Kinetics of Burning Side Reaction in the Liquid-phase Oxidation of p-Xylene*
2009-05-12ChengYouwei成有为PengGe彭革WangLijun王丽军andLIXi李希
Cheng Youwei (成有为), Peng Ge (彭革), Wang Lijun (王丽军),* andLI Xi (李希)
Kinetics of Burning Side Reaction in the Liquid-phase Oxidation of-Xylene*
Cheng Youwei (成有为)1, Peng Ge (彭革)2, Wang Lijun (王丽军)1,*andLI Xi (李希)1
1Department of Chemical and Biochemical Engineering, Zhejiang University, Hangzhou 310027, China2Chemical Engineering Department, Ningbo University of Technology, Ningbo 315010, China
During the liquid-phase oxidation of-xylene, over-oxidation of reactant, intermediates and solvent to carbon dioxide and carbon monoxide is generally known as the burning side reaction. Batch and semi-continuous experiments were carried out, and the experimental data of the burning side reaction were analyzed and reported in this paper. The results showed that the rates of burning side reactions were proportional to the rates of the main reaction, but decreased with the increasing concentrations of reactant and intermediates. The inter-stimulative and competitive relationship between the burning side reaction and the main reaction was confirmed, and the rates of the burning side reaction could be described with some key indexes of the main reaction. According to the mechanism of the side reactions and the kinetics model of main reaction which were proposed and tested in the previous papers, a kinetic model of the burning side reactions involving some key indexes of the main reaction was developed, and the parameters were determined by data fitting of the COrate curves. The obtained kinetic model could describe the burning side reactions adequately.
kinetics, burning side reaction,-xylene oxidation
1 INTRODUCTION
Liquid-phase oxidation of methyl aromatic hydrocarbons is of great scientific, technological, and commercial importance. One of the most successful commercial applications is the production of terephthalic acid (TA) by liquid-phase oxidation of-xylene (PX) with air over a Co-Mn-Br catalyst system (cobalt acetate, manganese acetate and hydrogen bromide) in acetic acid (HOAc) solvent at 150-210°C. As practiced, this reaction is known as the MC (Mid-Century) process[1-3].
The oxidation of PX follows the classical radical chain reaction mechanism involving the initiation, propagation, and termination steps[4, 5], in which two methyl groups on the benzene ring are oxidized and produce various kinds of intermediates and final products, such as-tolualdehyde (TALD),-toluic acid (PT), and 4-carboxybenzaldehyde (4-CBA) and TA. The brief reaction scheme is shown in Fig. 1. The efficacy of the Co-Mn-Br catalyst system is due to the fact that the catalytic cycles of cobalt, manganese, and bromide become coupled to produce synergistic results. Detailed studies on the kinetics have been conducted the last decade[6-19].
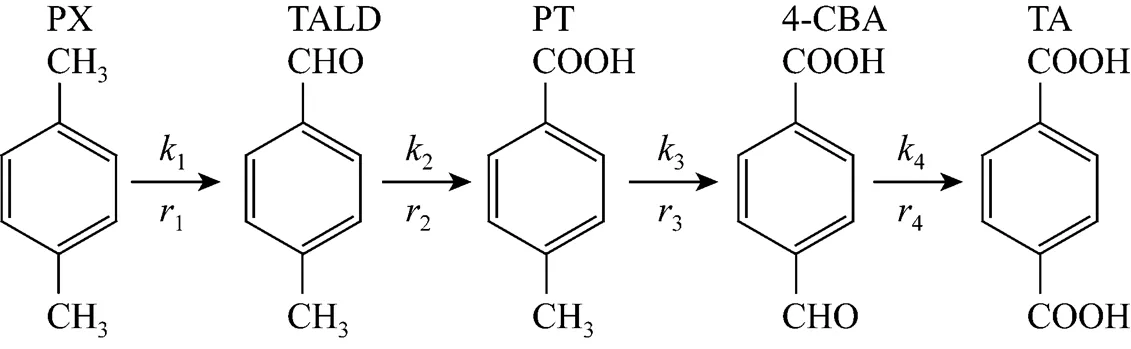
Figure 1 The main reaction scheme for the lumped kinetics of oxidation of-xylene to terephthalic acid
During the main reaction of PX oxidation to TA, a certain part of hydrocarbon reactant, intermediates and solvent get lost by side reactions of decarboxylation and decarbonylation, and are “over-oxidized”into carbon dioxide (CO2), carbon monoxide (CO), water (H2O), benzoic acid (BA), methyl acetate (MA) and methyl bromide,. They are generally known as the burning side reactions [2, 5, 20-24].Considering the large-scale production of TA, the loss of reactant, intermediates and HOAc solvent reaches a considerable amount. The generation of CO(CO2and CO) is generally considered as the burning side reaction rate index [5]. Ariko.studied the decarboxylation of acetic acid during the catalytic oxidation of-xylene, and found that the extent of the burning side reactions depended on the catalyst/promoter ratio but not on their absolute amounts [20, 21]. Ge reported the burning kinetics of the oxidation of pure acetic acid solvent with air over the MC catalyst system, but did not consider any function of PX oxidation to speed the solvent burning [22]. Roffia. studied methyl acetate formation in PX oxidation, and found that the recycle of methyl acetate to the oxidation medium appears a valid solution to recover acetic acid solvent [23]. Some studies show thatdecarboxylation and decarbonylation reactions of acetic acid solvent and intermediates were related to the concentration of the higher-valency form of cobalt, Co(III): more Co(III), more burning side reactions[2, 24]. The simplified mechanism of decarboxylation of acetic acid by Co(III) was shown as [2, 3, 24]

where Co(III) coordination compounds decarboxylate acetic acid ligands to form carbon dioxide and methyl acetate.
In our previous works,a large number of batch experiments were carried out to study the burning side reactions [5, 14, 18].The experimental results showed that the rate of burning side reactions was related to the main reaction closely and varied insignificantly during the PX oxidation. Considering the detailed radical chain reaction mechanism involved in the burning side reactions and several reasonable assumptions, we had hammered outa fractional-like kinetic model of burning side reactions:
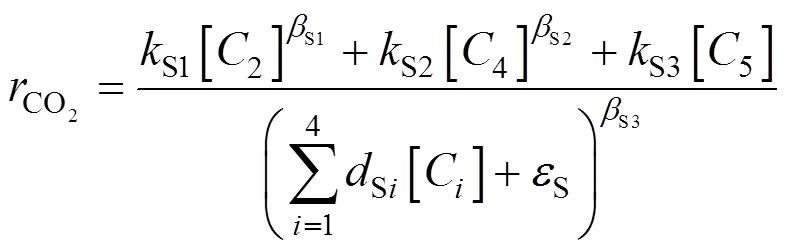

which can describe well the batch experimental dynamic curves of the generation rate of CO. The model Eq. (2) explained that the aldehyde intermediates and acetic acid were the primary contributors to the generation ofCO. This model was explained in detail in Refs. [5, 14].
Recently, we carried outa number of semi-continuous experiments of PX oxidation to TA, and found that the percentage error between the experimental burning rate and that predicted by Eq. (2) was up to 80% or more [19].It indicated that Eq. (2) was faulty in describing the burning side reaction in semi-continuous experiments. Therefore, a more practical kinetic model is required to be derived from the batch and the semi-continuous experimental data in connection with the mechanism of the burning side reaction, and the model parameters are to be determined based on both the batch and semi-continuous experimental results.
The purpose of this work is to report the relation between the burning side reactions and the main reaction during the liquid-phase oxidation of-xylene to terephthalic acid and more reliable kinetics of the burning side reactions are obtained. This work will be helpful for the optimizing control and reduction of consumption in the commercial plant.
2 EXPERIMENTAL
As the milder conditions alleviate the burning side reaction, the low-temperature oxidation technique is likely more competitive [25-27]. Therefore, several experiments at 160°C involved oxidation of PX to TA were carried out to study the kinetics of the burning side reaction in the present paper, including batch experiments and semi-continuous experiments.
2.1 Batch experiment

2.2 Semi-continuous experiment

2.3 Analysis
The reproducibility of the experimental runs was verified by repeating each of them at least twice. The liquid components of solvent, reactant, intermediates and product such as HOAc, PX, TALD, PT, 4-CBA and TA were analyzed by the Shimadzu GC-9A gas chromatography (GC) and Agilent 1100 liquid chromatograph (HPLC). Toluene was used as the internal standard substance to correlate the data obtained from GC and HPLC analysis.The analytical methods used in this work were described in detail by Cheng[5, 10, 15].


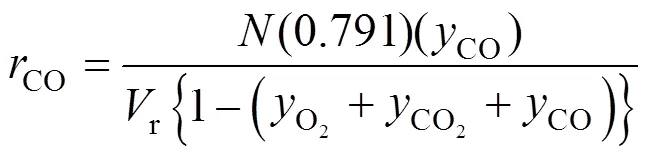
3 RESULTS AND DISCUSSION
3.1 Characteristics of the burning side reactions
The batch experimental data of the COgeneration rate were shown in Figs. 2 (a) and 2 (b). According to these experimental curves, it is evident that the generation rate of COcan be nearly divided into three stages: in the initial stage of the reaction, with the going on of the main reaction of PX oxidation, the generation rate of COalso increases sharply, and then the first peak value of the COgeneration rate appears; the generation rate of COdecreases at a slow rate in the middle portion of the reaction; on the later stage, the generation rate of COrises again, and drops suddenly and form a step at the end of main reaction. Combine the PX oxidation kinetic discussed in our previous works we can know that these characteristics are related with the main reaction closely. These will be described in detail in Section 3.3.
Figure 2 Rate of COgenerationtime in batch oxidation experiment(HOAc)/(PX):□ 20/1, exp.;○ 10/1, exp.;△ 5/1, exp.; ▽ 3/1, exp. ——: model fitting
Like the batch experimental results, the COgeneration rate presents the similar characteristic during the semi-continuous oxidation of-xylene. The generation rate of COincreases quickly at the beginning of the reaction; after the value reaches a platform, the rate remains invariable relatively during the oxidation; at the end of the semi-continuous oxidation, the generation rate of COdrops suddenly with the shutdown of the feed of-xylene. The semi-continuous experimental data of the COgeneration rate were shown in Figs. 3 (a) and 3 (b).
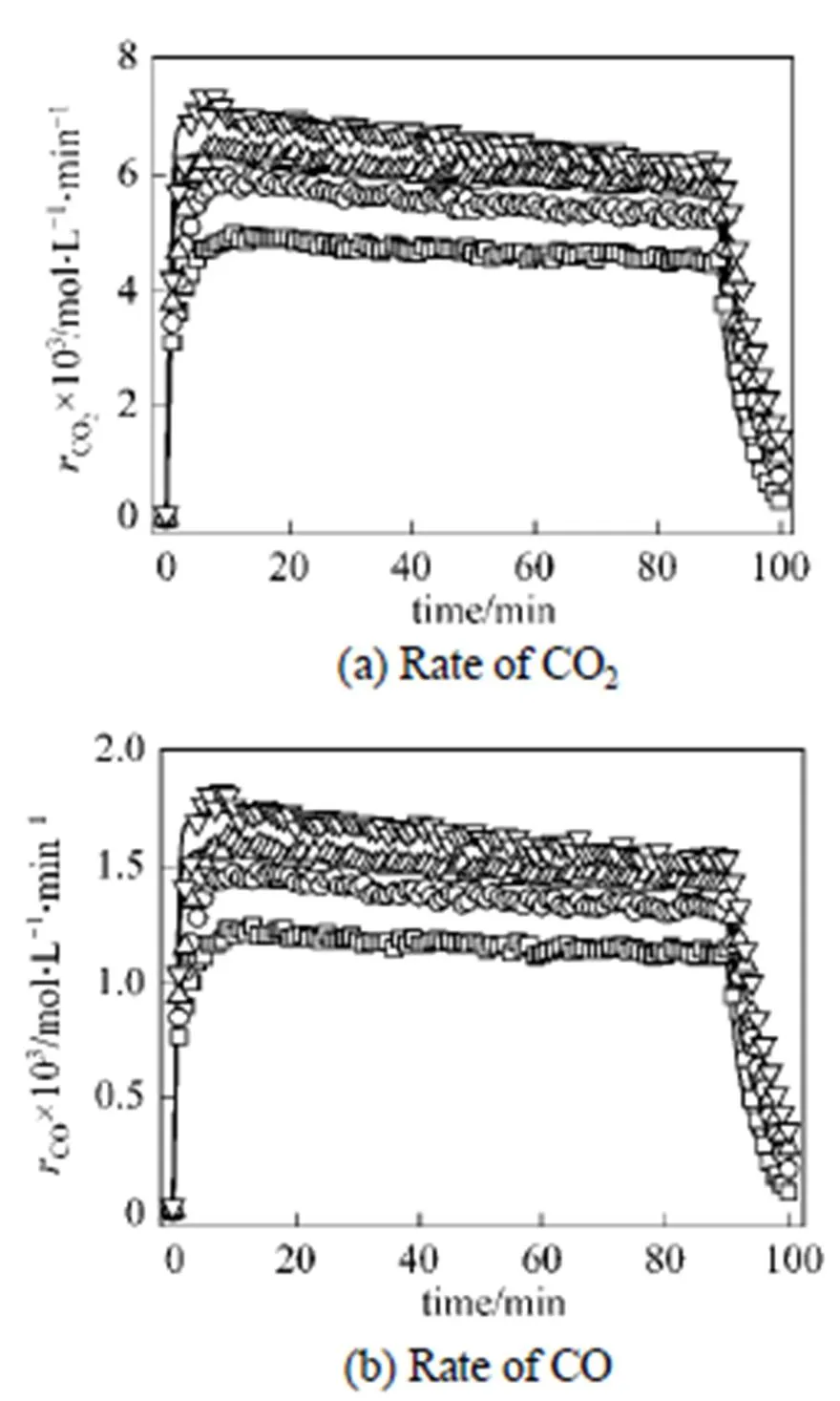
Figure 3 Rate of COgenerationtime in semi-continuous experimentPX/mol·min-1:□ 2.61×10-2, exp.;○ 3.17×10-2, exp.; △ 3.55×10-2, exp.;▽ 4.00×10-2, exp. ——: model fitting
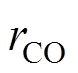


3.2 Mechanisms of the burning side reactions
3.2.1
Like the main reaction, the burning side reactions follow the radical chain reaction mechanism, too. They include the over-oxidation of reactant and intermediates, and the decarboxylation of the solvent acetic acid. Several active hydrocarbon radical, peroxide radical or oxygenic radical will be produced during the oxidation of PX [5]. Among these free radicals, RCO· and RCOO· mostly capture the hydrogen atoms of the reactant and intermediates and form products. At the same time, a small part of RCOO· and RCO· undergoes decarboxylation and decarbonylation and produces CO2and CO. Theconceivable reaction mechanism is shown in reactions (5)-(9):

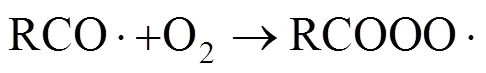
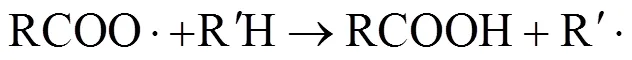



As the acetic acid solvent is one hydrogen-rich reactable compounds, it can be attacked by active free radicals and higher-valency metal ions, and produces CH3COO· or CH2·COOH radicals. They mostly can capture other hydrogen atoms of reactant to reduce into the acetic acid mostly, but a part of CH3COO· and CH2·COOH can also carry out decarboxylation and decarbonylation and over-oxidized into CO2, CO, water, methyl acetate and methyl bromide,. Theconceivable reaction mechanism consists of reactions (10)-(14):





where I· stands for active free radicals and higher- valency metal ions such as Co (III ) and Mn (III ),.
3.2.2
We have developed a fractional kinetic model:



Table 1 Parameters in the kinetics model Eq. (15) [19]

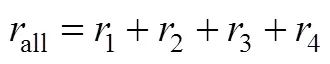

3.2.3
The active free radicals and higher-valency metal ions are produced continually along with the main reaction, and these active free radicals and higher-valency metal ions are essential for the occurrence of the burning side reactions. Meanwhile, some active free radicals and higher-valency metal ions will be consumed during the burning side reactions, as shown in Eqs. (5)-(14),., it may reduce the opportunity that the reactant being attacked by the active components. Therefore, the two may have certain competition relations.
To promote more systematic and deeper studies on the burning side reaction, analysis and comparison between the main and the side reaction are needed. Some indexes for the main and burning side reaction under the typical conditions are compared in this section.


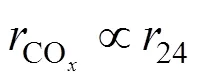
This correlation was also indicated by the semi- continuous experiments shown in Fig. 5.


3.3 Kinetics of the burning side reactions
According to the above mechanism analysis and data analysis, there exist both inter-stimulative and competitive relations between the burning side reaction and the main reaction, and the rate of the burning side reaction can be described with some key indexes of the main reaction, such as Eqs. (16) and (17). Combining Eqs. (16), (17) and (4), the kinetics of the burning side reaction can be described as follows:


whereCstand for the mole concentration (mol·L-1) of reactant and intermediates;rstand for the rate of corresponding step main reaction that shown in Fig. 4, which is calculated from Eq. (15);α,andare model parameters introduced and determined by experimental data fitting.


Table 2 Model parameters for the kinetics of burning side reaction in Eq. (18)

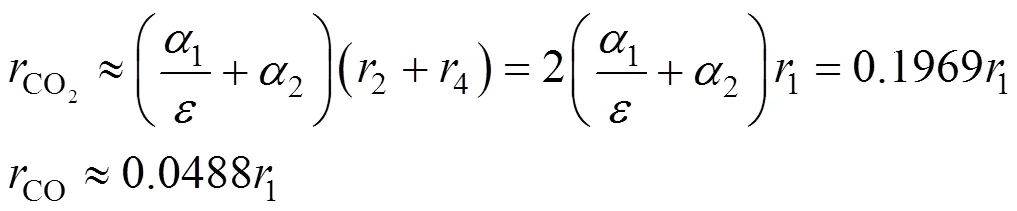


Table 3 Comparison of model estimated and measured results in industry reactor
3.4 Correlation of the selectivity of TA with burning side reactions
The mol selectivity of TA can be calculated from the mass of TA product and PX charged into the reactors. On account of the TA product consumption during sampling, separation and the leftover in the reactor, it was difficult to get accurately the total TA mass, which were statistic averages of 3 to 5 repeated experiments. The average generation rate of COin each run could be estimated by the experimental generation rate of CO2and CO..,


4 CONCLUSIONS
In this paper, batch and semi-continuous experiments were carried out to investigate the burning side reactionduring the MC catalytic oxidation of-xylene to terephthalic acid by molecular oxygen. The rate of generation of CO(CO2and CO) can be generally considered as the burning side reaction rate index. The experimental data showed that there were two factors that can influence the rates of burning rate markedly. One is the concentrations of reactant and intermediates, and another is the rates of the main reaction. The burning rateswere proportional to the rates of the main reaction, but decreased with the increasing of the concentrations of reactant and intermediates. According to the mechanism analysis and data analysis, the inter-stimulative and competitive relations between the burning side reaction and the main reaction were confirmed.
Furthermore, a kinetic model of the burning side reaction was developed as Eq. (18), and the model parameters were determined by data fitting.The obtained kinetics model could describe the burning side reaction adequately.


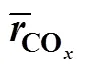
NOMENCLATURE
all1+2+3+4, mol·L-1
Cconcentration ofth component, mol·L-1
k rate constants of the main reaction, min-1
Si,Si,Si,Sformer kinetics model parameters from batch experiments
flow rate of air inlet, mol·min-1
all1+2+3+4, mol·L-1·min-1
COrate of CO generation, mol·L-1·min-1
rrate of theth step of the main reaction, mol·L-1·min-1
242+4, mol·L-1·min-1
rreaction volume, L
1,2,kinetic parameters from batch and semi-continuous experiments
model parameters, mol·L-1
1 Raghavendrachar, P., Ramachandran, S., “Liquid-phase catalytic oxidation of-xylene”,...., 31, 453-462 (1992).
2 Partenheimer, W., “Methodology and scope of metal/bromide autoxidation of hydrocarbons”,., 23, 69-150 (1995).
3 Cesar, M.A., PEP Report 9F: Terephthalic Acid, SRI Consulting (2005).
4 Suresh, A., Sharma M., Sridhar, T., “Engineering aspects of industrial liquid-phase air oxidation of hydrocarbons”,...., 39, 3958-3997 (2000).
5 Cheng, Y.W., “Studies on MC process of hydrocarbon liquid phase catalytic oxidation”, Ph.D. Thesis, Zhejiang University, Hangzhou, China (2004). (in Chinese)
6 Cao, G., Massimo, P., Massimo, M., “A lumped kinetic model for liquid-phase catalytic oxidation of-xylene to terephthalic acid”,...,49, 5775-5788 (1994).
7 Cao, G., Alberto, S., Massimo, P., “Kinetics of-xylene liquid-phase catalytic oxidation”,., 40, 1156-1166 (1994).
8 Cincotti, A., Orru, R., Bori, A., Cao, G., “Effect of catalyst concentration and simulation of precipitation processes on liquid-phase catalytic oxidation of-xylene to terephthalic acid”,...,52, 4205-4213 (1997).
9 Cincotti, A., Orru, R., Cao, G., “Kinetics and related engineering aspect of catalytic oxidation of-xylene to terephthalic acid”,., 52, 331-347 (1999).
10 Cheng, Y.W., Zhang, L., Xie,G., Li, X., “Experiment technique of-xylene liquid phase catalytic oxidation”,... (), 19, 182-187 (2003). (in Chinese)
11 Wang, L.J., Li, X., Xie,G., Cheng, Y.W., Sima, J., “Studies on the kinetics of the-xylene liquid phase catalytic oxidation (I) Mechanism and kinetic model”,....(), 54, 946-952 (2003). (in Chinese)
12 Xie, G., Li, X., Niu, J., “Studies on the kinetics of the-xylene liquid phase catalytic oxidation (II) Temperature effect”,....(), 54, 1013-1016 (2003). (in Chinese)
13 Cheng, Y.W., Li, X., Niu, J., “Studies on the kinetics of the-xylene liquid phase catalytic oxidation (III) Catalyst effect”,.(), 55, 580-585 (2004). (in Chinese)
14 Cheng, Y.W., Li, X., Sima, J., “Studies on the kinetics of the-xylene liquid phase catalytic oxidation (IV) Kinetics for PX and solvent burning”,....(), 55, 1894-1899 (2004).(in Chinese)
15 Wang, Q.B., Li, X., Wang, L.J., Cheng, Y.W., Xie, G., “Kinetics of-xylene liquid-phase catalytic oxidation to terephthalic acid”,....,44, 261-266 (2005).
16 Wang, Q.B., Li, X., Wang, L.J., Cheng, Y.W., Xie, G., “Effect of water content on the kinetics of-xylene liquid-phase catalytic oxidation to terephthalic acid”,...., 44, 4518-4522 (2005).
17 Cheng, Y.W., Li, X., Wang, L.J., Wang, Q.B., “Effects of guanidine on the liquid-phase catalytic oxidation of-xylene to terephthalic acid”,....,44,7756-7760 (2005).
18 Cheng, Y.W., Li, X., Wang, L.J., Wang, Q.B., “Optimum ratio of Co/Mn in the liquid-phase catalytic oxidation of-xylene to terephthalic acid”,....,45, 4156-4162 (2006).
19 Wang, Q.B., “Reactive crystallization in the oxidation of-xylene”, Ph.D. Thesis, Zhejiang University, Hangzhou, China (2006). (in Chinese)
20 Ariko, N.G., “Effect of deuteration of solvent on process of catalytic oxidation of-xylene and associated decarboxylation of acetic acid”,.., 32, 757-761 (1992).
21 Kenigsberg, T.P., Ariko, N.G., Agabekov, V.E., “Effect of catalyst composition on decreasing of CO2and CO formation in synthesis of aromatic acids”,.., 36, 677-680 (1995).
22 Ge, X., “Studies on catalytic oxidation kinetic oxidation of acetic acid-xylene system in liquid phase”,(), 22, 715-721 (1993).(in Chinese)
23 Roffia, P., Calini, P., Tonti, S., “Methyl acetate: byproduct in the terephthalic acid production process. Mechanisms and rates of formation and decomposition in oxidation”,....,27, 765-770 (1988).
24 Partenheimer, W., “A chemical model for the amoco MC oxygenation process to produce terephthalic acid”, In: Catalysis of Organic Reactions, Blackburn, D.W., eds., Marcel Dekker, New York, 321-346 (1990).
25 Wonders, A.G., Lavoie, G.G., Sumner, C.E., “Optimized liquid-phase oxidation”, US Pat., 20060205976 (2006).
26 Sumner, C.E., Hembre, R.T., Lange, D., “Processes for producing terephthalic acid”, US Pat., 20060205977 (2006).
27 Lin, R., “Process for energy recovery in processes for the preparation of aromatic carboxylic acids”, US Pat., 7049465 (2006).
28 Wang, Q.B., Cheng, Y.W., Wang, L.J., Li, X., “Aging of crude terephthalic acid crystals at high temperatures”,...,46,7367-7377 (2007).
29 Wang, Q.B., Cheng, Y.W., Wang, L.J., Li, X., “Semicontinuous studies on the reaction mechanism and kinetics for the liquid-phase oxidation of-xylene to terephthalic acid”,..., 46, 8980-8992 (2007).
2008-02-18,
2008-11-11.
the Natural National Science Foundation of China (20080672) and the Research Fund for the Doctoral Program of Higher Education of China (200803351111).
** To whom correspondence should be addressed. E-mail: wang_lijun@zju.edu.cn
猜你喜欢
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Design and Performance Analysis of Micro Proton Exchange Membrane Fuel Cells*
- Isolation of Cordyceps ophioglossoides L2 from Fruit Body and Optimization of Fermentation Conditions for Its Mycelial Growth*
- Efficient and Comprehensive Utilization of Hemicellulose in the Corn Stover*
- Simulating Surface Aeration Systems at Different Scale of Mixing Time*
- Kinetics of Reaction-Crystallization of Struvite in the Continuous Draft Tube Magma Type Crystallizers—Influence of Different Internal Hydrodynamics
- Preparation and Characterization of Tungsten-substituted Molybdophosphoric Acids and Catalytic Cyclodehydration of 1,4-Butanediol to Tetrahydrofuran*
