237-244.
Berman CM, Li JH. 2002. Impact of translocation, provisioning and range restriction on a group ofMacaca thibetana[J].Int J Primatol, 23: 383-397.
Connor RC, Smolker R, Bejder L. 2006. Synchrony, social behavior and alliance affiliation in Indian ocean bottlenose dolphins,Tursiops aduncus[J].Anim Behav, 72: 1371-1387.
Danley PD, de Carvalho TN, Fergus DJ, Shaw KL. 2007. Reproductive asynchrony and the divergence of Hawaiian crickets [J].Ethology, 113: 1125-1132.
Eberle M, Kappeler PM. 2004. Sex in the dark: determinants and consequences of mixed male mating tactics inMicrocebus murinus, a small solitary nocturnal primate [J].Behav Ecol Socialbiol,57: 77-90.
Gordon TP, Bernstein IS. 1973. Seasonal variation in sexual behavior of all-male rhesus troops [J].Am J Phys Anthropol, 38: 22-225.
Herndon JG. 1983. Seasonal breeding in rhesus macaques: Influence of the behavioral environment [J].Am J Primatol, 5: 197-204.
Kappeler PM. 1998. To whom it may concern: the transmission and function of chemical signals inLemur catta[J]. Behav Ecol Socialbiol, 42: 411-421.
Lack D. 1968. Ecological Adaptations for Breeding in Birds [M]. London: Methuen.
Li JH. 1999. The Tibetan Macaque Society: A Field Study [M]. Hefei: Anhui University Press. (in Chinese)
Li JH, Wang QS, Han DM. 1996. Fission in a free-ranging Tibetan macaque troop at Huangshan Mountains, China [J].Chn Sci Bull, 41:1377-1381.
Li JH, Yin HB, Wang QS. 2005. Seasonality of reproduction and sexual activity in female Tibetan macaques (Macaca thibetana) at Huangshan, China [J].Acta Zool Sin, 51(3): 365-375.
Li JH, Yin HB, Zhou LZ. 2007. Non-reproductive copulation behavior among Tibetan macaques (Macaca thibetana) at Huangshan, China[J].Primate, 48: 64-72.
Lyengar VK, Starks BD. 2008. Sexual selection in harems: male competition plays a larger role than female choice in an amphipod [J].Behav Ecol, 19(3): 642-649.
MacFarlane AM, Coulson G. 2005. Synchrony and timing of breeding influences sexual segregation in western grey and red kangaroos (Macropus fuliginosusandM. rufus) [J].J Zool,267: 419-429.
Moore IT, Bonier F, Wingfield JC. 2005. Reproductive asynchrony and population divergence between two tropical bird populations [J].Behav Ecol, 16: 755-762.
Nakatsuru K, Kramer DL. 1982. Is sperm cheap? Limited male fertility and female choice in the lemon tetra (Pisces characidea) [J].Science, 216: 753.
Norusis M. 2005. SPSS 13.0 Advanced Statistical Procedures Companion [M]. Upper Saddle River, NJ: Prentice Hall.
Palagi E, Telara S, Tarli SMB. 2004. Reproductive strategies inLemur catta: balance among sending, receiving, and countermarking scent signals [J].Int J Primatol, 25(5): 1019-1031.
Pereira ME. 1991. Asynchrony within estrous synchrony among ring-tailed lemurs (Primates: Lemuridae) [J].Physiol Behav, 49: 47-52.
Prendergast BJ, Nelson RJ, Zucker I. 2002. Mammalian seasonal rhythms: behavior and neuroendocrine substrates [C]// Pfaff DW, Amold A, Etgen A, Fahrbach S, Rubin R. Hormones, Brain and Behavior. San Diego: Academic Press.
Roughgarden J, Oishi M, Akcay E. 2006. Reproductive social behavior: Cooperative games to replace sexual selection [J].Science, 311(5763): 965-969.
Setchell JM, Wickings EJ. 2004. Social and seasonal influences on the reproductive cycle in female mandrills (Mandrillus sphinx) [J].Am J Phys Anthropol, 125: 73-84.
Sharma AK, Singh M, Kaumanns W, Krebs E, Singh M, Kumar MA, Kumara HN. 2006. Birth patterns in wild and captive Lion-tailed macaques (Macaca silenus) [J].Int J Primatol, 27(5): 1429-1439.
Strier KB. 2003. Primate Behavioral Ecology [M]. New York: Allyn and Bacon Press.
Thomsen R, Soltis J, Matsubara M, Matsubayashi, K, Onnma M, Takenaka O. 2006. How costly are ejaculates for Japanese macaques? [J]Primates, 47: 272-274.
Trivers R. 1972. Parental investment and sexual selection [C]// Campbell B. Sexual Selection and the Descent of Man. Chicago: Aldine Press, 136-179.
Wada K, Xiong CP. 1996. Population changes of Tibetan monkeys with special regard to birth interval [C]// Shotake T, Wada K. Variations in the Asian Macaques. Tokyo: Tokai University Press, 133-145.
Wada K, Xiong CP, Wang QS. 1987. On the distribution of Tibetan and rhesus monkeys in Southern Anhui, China [J].Acta Theriol Sin, 7: 148-176. (in Chinese)
Wallen K, Zehr JL. 2004. Hormones and history: the evolution and development of primate female sexuality [J].J Sex Res,41(1): 101-112.
Wallen K. 2001. Sex and context: hormones and primate sexual motivation [J].Horm Behav, 40: 339-357.
Xia DP, Li JH, Zhu Y, Chen R, Sun BH. 2008a. Seasonal variations of testosterone in wild adult male Tibetan macaques (Macaca thibetana) at Huangshan, China [J].Acta Theriol Sin28(3): 287-292. (in Chinese)
Xia D, Li J, Kyes RC, Zhu Y, Street S, Ferguson B. 2008b. Genetic assessment of the Tibetan macaques (Macaca thibetana) at Huangshan National Reserve, Anhui, China [J].Am J Primatol, 70(suppl.1): 36.
Xiong CP. 1998. Sexual behavioral pattern in the female Tibetan monkeys (Macaca thibetana) [J].Acta Theriol Sin, 18(4): 247-253. (in Chinese)
Zhang M, Li J, Zhu Y, Wang X, Wang S. 2010. Male mate choice in Tibetan macaquesMacaca thibetanaat Mt. Huangshan, China [J].Curr Zool, 56(2): 213 - 221
野生雄性黄山短尾猴性行为季节性变化同步性
夏东坡1,2,3,李进华1,2,3,朱 勇1,2,3,孙丙华1,2,3,Lori K SHEERAN4,Megan D MATHESON5
(1. 安徽大学 生命科学学院, 安徽 合肥 230039;2. 安徽省生物工程与生物技术重点实验室, 安徽 合肥 230039;3. 安徽省生态经济技术中心, 安徽 合肥 230039;4.Department of Anthropology, Central Washington University, Ellensburg, WA98926;5.Department of Psychology, Central Washington University, Ellensburg, WA98926)
季节性繁殖是非人灵长类动物的普遍特征,而野生雄性个体的性行为季节性变化特点仍有待研究。该研究于2005年10月至2006年9月对黄山短尾猴鱼鳞坑YA2群5只成年雄性个体进行全年观察,以期探讨雄性黄山短尾猴性行为季节性变化特征。结果表明:繁殖季节交配行为和性动机行为(性追赶,做鬼脸和性检查)频次显著高于非繁殖季节,季节性差异显著。成年雄性个体交配行为和性动机行为季节性变化呈现显著同步性。该研究为进一步阐明短尾猴和同属季节性繁殖非人灵长类动物的雄性竞争和雄性策略提供理论依据。
短尾猴;雄性;性行为;季节性;同步性
Q959.848;Q958.112;Q492.4
A
0254-5853-(2010)05-0509-07
;2010-01-27;接受日期:2010-07-29
国家自然科学基金(30770288;30970414);教育部博士点基金;安徽省人才开发基金;安徽省自然科学基金年度重点项目
夏东坡(1981-),男,博士研究生,研究方向:行为生态学与分子生态学
10.3724/SP.J.1141.2010.05509
date: 2010-01-27; Accepted date: 2010-07-29
*通信作者(Corresponding author),Email: jhli@ahu.edu.cn
猜你喜欢
杂志排行
Zoological Research的其它文章
- Rates and patterns of microsatellite mutations in common carp (Cyprinus carpio L.)
- Ethogram of Yangtze finless porpoise calves (Neophocaena phocaenoides asiaeorientalis)
- Embryonic development of the concave-eared torrent frog with its significance on taxonomy
- Identification of zRAP55,a gene .preponderantly expressed in StagesⅠandⅡ oocytes of zebrafish
- Niche partitioning between sympatric rhesus macaques and Yunnan snub-nosed monkeys at Baimaxueshan Nature Reserve, China
- 小杜鹃对强脚树莺的巢寄生及其卵色模拟
