健康成年小鼠和AD模型小鼠在GABA受体调节后脑电与记忆的不同相关模式
2022-06-08周正李连娥段梦思孙华英易虎付玉
周正,李连娥,2,段梦思,孙华英,易虎,付玉
1. 昆明理工大学医学院,云南 昆明 650500
2. 重庆三峡学院生物与食品工程学院,重庆 404100
3. 云南中医药大学中药学院,云南 昆明 650500
4. 云南大学信息科学与工程学院,云南 昆明 650091
Alzheimer's disease (AD) is an important social health concern. As life expectancy increases,the morbidity of neurodegenerative diseases,such as AD,also rapidly increases. AD is the most common cause of dementia in the elderly. According to the 2015 World Alzheimer Report,the incidence of dementia increases exponentially with increasing age,with the most rapid increase occurring after 70 years old. Evidence suggests that brain senescence contributes to AD pathogenesis[1-2],and a link exists between the aging process and disease development[3-5]. As aging is an important risk factor for AD, it would be interesting to compare differences between AD and normal aging, and thus help to clarify the neural mechanisms of AD.
Normal aging and AD are both associated with brain atrophy, neuronal loss, neurotransmitter dysfunction, impaired functional connectivity, and cognitive decline, although these issues are more pronounced in AD than in normal aging[6]. For example, AD patients exhibit worse memory dysfunction and deficits in the early stages compared with normal aging[6]. Therefore, whether AD pathological characteristics represent an acceleration of the aging process has been a topic of great interest[6-8].
Accumulating evidence suggests that an imbalance in excitation and inhibition (E/I) especially that originated from gamma-aminobutyric acid(GABA) inhibition dysfunction is involved in both AD and aging[6,9]. Indeed, normal aging is accompanied by degeneration of GABA inhibition[10], with recent evidence also suggesting that GABA system dysfunction is involved in AD neuropathology[11-12].Furthermore, changes in GABA neurotransmitters are thought to be related to cognitive deficits in AD[13],and GABA receptor-related drugs have been used for cognitive recovery in AD treatment[14].
We previously highlighted the potential use of low-dose GABAAdrugs to improve cognition and restore neural network activity in AD[15]. Notably,GABAAagonists and antagonists may adjust E/I balance differently, resulting in brain function measures distributed scatteredly. In the current study,based on these scattered distributions, we analyzed the relationship between behavioral cognitive performance and cortical electrical activity in normal and AD mice. Both the AD and normal mice used in this study were 12 months old and the degree of senescence, according to the lifespan, corresponded to 42.5 years of human age[16]. Thus, our results should provide information on the similarities and differences between normal aging and AD.
1 Methods
In the present study, electrophysiological recordings were used following previous experiments (15),in which EEGs were recorded from mice with GABAAintervention during Y-maze task performance(Fig. 1A). The work revealed changes in EEG frequency activity and behavioral memory after GABAAagonist and antagonist treatments as well as the role of GABAAintervention in AD model mice (Fig. 1B).In the current study, we pooled all drug-group data together for correlation analyses between EEGs and behavioral changes and focused on differences in the correlation patterns between normal middle-aged and AD mice (Fig.1C). Please see Fu et al.[15]for detailed information regarding electrophysiological recordings in mice.
1.1 Animals and drugs
All of the procedures involving animal care and sacrifice (using pentobarbital) were carried out in accordance with the guidelines for the National Care and Use of Animals and were approved by the National Animal Research Authority (approval number:2016024). APP/PS1 double transgenic mice (amyloid precursor protein/presenilin-1 double transgenic,APPswe/PSEN1dE9; abbreviated to AD mice) and wild-type (WT) littermates were purchased from the Nanjing Biomedical Research Institute of Nanjing University (China). This mouse strain develops betaamyloid (Aβ) deposits in the brain by 6-7 months of age. All mice included in this study were male and~12 months of age(53-55 weeks). They were housed in a temperature- and humidity-controlled room on a natural light-dark cycle with food and water availablead libitum.
After the experiments, the locations of the recording electrodes were confirmed histologically.They were anesthetized with pentobarbital and perfused with saline followed by 4% paraformaldehyde (Tianjin Guangfu Fine Chemical Research Institute, China). The brains were taken out and frozen sectioned to confirm the recording locations by Nissl staining. Data were excluded from any mice in which the recording locations were misplaced[15].
The GABAAreceptor agonist muscimol hydrobromide (Sigma-Aldrich, St. Louis, MO, USA) and antagonist (+) -bicuculline (Selleck, Houston, TX,USA) were dissolved in 0.9% saline (Shandong Kangning Pharmaceutical Co.,Ltd.,China). In total,22 AD mice and 19 WT mice were separately divided into three drug groups with 6-8 mice in each group.Mice were intraperitoneally (i.p.) injected with a single dose (0.1 mg/kg, 0.1 mL/10 g body weight)of muscimol (Mu) or bicuculline (Bi). Control mice received saline only. Drug administration was performed 30 min before the Y-maze task(Fig.1A).
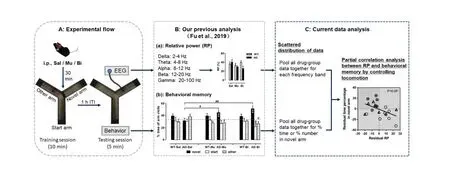
Fig.1 Experimental design and data analyses
1.2 EEG experiment[15]
Before the EEG experiment,mice were implanted with three recording electrodes over the right hippocampus (Hip: AP: -1.82 mm, ML: +1.5 mm, DV:-1.7 mm from skull), right prefrontal cortex (PFC:AP: +2.95 mm, ML: +1.5 mm, DV: -0.75 mm from dura), and left occipital cortex (Ctx, as a control:AP:-2.80 mm, ML: -2.25 mm), respectively. The implantation surgery was conducted under pentobarbital anesthesia (60 mg/kg, i. p.; dissolved in 0.9%sodium, 10 mg/mL, Merck, Darmstadt, Germany).The Hip and PFC recording electrodes consisted of two twisted pairs of perfluoroalkoxy (PFA)-coated stainless steel wires (diameter 0.002″, A-M systems,WA, USA), and that in the Ctx was a stainless-steel watch screw (M 1.0 mm × L 2.0 mm, RWD). In addition, two watch screws were placed in contact with the dura, one above the left olfactory bulb as a reference electrode and the other in the central cerebellum as a ground electrode. All five electrodes were attached to a five-pin array and secured with dental acrylic. Animals were allowed at least one week to recover from surgery.
The Y-maze EEG recordings were performed in a shielding cage with a ceiling-mounted CCD camera monitoring the behaviors of mice. The EEGs were recorded by a signal acquisition system (Intan Technologies, Los Angeles, CA, USA) consisting of an RHD2132 amplifier, RHD2000 USB interface board,and RHD2000 Interface GUI Software. The EEG sampling rate was 1 kHz. The five-pin array on the head of each mouse was connected to the amplifier,then to the interface board,and finally to the computer.
The Y-maze consisted of three arms (included angle of 120°), which was placed in the shielding cage. The walls of the cage and maze were decorated with a few visual spatial cues. The Y-maze task consisted of two sessions with a 1-h interval between them (Fig. 1A): (1) 10-min training session, in which the mouse was allowed to explore only two arms; (2)5-min testing session, in which the mouse was allowed to explore all three arms. The arm blocked in the first session and open in the second session was novel to the animal,i.e.,the novel arm.
1.3 Data analysis
The EEG signals were analyzed off-line by MATLAB. Continuous EEG data were first separated into segments, each including 1 024 sample points.After rejection of segments with artifacts, each segment was filtered with no 50 Hz notch filter for five frequency bands: (1) delta: 2-4 Hz, (2)theta: 4-8 Hz, (3) alpha: 8-12 Hz, (4) beta: 12-20 Hz, and (5) gamma: 20-100 Hz. For each segment,the absolute power for each frequency band was calculated as
P=Σx2/1 024.
Total power was the sum of the power of all frequency bands. The relative power (RP) for each frequency band was the percentage of its absolute power to the total power. The mean RP of the testing session during the Y-maze task was used for further analysis.
Behavioral memory ability in each mouse was evaluated by the percentages of time spent in the novel arm (%Time) and number of entries in the novel arm (%Number) to all three arms during the Y-maze testing session. In addition, the total number of entries in all arms was used as an indicator of locomotion.
To investigate the correlation between EEG activity and behavioral memory, partial correlation analysis was used with locomotion as a covariate in the WT and AD groups. For each group,all drug data were pooled together for analysis. Partial correlation residuals were obtained by linear regression analysis,with EEG or behavioral memory ability as the dependent variables and locomotion as the independent variable. In the scatter plots of partial correlation residuals, the areas of interest (AOIs) were the upper-left corner for negative correlations (e. g., dotted area in Fig. 2A) and upper-right corner for positive correlations (e. g., dotted area in Fig. 3A). The animals distributed in these AOIs were those with better memory scores and decreased or increased EEG frequency activities. The ratios of the number of animals distributed in each AOI to total number of animals were compared among the different drug treatments by chi-squared tests.P-values of < 0.05,<0.01, and < 0.001 were considered significant,highly significant, and very highly significant, respectively.
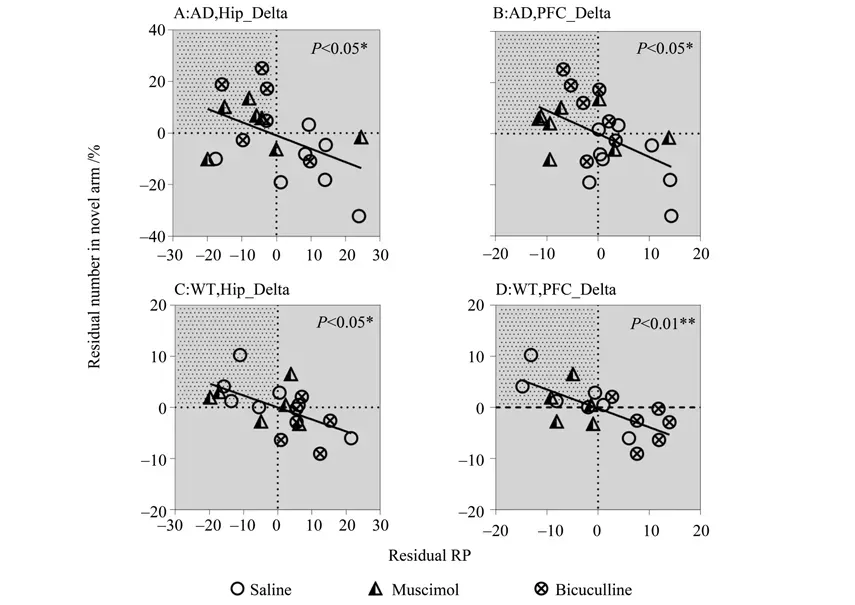
Fig.2 EEG activity in delta band showing negative correlations with memory performance in AD (A-B)and WT(C-D)mice
2 Results
Table 1 displays the results of partial correlation analyses between EEG activity and behavioral memory ability in the WT and AD mice. Results showed both similar and different correlations between the two groups. In both groups, Hip and PFC EEG activities in the delta band were significantly negatively correlated with time spent (%) or number of entries (%) in the novel arm (see Table 1 for correlation coefficients andP-values, same below). In the WT group, Hip EEG activity in the beta-gamma bands and PFC EEG activity in the alpha-gamma bands were significantly positively correlated with number of entries (%) in the novel arm; in the AD group, Hip EEG activity in the theta band showed a significant positive correlation with the number of entries(%)in the novel arm.No significant correlations between Ctx EEG activity and behavioral memory ability were found in any group.
The significant correlations above are also shown as scatter plots in Fig. 2-3, with the partial correlation residuals for EEG and memory indices presented. The plots also show the distribution of animals injected with muscimol, bicuculline, or saline along the regression lines. Interestingly, different distributions were found between the WT and AD groups according to different drug administration,especially for bicuculline. For the negative correlations (Fig. 2), bicuculline treatment decreased Hip and PFC EEG activity in the delta band but improved memory performance in AD mice (Fig. 2A-B). In contrast, bicuculline treatment showed the opposite effect on WT mice (Fig. 2C-D). As seen in Table 2,compared with the saline-treated mice, bicuculline administration significantly increased the number of AD animals in the AOI, but significantly decreased that number in the WT group (see Table 2 for chi-square andP-values, same below). For the positive correlations (Fig. 3),bicuculline treatment mainly affected the WT mice, rather than the AD mice. In this group, drug treatment decreased Hip and PFC EEG activity in the mid-high frequency bands(alpha-gamma) and decreased memory performance(Fig. 3B-F). Furthermore, the number of animals in the AOI significantly decreased after bicuculline treatment compared with that after saline treatment(Table 3,rows 2-6).
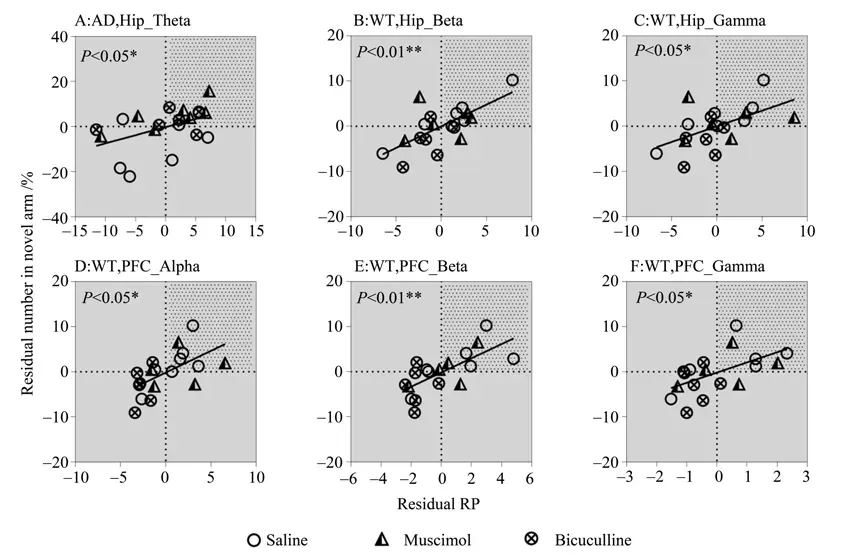
Fig.3 EEG activity in theta-gamma bands showing negative correlations with memory performance
In addition, muscimol treatment decreased Hip and PFC EEG activity in the delta band and improved memory in AD mice but not in WT mice (Fig. 2-3).The number of animals in the AOI was significantly higher after muscimol treatment than that after saline treatment(Table 2,rows 1-2).
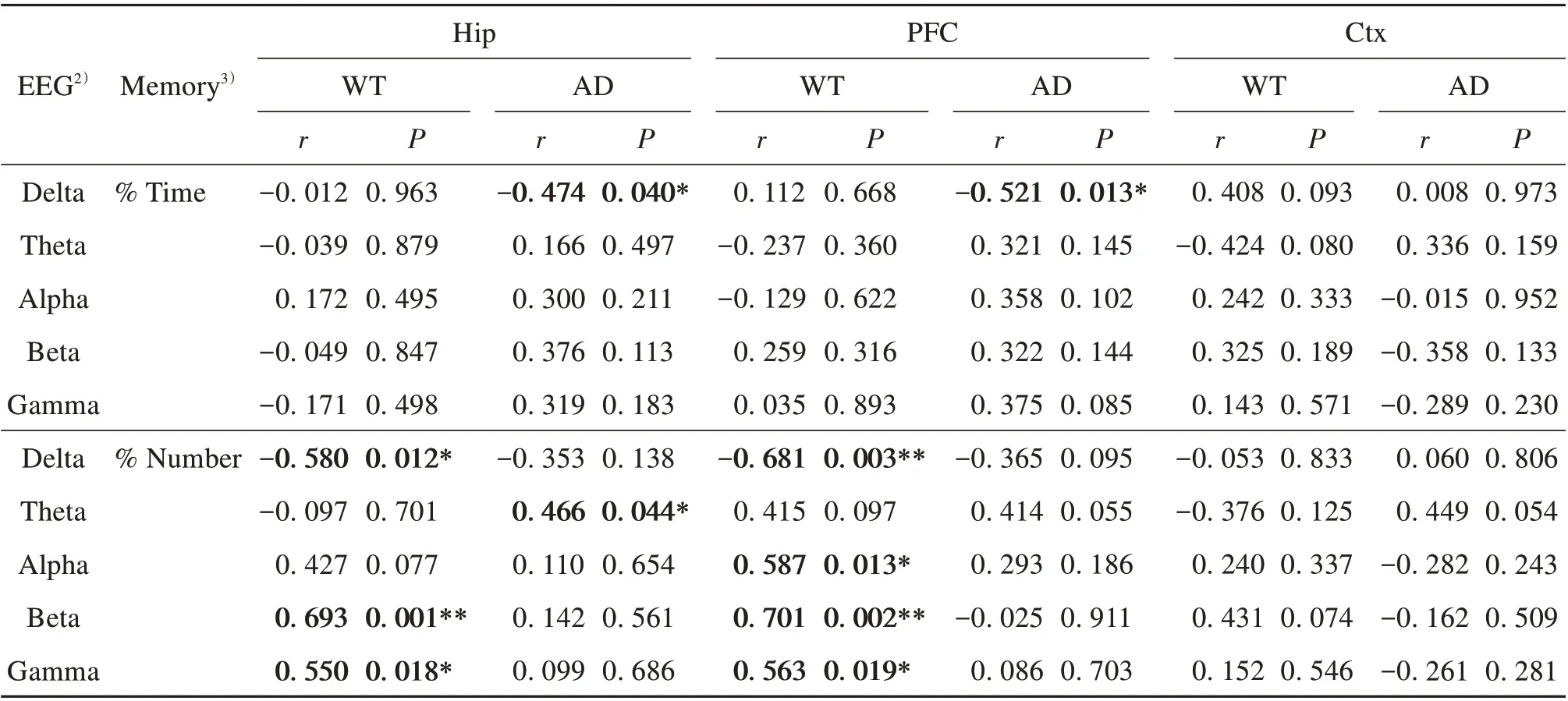
Table 1 Partial correlation analysis(controlling for locomotion)between EEG activity and memory performance in WT and AD mice1)
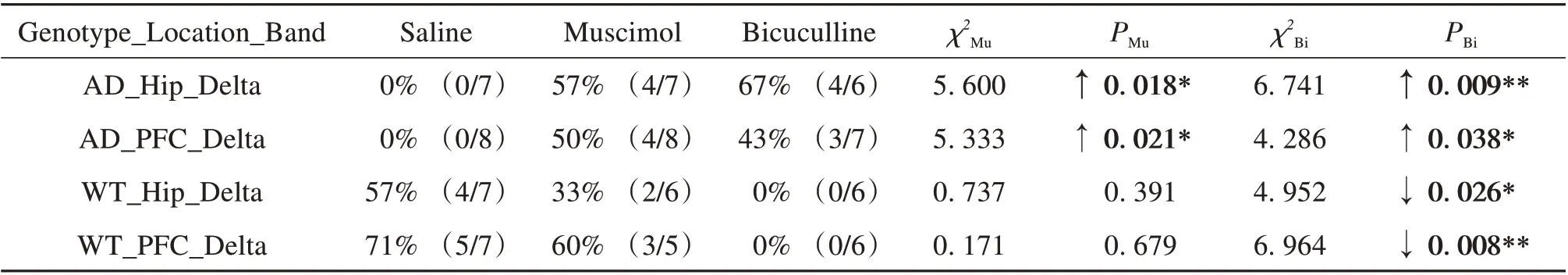
Table 2 Comparison of number of animals in area of interest(AOI)among drug treatments for negative correlations1)
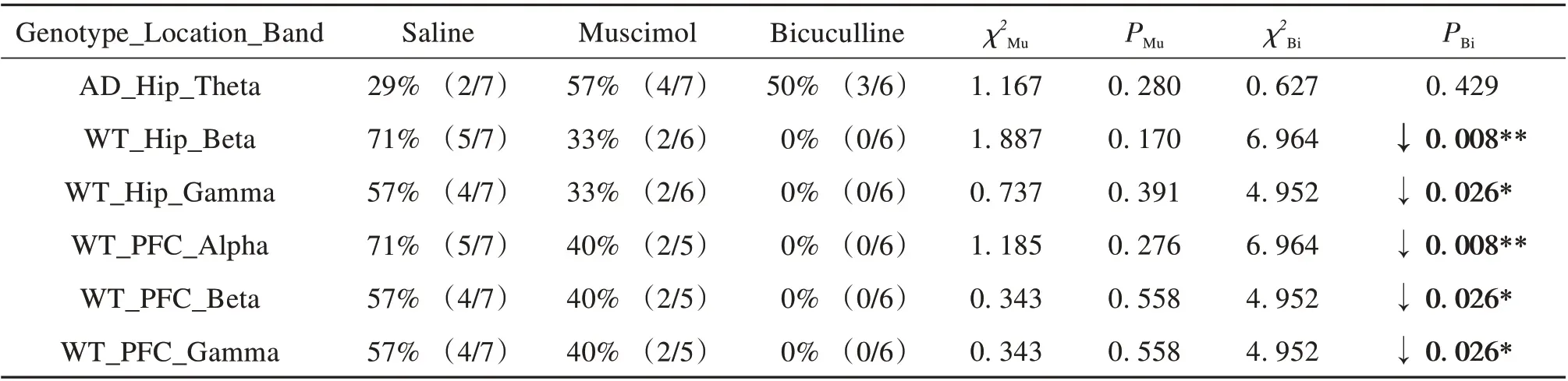
Table 3 Comparison of number of animals in area of interest(AOI)among drug treatments for positive correlations1)
3 Discussion
In this study, we found that: (1) WT and AD mice showed different correlation patterns between Hip/PFC EEG activity and behavioral memory ability;(2) based on regression correlations, treatment with the GABAAantagonist bicuculline improved brain function in AD mice,but resulted in poorer brain function in WT mice; (3) treatment with the GABAAagonist muscimol also benefited AD mice, but showed no obvious effect on WT mice.
Previous researches have suggested that EEG oscillations in specific frequency bands can reflect behavioral or cognitive states[17]. In the present study,correlations between Hip/PFC EEG activity in various frequency bands and behavioral memory abilitydiffered in WT and AD mice. Correlations in EEG activity could be detected across many frequency bands (except theta) in WT mice but were mainly found in the low-frequency bands (delta-theta) in AD mice. Because the WT and AD mice performed the same task, divergence in the behavior-EEG correlations might indicate differences in Hip/PFC functional state between them. Our results are similar to those of other studies. For example, earlier research showed that Hip-PFC connection strength and information transfer efficiency increased in control animals during working memory tasks, but showed no change in Aβ-injected animals[18].
In the current study, we found that cognitive performance in AD mice was associated with EEG activity in the delta-theta frequency bands, which is worthy of attention. Most EEG studies in AD patients or animals are performed during spontaneous state,and relatively few studies are conducted during task performance[19]. However, EEG slowing, especially increases in delta power, is a typical phenomenon accompanying cognitive symptoms in the AD brain[17,20-21]. Delta power could also be detected to positively correlate with Aβ1-42 peptide levels[21]. In addition, disrupted theta oscillations were observed in rats following exposure to Aβ and in transgenic AD mice with Aβ pathology[17]; and reduced theta activity was found in AD mice during cage exploration[20].Importantly, EEG anomalies in the two frequency bands occurred during the early stage of AD[22-23].Thus, we found that spatial memory deficits were significantly related to delta-theta EEG activity,which may provide a direction for future AD treatment,e.g.,decreasing delta and increasing theta EEG activity to aid cognitive recovery. Indeed, several current noninvasive interventions involve the gradual improvement of the functional state of the AD brain via transcranial alternating current stimulation[24]or visual/auditory stimulation[25]at a specific frequency band.
Interestingly, in our study, treatment with the GABAAantagonist bicuculline had the opposite effect on the behavior-EEG correlations in WT and AD mice. Delta EEG activity was negatively correlated with memory performance in both groups. However,bicuculline treatment in AD mice decreased delta activity and improved memory performance, contributing to better brain function, whereas bicuculline treatment in WT mice showed the opposite pattern,leading to poorer brain function. The negative effects of bicuculline in WT mice were also supported by decreased alpha-gamma EEG activity and impaired memory performance.
On one hand,we previously discussed that modulation of E/I balance can contribute to recovery of brain function in AD mice[15]. The benefits of the GABAAantagonist may relate to the restoration of synaptic plasticity, compensation for the dampening of hyperexcitation, and cooperation with the cholinergic system[26-29]. On the other hand, WT mice were at an early stage of senescence. Aging can impair the GABA inhibitory system, leading to E/I imbalance[4].From this point of view, GABA antagonists may potentiate the E/I imbalance toward excitation in aged animals. This imbalance could cause changes in neural network activity[4]and impair different forms of memory function, including Hip/PFC-dependent memory[30]. This may explain why exposure to bicuculline in the normal-aged WT mice resulted in changes in the behavior-EEG correlations and poorer brain function.
Our study also showed that administration of the GABAAagonist muscimol improved the behavior-EEG correlations in AD mice but had no significant effect on WT mice. The apparent paradox of muscimol and bicuculline having similar effects on AD mice has been discussed previously[15]. A possible explanation is that while muscimol is widely used as a selective GABAAagonist but, in fact, it has more potent action as a partial agonist at GABACreceptors[31]. However, GABAAand GABACreceptors might have opposing action on memory[32]. Muscimol may activate both GABAAand GABACreceptors together, whereas bicuculline blocks only GABAAreceptors, thus confounding the opposing involvement of these receptors on some aspects of memory.The benefits of muscimol in our AD mice may also relate to neuroprotective effects, inhibition of Aβinduced cascade, and normalization of GABA expression[15]. Unexpectedly, we have found that muscimol,as a GABAAagonist, had no significant effect on the normal middle-aged (WT) mice. As GABA-mediated inhibition degrades during aging, muscimol should be effective on old animals[33]. The reason for this may be due to the small sample size,relatively low dose of administration, and relatively young middle-aged animals. It would be interesting to determine whether muscimol is effective in older animals by adjusting these variables in future studies. In addition, the different expression of GABA receptors and their subunits between AD and aging and between different aging processes may also be an important reason, but the question requires further investigations. It has been concluded that while aging and AD-related changes in GABA receptor subunits are modest or preserved[34-35], the mechanisms that compensate for these changes may alter the functional properties of the receptors[36]. It is therefore crucial to understand the subunit composition of GABA receptors and their functional changes in normal aging process and AD.
Whether AD represents an acceleration of the aging process has been discussed previously[37]. Here,because the normal-aged mice showed different behavior-EEG correlation patterns from the AD mice,and because the GABAAantagonist produced the opposite effects on the two groups, we suspect that AD exhibits distinct and unique patterns of disrupted brain functional state. Thus, the data may not support the accelerated aging hypothesis of AD. Our results are in accordance with several previous studies[6,8,37],which suggest that normal aged brains exhibit general changes, but AD brains manifest changes in specific areas[37].
In summary, we found different correlation patterns between EEG and memory ability after E/I balance adjustment in normal middle-aged mice and AD mice. This work provides new insights into future AD treatment based on low-frequency EEG activity to aid cognitive recovery.
