Synthesis of 4,4′-MDC in the Presence of Sulfonic Acid-functionalized Ionic Liquids*
2009-05-15GENGYanlou耿艳楼HULiyan胡利彦ZHAOXinqiang赵新强ANHualiang安华良andWANGYanji王延吉
GENG Yanlou (耿艳楼), HU Liyan (胡利彦), ZHAO Xinqiang (赵新强)**, AN Hualiang (安华良) and WANG Yanji (王延吉)
Synthesis of 4,4′-MDC in the Presence of Sulfonic Acid-functionalized Ionic Liquids*
GENG Yanlou (耿艳楼), HU Liyan (胡利彦), ZHAO Xinqiang (赵新强)**, AN Hualiang (安华良) and WANG Yanji (王延吉)
Hebei Provincial Key Lab of Green Chemical Technology and High Efficient Energy Saving, Hebei University of Technology, Tianjin 300130, China
ionic liquids, methyl-phenyl carbonate, formaldehyde, methylene diphenyl dimethylcarbamate, Brönsted acidity, catalyst recycling
1 INTRODUCTION
Diphenylmethane diisocyanate (MDI) is an important raw material for the manufacture of polyurethanes. At present, MDI is industrially produced by a phosgene route, which uses toxic phosgene as substrate, generates highly corrosive hydrochloride as a byproduct, and produces the desirable product with rather high level of residual chlorine anion. For the purpose of eliminating all these drawbacks, several non-phosgene routes [1] have been developing. Among them, the dimethyl carbonate (DMC) route is the most competitive, which comprises of synthesis of methyl-phenyl carbamate (MPC) from aniline and DMC, condensation of MPC with formaldehyde (HCHO) to diphenylmethane dicarbamate (MDC), and then thermal decomposition of MDC to MDI.
So far, much more research work has been done on the first and third step and impressive progress has been made [2-5]. At the moment, the condensation step has attracted increasing attention because of its lower product yield, selectivity and unsatisfactory catalyst. The catalysts used for this reaction include liquid acids (such as HCl, H2SO4, H3PO4, CF3SO3H, mixed acid) and solid acids (such as SbCl5, ZnCl2, BF3, ion-exchange resin, zeolites) [6-9]. The liquid acid catalysts cause corrosion to the equipment and result in environmental pollution, while the solid acid catalysts not only deactivate rapidly but also necessitate environmentally-unfriendly organic solvents in the reaction. Therefore, it is necessary to develop novel environmentally benign and highly active catalysts for the condensation reaction of MPC with HCHO to MDC.
Ionic liquids, due to their low volatility, high thermal and chemical stability, have received much attention as an environmentally friendly substitute for traditional volatile organic solvents in catalysis and organic synthesis reactions. Furthermore, task-specific ionic liquids can be used as dual solvent-catalyst. We evaluated the catalytic activity of acidic ionic liquid [emim]BF4and [HSO3-bpy]CF3SO3for the condensation reaction in previous studies [10, 11], and the experimental results show that the two ionic liquids have higher acid strength and better catalytic performance. Their acid strength is well consistent with their catalytic activity in the condensation reaction of MPC with HCHO to MDC.
In this work, in order to find a new substitute for the conventional catalyst and solvent, we synthesized five Brösted acidic ionic liquids with an alkyl sulfonic acid group in its imidazolium cation, and investigated the synthesis of 4,4′-MDC from MPC and HCHO in the presence of the ionic liquids as dual solvent-catalyst. Both the relationship of acid strength-catalytic activity and the recycle of the ionic liquid were also discussed.
2 EXPERIMENTAL
2.1 Materials and reagents
Main reagents in the experiment and their producers were listed in Table 1, and the reagents were used without further purification unless stated otherwise. MPC was prepared as follows: Under stirring, phenyl isocyanate (0.1 mol) and methanol (0.15 mol) were mixed in a 100 ml beaker at room temperature, and the exothermic reaction took place quickly, then the crude product was collected by filtration and re-crystallized using cyclohexane as a solvent, and a white crystal (MPC) was obtained.
Table 1 Main raw materials and chemical reagents
2.2 Preparation of ionic liquids
The sulfonic acid-functionalized ionic liquids (SFILs) were synthesized according to the method described in the literatures [12-15]. The equimolar-methylimidazole (32 ml, 0.4 mol) and 1,4-butane sultone (41 ml, 0.4 mol) were charged into a 250 ml round-bottom flask, and the mixture was stirred at 40°C for 10 h. In order to remove the non-ionic residues, the resultant 1-methyl-3-(butyl-4-sulfonate) imidazolium ([HSO3-bmim]) was washed for three times with tolueneand ethyl ether, respectively, and dried in vacuum. Then,the equimolar [HSO3-bmim] and each acid were mixed and stirred for 2-24 h at 80°C to form the SFILs. The resultant SFILs were also washed three times with tolueneand ethyl ether, respectively, and then dried in vacuum.
2.3 Characterization procedures
The Fourier transform infrared (FT-IR) spectra of MDC and the ionic liquids were recorded on a VECTOR22 infrared spectroscopy (KBr pellets / film) in a frequency range of 400-4000 cm-1. On compared with the standard spectrum [16], the reaction product MDC was identified as-4,4′-MDC. Pyridine was used as a probe molecule to determine the acidity of the SFILs while the acid strength of SFILs was determined by the Hammett method with UV-visible spectroscopy, which were recorded on a Cary 300 UV-visible spectroscope (Varian Inc., USA) at room temperature. 4,4′-MDC was quantitatively analyzed by Waters 515 HPLC (Waters Co., USA) with a Nova-pak C18 (3.9 mm×150 mm) column, methanol-water (3/2 by volume) as mobile phase at a flow of 0.6 ml·min-1and 254 nm UV detector.
2.4 Condensation reaction
In the condensation reaction, MPC, SFILs and HCHO were charged into a 100 ml round-bottom flask immersed in a water bath and fitted with a reflux condenser. The mixture was stirred at a certain temperature for a period of time. When the reaction finished, some water was added into the reaction mixture to make white MDC precipitated. After that the mixture was filtered. SFILs could be reused after the removal of produced water by vacuum distillation.
In this work, the yield of MDC is calculated based on HCHO added, while the selectivity of 4,4′-MDC is calculated with MPC consumed as the basis because the concentration of HCHO after reaction is difficult to determine.
3 RESULTS AND DISCUSSION
3.1 Effect of kind of ionic liquids
The catalytic activities of different SFILs in the condensation reaction of MPC with HCHO are listed in Table 2. It can be seen that the catalytic activities of the SFILs depend on the kind of anions, and [HSO3-bmim]CF3SO3shows the best catalytic activity while [HSO3-bmim]HSO4exhibits a slightly lower activity. However, the activities of [HSO3-bmim]- TSA and [HSO3-bmim]CH3SO3are much lower while [HSO3-bmim]CF3COO even has no activity.
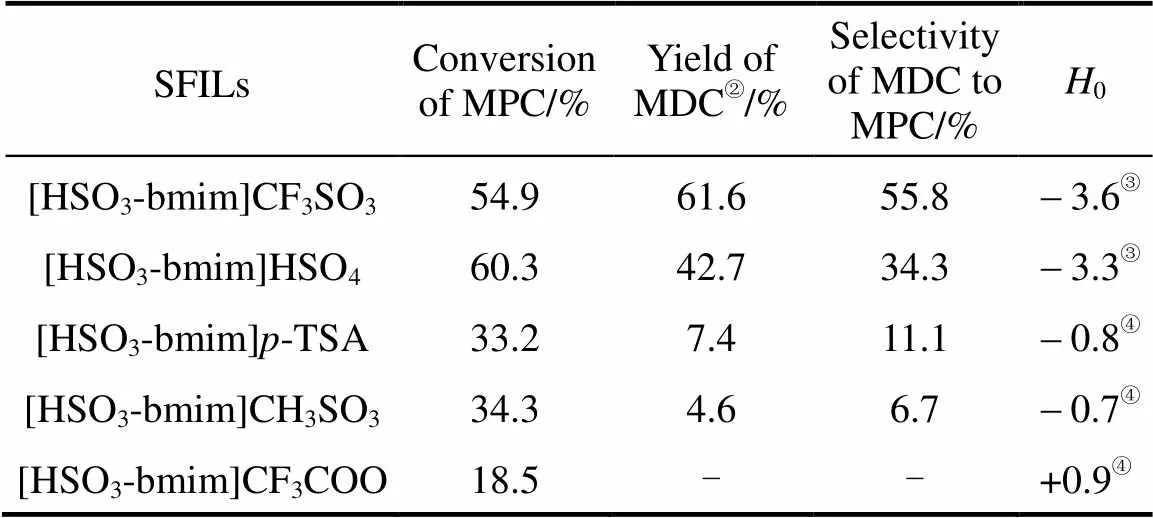
Table 2 Catalytic activity of different ionic liquids and H0① values
②Calculated based on HCHO. Reaction conditions: 10 ml ILs; 20 mmol MPC; molar ratio of MPC:HCHO is 4︰1; 70°C; 1 h.
③ Indicator: 4-nitrodiphenylamine.
④ Indicator:-nitraniline.
The acidity of the SFILs has been determined by FT-IR using pyridine as a probe molecule. On mixing pyridine with ionic liquids, the spectra of all the ionic liquids show a band near 1540 cm-1, indicating the presence of Brönsted acid sites due to the formation of pyridinium ions (Fig. 1).
The acid strength values (0) of the SFILs were determined by the Hammett method with UV-visible spectroscopy at room temperature [17]. From Table 2 it can be seen that0values of the SFILs depends on their anions and is well consistent with their catalytic activity for the synthesis of 4,4′-MDC. Similar result has been attained when sulfonic acid-functional butyl pyridine ionic liquids were used as dual solvent-catalyst in this reaction [10].
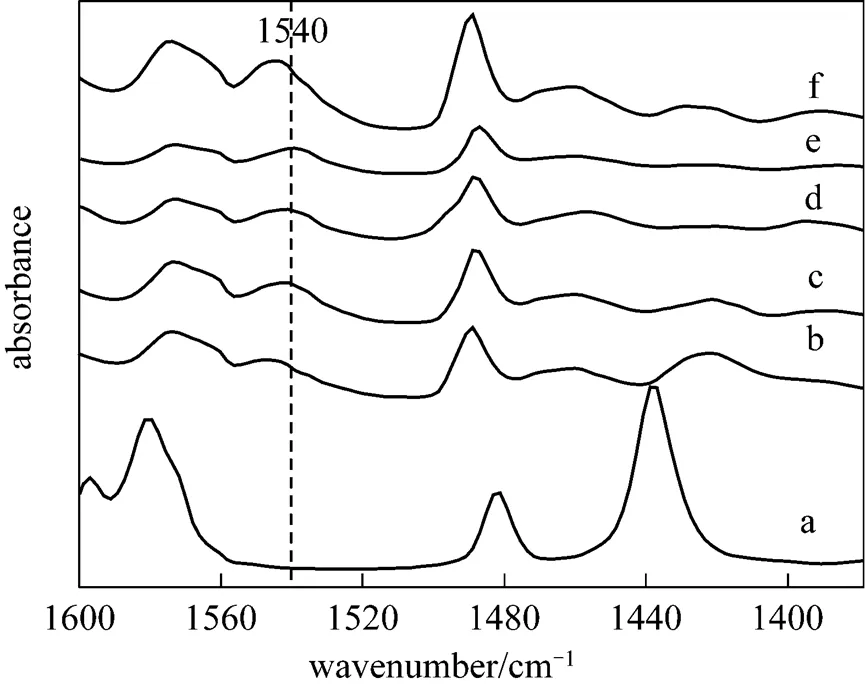
Figure 1 FT-IR spectra
a—pure pyridine; b—pyridine with [HSO3-bmim]CF3COO; c—pyridine with [HSO3-bmim]CH3SO3; d—pyridine with [HSO3-bmim]-TSA; e—pyridine with [HSO3-bmim]HSO4; f—pyridine with [HSO3-bmim]CF3SO3
Since ionic liquid [HSO3-bmim]CF3SO3showed the best catalytic activity,subsequent experiment was done using it as the medium.
3.2 Effect of reaction time
Figure 2 shows the effect of reaction time on the condensation reaction of MPC with HCHO in the presence of ionic liquid [HSO3-bmim]CF3SO3. The conversion of MPC keeps constant with the reaction time, while the yield of MDC increases in the early stage of the reaction and reaches 67.0 % at 40 min and then decreases afterwards because a further reaction of MDC forms polymethylene polypenylcarbamates [18].
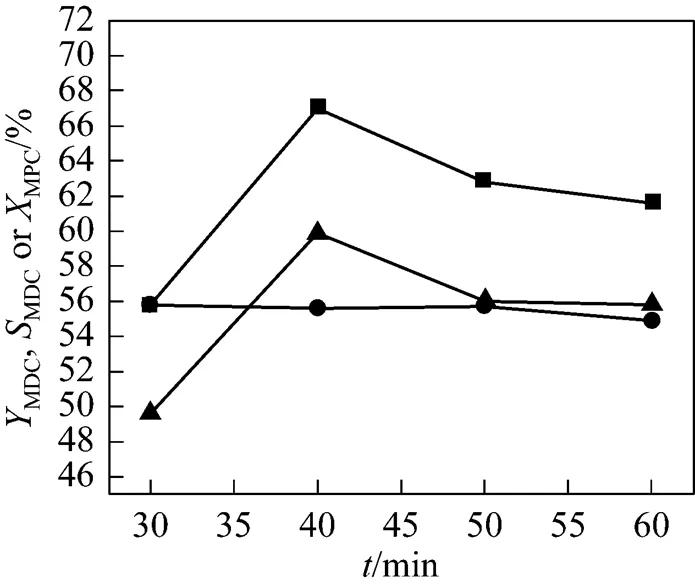
Figure 2 Effect of reaction time on the condensation reaction
(Reaction conditions: 10 ml ILs; mass ratio of ILs to MPC is 4.5︰1; molar ratio of MPC to HCHO is 4︰1; 70°C)
■MDC;▲MDCto MPC;●MPC
3.3 Effect of reaction temperature
The effect of reaction temperature on the condensation reaction is shown in Fig. 3. The conversion of MPC increases with the rising of the reaction temperature.When the temperature changes from 50°C to 70°C, both the yield and selectivity of MDC increase and reach the highest value of 67.0% and 59.9%, respectively. However, both the yield and selectivity of MDC decrease when the temperature surpasses 70°C due to the formation of polymethylene polypenylcarbamates.
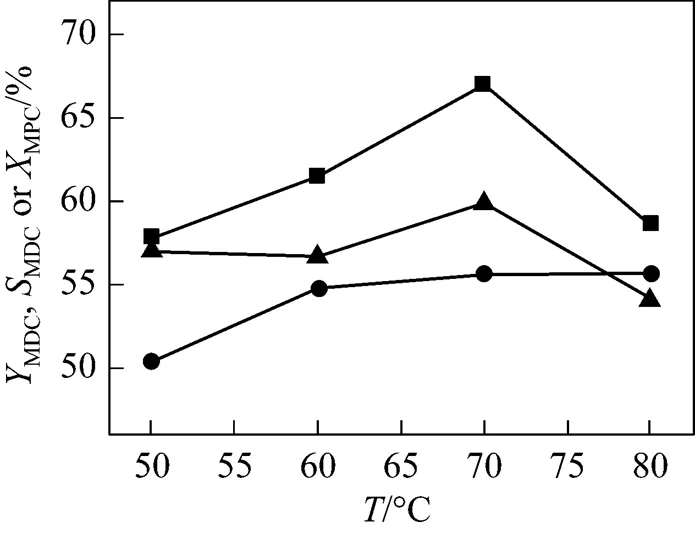
Figure 3 Effect of reaction temperature on the condensation reaction
(Reaction conditions: 10 ml ILs; mass ratio of ILs to MPC is 4.5︰1; molar ratio of MPC to HCHO is 4︰1; 40 min)
■MDC;▲MDCto MPC;●MPC
3.4 Effect of the molar ratio of MPC to HCHO
The effect of the molar ratio of MPC to HCHO (MPC/HCHO) on the condensation reaction is illustrated in Fig. 4. AsMPC/HCHOincreases, the conversion of MPC decreases monotonously while the yield of MDC passes a maximum of 89.9% at 10/1. WhenMPC/HCHOis lower than 10/1, polymethylene polyphenylcarbamates byproducts are easily formed because of the higher concentration of HCHO. WhenMPC/HCHOsurpasses 10/1, the lower concentration of HCHO slows down the reaction rate and restrains the by-reactions, and the yield of MDC decreases while the selectivity of MDC increases. Consequently, the optimal molar ratio ofMPC/HCHOis 10/1.
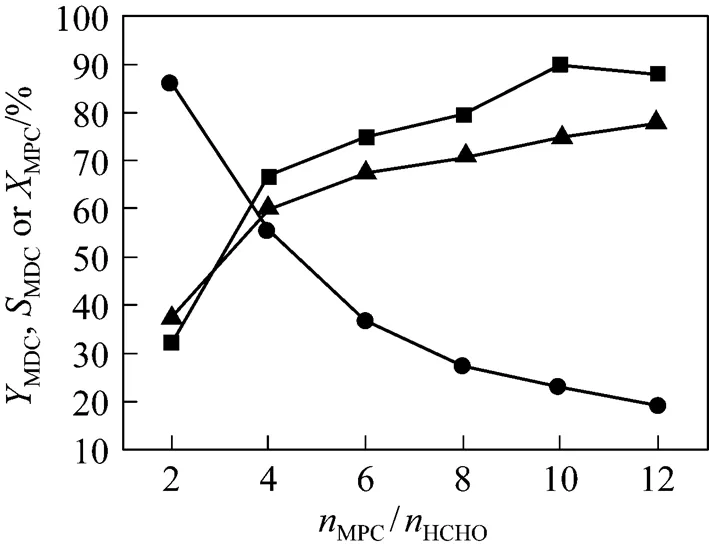
Figure 4 Effect of molar ratio of MPC to HCHO on the condensation reaction
(Reaction conditions: 10 ml ILs; mass ratio of ILs to MPC is 4.5︰1; 70°C; 40 min)
■MDC;▲MDCto MPC;●MPC
3.5 Effect of ionic liquid amount

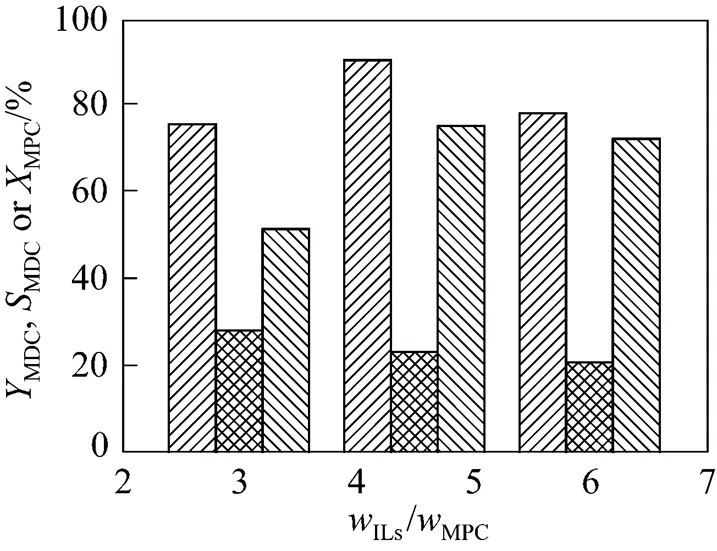
Figure 5 Effect of the amount of ionic liquid on the condensation reaction
(Reaction conditions: 10 ml ILs; molar ratio of MPC to HCHO is 10︰1; 70°C; 40 min)

3.6 Recycle of ionic liquid
Because [HSO3-bmim]CF3SO3is a viscous liquid with certain solubility of MDC, MDC can not be separated by filtration. Considering that the ionic liquid can be entirely miscible with water while MDC is insoluble in water, some water was added into the reaction mixture. And then the white MDC precipitation was recovered by filtration. After the water in the filtrate was removed by vacuum distillation at 80°C, the concentration of MPC and MDC in the filtrate (purified ionic liquid) was much lower and had no influence on the reuse in the next run. The purified ionic liquid was reused 4 times. The results listed in Table 3 indicates that the catalytic activity of ionic liquid [HSO3-bmim]CF3SO3slightly decreases in the first reuse and afterwards no obvious loss in its catalytic activity is observed.
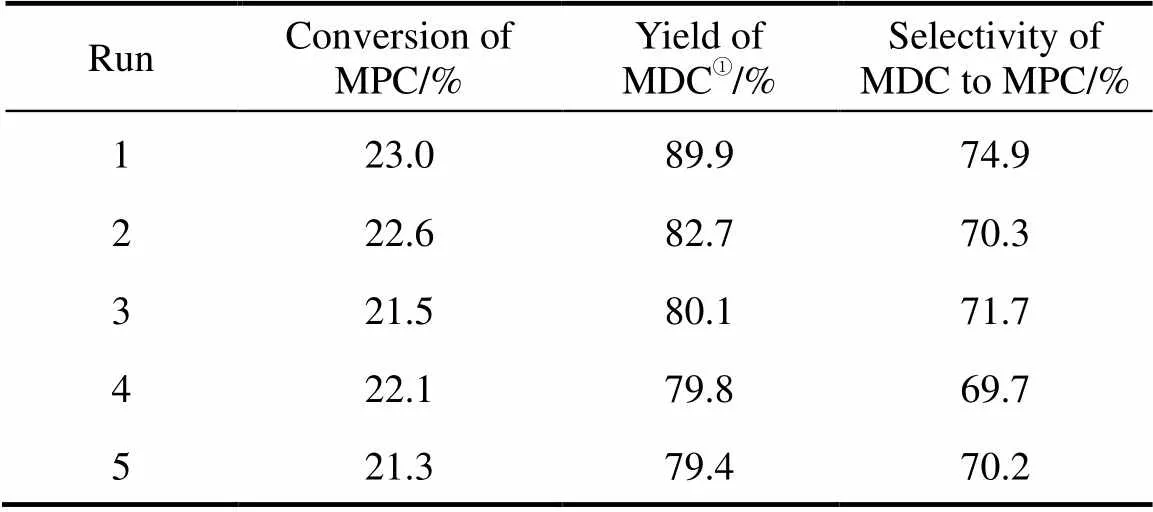
Table 3 Reuse of ionic liquid in the condensation reaction (Reaction conditions: 10 ml ILs; mass ratio of ILs to MPC is 4.5︰1; molar ratio of MPC to HCHO is 10︰1; 70°C; 40 min)
① Calculated based on HCHO.
4 CONCLUSIONS

1 Fukuoka, S., Chono, M., Watanabe, T., Kohno, M., “Method for manufacture of diphenylmethane diisocyanates”, U.S. Pat., 4547322 (1985).
2 Li, F., Miao, J., Wang, Y.J., Zhao, X.Q., “Synthesis of methyl-phenyl carbamate from aniline and dimethyl carbonate over supported zirconia catalyst”,...., 45 (14), 4892-4897 (2006).
3 Tsutomu, Y., Masaaki, S., Fumiaki, H., Yukio, K., Koji, I., Akio, I., Kazuhito, M., Tsuneo, E., Toshihide, B., “Highly selective methoxycarbonylation of aliphatic diamines with methyl phenyl carbonate to the corresponding methyl-alkyl dicarbamates”,..., 289 (2), 174-178 (2005).
4 Zhao, X., Wang, Y.J., Wang, S.F., Yang, H.J., Zhang, J.Y., “Synthesis of MDI from dimethyl carbonate over solid catalysts”,...., 41 (21), 5139-5144 (2002).
5 Grzegorz, L., Eugeniusz, M., “Thermal decomposition of methylene- 4,4′-di(ethylphenyl- carbamate) to methylene-4,4′-di(phenylisocyanate)”,..., A119, 19-24 (2005).
6 Kim, S.D., Lee, K.H., “Control of regioselectivity by cation-exchanged sulfonie acid resin catalysts”,...., 78 (2), 237-248 (1993).
7 Wang, F.Q., Chen, T., Ma, F., Yao, J., Wang, G.Y., “Synthesis of methylene diphenyl dicarbamate over silicotungstic acid catalyst”,, 35 (3), 260-263 (2006). (in Chinese)
8 Clerici, M.G., Bellussi, G., Romano, U., “Process for the preparation of 4, 4′-diaminodiphenyl -methane and its derivatives”, U.S. Pat., 5241119 (1993).
9 Zhao, X.Q., Wang, Y.J., Li, F., Zhang, W.H., Hao, D.Z., “Study of cleansynthesis process for methylene diphenyl diisocynate II Catalytic synthesis and cleavage of methylene diphenyl dicarbamate”,.(), 17, 53-58 (2001). (in Chinese)
10 Wang, N., Zhao, X.Q., Wu, X.L., Wang, Y.J., “MDC synthesis reaction catalyzed by [HSO3-bpy ]CF3SO3ionic liquid”,.... (), 59 (4), 887-891 (2008). (in Chinese)
11 Hu, L.Y., Geng, Y.L., Zhao, X.Q., Wang, Y.J., “Synthesis of 4,4′-MDC in the presence of [emim]BF4ionic liquid modified with H+”,....., 21 (3), 467-470 (2007). (in Chinese)
12 Gu, Y.L., Shi, F., Deng, Y.Q., “Esterification of aliphatic acids with olefin promoted by Brönsted acidic ionic liquids”,...:., 212 (1/2), 71-75 (2004).
13 Gui, J.Z., Ban, H.Y., Cong, X.H., Zang, X.T., Hu, Z.D., Sun, Z.L., “Selective alkylation of phenol with-butyl alcohol catalyzed by BrÖnsted acidic imidazolium salts”,...., 225 (1), 27-30 (2005).
14 Amanda, C.C., Jessica, L.J., Loanna, N., Kim, L.T., Kristin, J.W., David, C.F., James, H.D., “Novel Brösted acidic ionic liquids and their use as dual solvent-catalysts”,...., 124 (21), 5962-5963 (2002).
15 Duan, Z.Y., Gu, Y.L., Zhang, J., Zhu, L.Y., Deng, Y.Q., “Protic pyridinium ionic liquids: Synthesis, acidity determination and their performances for acid catalysis”,...., 250 (1/2), 163-168 (2006).
16 Hocker, J., Born, L., “The structure of two modifications of the MDI-bismethylurethane as a model substance for hard segments in polyurethane elastomers”,......, 17 (11), 723-730 (1979).
17 Xing, H.B., Wang, T., Zhou, Z.H., Dai, Y.Y., “The sulfonic acid-functionalized ionic liquids with pyridinium cations: Acidities and their acidity-catalytic activity relationships”,...., 264 (1/2), 53-59 (2007).
18 Fukuoka, S., Watanabe, T., “Process for producing diphenylmethane dicarbamates”, U.S. Pat., 4552974 (1985).
2008-12-25,
2009-08-17.
the National Natural Science Foundation of China (20576025). the National Key Basic Project of China (2005CCA06100), the Science and Technological Research and Development Project of Hebei Province (07215602D) and the Natural Science Foundation of Hebei Province (B2007000010).
** To whom correspondence should be addressed. E-mail: zhaoxq@hebut.edu.cn
猜你喜欢
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Molecular Simulation of CO2/H2 Mixture Separation in Metal-organic Frameworks: Effect of Catenation and Electrostatic Interactions*
- Deactivation Kinetics of Nitrile Hydratase in Free Resting Cells*
- Corrosion Behavior of TP316L of Superheater in Biomass Boiler with Simulated Atmosphere and Deposit
- Influence of A-type Zeolite on Methane Hydrate Formation*
- Effects of Sintering Atmosphere on the Microstructure and Surface Properties of Symmetric TiO2Membranes*
- Improvement of Isomerization Process of Crude Isoamylene with Tertiary-amyl-alcohol Addition
