蚯蚓修复污染土壤的作用与机理研究进展
2021-11-19顾浩天袁永达张天澍滕海媛常晓丽王冬生
顾浩天 袁永达 张天澍 滕海媛 常晓丽 王冬生

摘要:蚯蚓是土壤生态系统中最大的动物区系,在维持土壤理化结构、提高土壤肥力、促进有机质降解及营养矿化循环、改善微生物群落等方面有重要生态作用。此外,蚯蚓可以抵御高浓度土壤污染,对无机物和有机物污染均有良好的修复效果,利用其修复污染土壤具有环境友好、高效、低成本等优势。蚯蚓对土壤污染物的吸收积累、转化降解作用较为复杂且与多种生物、非生物因素相关。本文综述了蚯蚓修复土壤污染的潜能、影响因素及机理,同时展望蚯蚓修复的应用前景。
关键词:蚯蚓;土壤;污染修复;重金属;有机污染物;机理
中图分类号:X53 文献标志码: A
文章编号:1002-1302(2021)20-0030-10
收稿日期:2021-01-08
基金项目:上海市科技兴农重点推广项目[编号:沪农科推字(2018)第4-14号]。
作者简介:顾浩天(1993—),男,辽宁阜新人,硕士,研究实习员,主要从事农药生态毒理效应及环境风险、农业害虫生理分子调控机理相关研究,E-mail:guhaotian@saas.sh.cn;共同第一作者:袁永达(1972—),男,上海人,硕士,副研究员,主要从事农药学、农业昆虫与害虫防治相关研究。
通信作者:王冬生,硕士,研究员,主要从事农药学、农业昆虫与害虫防治相关研究。E-mail:zb3@saas.sh.cn。
近年来,土壤污染随着城镇化、工业化的发展日趋严峻,已成为全球普遍关注的热点问题[1]。土壤污染物会对生物的生长、繁殖、多样性等造成负面影响,甚至通过食物链的富集级联威胁人类健康[2]。Zeng等调查了我国1 781个农田土壤样点的5 597个样品复合污染的状况,结果表明:我国农田土壤受污染的比例为22.1%,其中1.23%的土壤样品为重度污染[3]。因此,消除或减轻土壤污染问题迫在眉睫[4]。
常见的土壤污染修复技术有物理、化学和生物修复技术[5]。生物修复技术旨在利用微生物、植物、动物降解或清除污染物[6]。现阶段,生物修复的研究大多关注微生物修复[7]、植物修复[1],而利用动物修复土壤的研究相对较少。蚯蚓修复旨在利用蚯蚓降解土壤污染物[8],可作为土壤生物修复的替代技术[5]。蚯蚓是土壤生态系统中生物量最大的无脊椎动物,因其具有较强的环境适应性、生殖力及对有机污染物表现出极强的耐性和抗性等优势[9],被广泛地应用于土壤污染生物修复中。蚯蚓掘穴、取食、呼吸、排泄等生理活动不仅可以改良土壤结构、理化性质,还可以改变土壤污染物的迁移转化、生物有效性及空间分布[10-11]。蚯蚓修复的机理主要为通过取食或表皮从土壤中吸收有毒有害物质[12];通过增加土壤微生物活性、营养物质生物有效性、植物生长等方式提高微生物修复或植物修复效率[13]。
已有学者研究了蚯蚓对土壤重金属[14]、多环芳烃(PAHs)[15]、农药[16]等的降解修复,但其中相关机理仍不清楚。鉴于此,本文综述了蚯蚓对污染土壤的修复作用与机理。同时对蚯蚓修复技术的应用前景进行了展望,以期为未来研究蚯蚓修复技术提供参考和依据。
1 利用蚯蚓修復污染土壤
1.1 消除土壤中重金属污染
蚯蚓对土壤中的重金属污染具有修复潜力[17]。蚯蚓肠组织中的黄色细胞(chloragogenous tissue)可以吸收较高浓度的重金属[18],且随着环境中铜(Cu)和镉(Cd)浓度升高,其在蚯蚓体内的含量也逐渐升高[19]。Lavelle研究表明,蚯蚓黏液中含有—COOH、—NH2、—CO等活性基团,能够络合、螯合重金属,提高土壤中重金属的活性[20]。Suthar等发现Eisenia fetida体内可积累高浓度的重金属,如铅(Pb)、Cd、铬(Cr)、Cu[21]。Suthar利用E. fetida处理酿酒厂淤泥后发现体内组织中锌(Zn)、锰(Mn)、Cu含量显著升高,而环境中的含量分别减少32%、31.8%、43.5%[22]。与上述结论一致,Wu等报道E. fetida处理Cd污染土壤后,消除了土壤环境中17.60% Cd含量[23]。
蚯蚓对重金属的生物积累作用也与重金属种类及形态相关。Cd、汞(Hg)、Zn因其生物富集系数(BAF)显著>1.0而易被蚯蚓吸收积累[24]。Nannoni等发现在蚯蚓Allolobophora rosea和Nicodrilus caliginosus中,几种重金属的生物富集系数从大到小依次为Cd > Zn > Cu>As=Pb=Sb[25]。lvarez等发现单甲基汞(monomethyl-mercury,MeHg)的生物富集系数远高于Hg[26],表明甲基化汞由于其高脂溶性更易被蚯蚓吸收积累[27]。蚯蚓对不同重金属的吸收也取决于重金属的生物有效性[28]。重金属的生物有效性受多种环境因素影响,如重金属的溶解性、与土壤有机质络合情况[29]等。
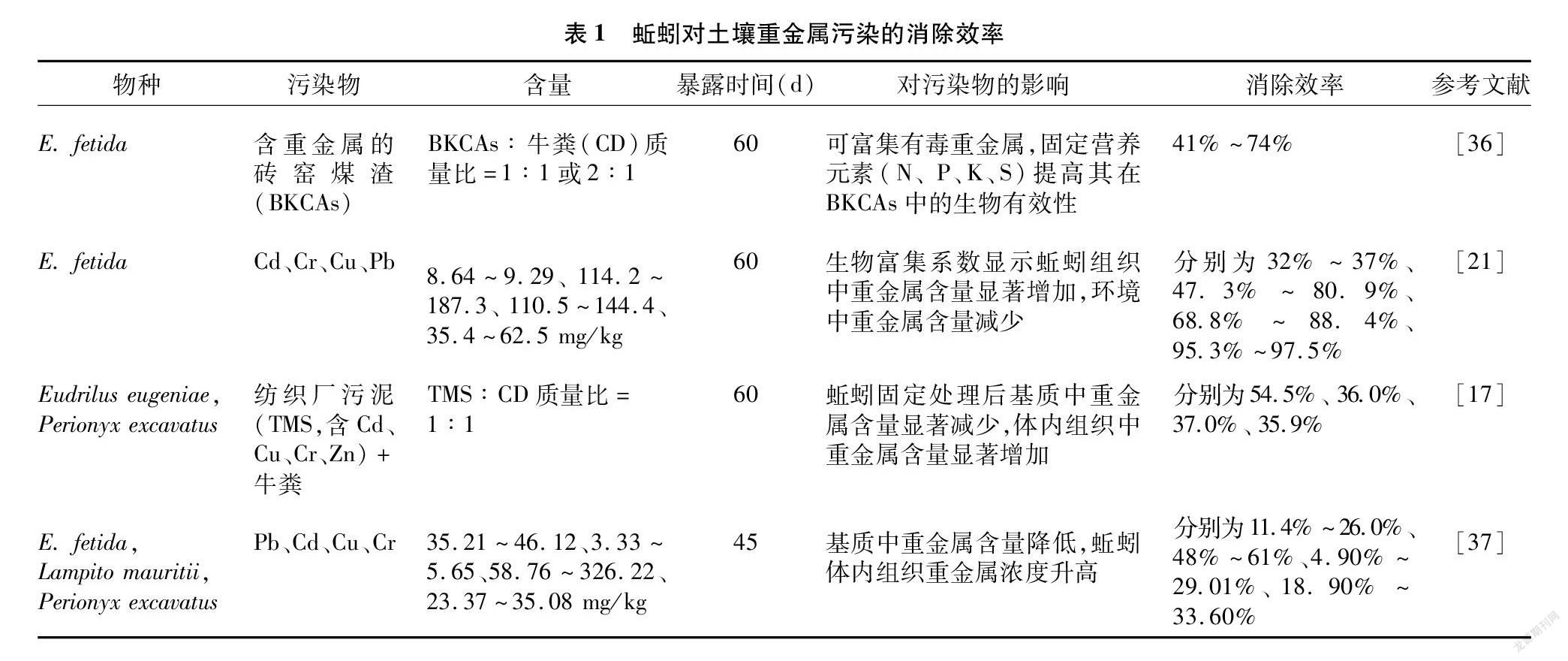
生物富集也与土壤性质、暴露时间及蚯蚓种类相关。Davies等报道E. fetida对Pb的吸收量与暴露时间成正比,且外源环境中Pb浓度越高,蚯蚓对Pb的积累速率越快[30]。Nannoni 等证明蚯蚓对重金属的积累能力受土壤有机碳、碳酸盐含量等理化性质的影响[31]。Wang等在我国湖南省原位污染的土壤中评估了Metaphire californica、Amynthas homochaetus、Amynthas pecteniferus、Amynthas heterochaetus几种蚯蚓对重金属吸收积累能力的差异,并发现生物富集系数从大到小为Cd (10.6~18.8) > Zn (1.15~1.75)>Cu (1.01~1.35)>Pb (0.56~0.95)[32];且相同生态类群的蚯蚓对重金属的生物富集系数接近[31-32],不同生态类群蚯蚓对重金属污染的耐受性不同[33-34]。此外,重金属在不同食料中的分布影响其在不同生态类群蚯蚓体内的积累,表明食物选择性和生态位分化对生物富集重金属的影响[35]。因此,评估重金属对蚯蚓生物积累的影响时,要考虑多种蚯蚓生态类群及重金属在环境中的本底浓度。利用蚯蚓修复重金属污染的研究结果如表1所示。
1.2 消除土壤中的有机污染物
土壤有机污染因其持久性、蓄积性、“三致”效应、毒性效应等特点而引起研究者们的广泛关注。我国农田土壤除受到传统有机污染物如多氯联苯(PCBs)、多环芳烃(PAHs)、农药、石油烃等污染外,还受到多种新型有机污染物如抗生素、酞酸酯(PAEs)、全氟化合物(PFASs)、微塑料等的污染[38]。研究表明,蚯蚓能够促进土壤中多种有机污染物的降解(表2),在土壤有机污染生物修复方面具有广阔的应用前景。
蚯蚓可以通过增加土壤通气性促进PCBs降解微生物的扩散分布,同时增加土壤中的碳、氮含量,改良土壤微生物群落[39]。蚯蚓生物堆肥处理使基质中总PCB含量降低55%~66%,而蚯蚓体内PCBs水平显著增加,表明PCBs主要被蚯蚓吸收富集[40]。Luepromchai等进行土壤柱試验,采用蚯蚓+投菌法可以消除9 cm表层土柱中约50% PCBs,而单独采用投菌法或添加蚯蚓仅能消除3 cm表层土柱中的PCBs。因此,蚯蚓不仅促进了PCBs降解菌的传播扩散,同时为微生物的生长和活动提供了适宜的环境条件,有利于土壤中PCBs污染的修复[41]。
蚯蚓通过取食含有有机污泥或废塑料的土壤而富集多溴联苯醚(PBDEs)[42]。有报道称PBDEs在蚓粪中含量比非根际土壤中低40%[43],表明PBDEs主要被蚯蚓吸收富集。外源添加Pb可以降低PBDEs的生物有效性,同时减少蚯蚓肠道吸收土壤中的PBDEs。Li等报道高剂量Pb会破坏蚯蚓细胞膜而增加渗透性[44];Zhang等发现250 mg/kg Pb暴露诱导更多BDE209被蚯蚓表皮吸收;而当Pb浓度为 500 mg/kg 时,蚯蚓体内吸收的BDE209含量下降[45]。
蚯蚓可以通过表皮被动吸收土壤中可溶态的PAHs,或经主动摄食由肠道吸收消化土壤中的PAHs[46-47]。蚯蚓通过排泄易降解的碳来增加微生物活性,进而促进土壤中PAHs的降解;同时通过取食、肠道消化吸收、形成土壤团聚体等间接影响微生物群落的结构和活性[15]。此外,蚯蚓活动不断混合扰动土壤,改善土壤的需氧条件进而加剧PAHs的降解过程[46]。E. fetida可以消除土壤中93%的蒽含量[48];在多环芳烃污染土壤中分别消除约91%芘、99%菲、91%蒽、16%苯并[a]芘(BaP)、43%荧蒽[49]。Hernández-Castellanos等发现培育112 d,蚯蚓Pontoscolex corethrurus从灭菌土壤中消除了26.6 mg/kg苯并[a]芘;而将Pontoscolex corethrurus添加到非灭菌土壤中,约有36.1 mg/kg BaP被消解,表明土壤微生物对BaP也具有降解作用[50]。因此,蚯蚓不仅增加了PAHs的生物有效性,同时提高了微生物对PAHs的消除能力[49,51]。蚯蚓对PAHs的生物积累也取决于蚯蚓种类,Parrish等研究发现蚯蚓E. fetida、Lumbricus terrestris可分别从PAHs污染土壤中吸收0.204、0.084 μg/g的PAHs[52]。
蚯蚓能够促进除草剂、杀虫剂等多种农药的降解[53-54]。Shan等研究蚯蚓Metaphire guillelmi粪便对二氯苯酚(2,4-DCP)、三氯苯酚(2,4,6-TCP)、五氯苯酚(PCP)的吸附能力,结果发现0、7、30 d后蚓粪对3种农药的吸附能力依次为PCP>2,4-DCP>2,4,6-TCP[55]。此外,蚯蚓也可以通过取食活动改变氯酚类化合物的生物有效性及环境归宿[54]。Schreck等发现添加蚯蚓消除了土壤中80%以上的毒死蜱、氯氟氰菊酯、灭菌丹、甲霜灵、腈菌唑等农药[56]。蚯蚓通过取食、转运土壤表面除草剂及促进土壤与除草剂吸附结合,从而降低除草剂的淋溶风险[57]。Lumbricus terrestris和 Aporrectodea caliginosa不仅可以转运污染物,还能改变莠去津的矿物化和吸附性。培育86 d后,蚯蚓将莠去津的矿物化成分14CO2—C含量从15.2% 降低至117%。同时,蚯蚓促进土壤中结合态莠去津形成[58]。不同种蚯蚓对莠去津的生物积累能力不同。当暴露于 4.25 mg/kg 莠去津污染的土壤中,深栖类蚯蚓Metaphire guillelmi的生物富集系数约是表栖类E. fetida的5倍,可能由于不同蚯蚓对受试物的吸收机制不同。M. guillelm的生物富集途径主要是肠道吸收,而E. fetida则主要通过表皮吸收途径[59]。同理,内栖类Amynthas robustus和表栖类E. fetida可以显著消除土壤中双对氯苯基三氯乙烷(DDT)污染,且 A. robustus具有相对较高的消除潜力[60]。
蚯蚓修复的效率与石油的组成、浓度、土壤中微生物群落、蚯蚓种类相关。蚯蚓活动混合土壤、增加土壤透气性,从而促进石油消解过程。Schaefer等用3种不同生态型的蚯蚓E. fetida、Allolobophora chlorotica、Lumbricus terrestris修复石油烃污染土壤,石油烃的降解速度明显加快,且L. terrestris的降解效果优于其他2种蚯蚓[61]。Chachina等采用蚯蚓、光合细菌、固氮细菌及真菌联合修复方法后土壤中机油消除效率高达99.9%,是无蚯蚓对照组的4~10倍[62]。同理,只使用植物修复对消除土壤中柴油污染效果不佳,而添加蚯蚓后表层土中柴油含量减少43%,深层土中柴油量减少52%[63]。蚯蚓对总石油烃(TPH)的降解速度约为90 mg/d[64]。对中浓度石油烃污染(<4 000 mg/kg)可考虑使用蚯蚓进行原位修复处理[61]。但当石油烃浓度高于 4 000 mg/kg 时,投放蚯蚓的死亡率上升,对生存生长造成不利影响[65]。因此,须注意利用蚯蚓来修复石油污染的土壤具有一定局限性。
2 蚯蚓修复过程及机理
蚯蚓对土壤有机物及重金属污染均有良好修复效果。蚯蚓对污染物的吸附、积累、消除作用较为复杂且与多种因素相关[8]。蚯蚓修复过程包括对污染物的吸收、转化及降解,其中涉及的机理分为内在和外在机理。内在机理包括改善土壤理化性质、刺激土壤微生物生长、影响微生物活性和代谢、提高植物吸收率等。外在机理包括蚯蚓生理活动,蚯蚓对污染物形态、迁移及生物有效性的影响等。如蚯蚓可以直接通过掘穴活动有氧氧化DDT脱氯化氢后形成DDE,同时肠道厌氧还原DDT或DDE形成DDD或DDMU[2,2-双-(对氯苯基)-1-氯乙烯];也可以通过
间接改变土壤理化性质,刺激本土DDT降解菌的生长和活性,从而促进DDT污染的消除[73]。
2.1 蚯蚓吸收积累污染物
蚯蚓从环境中吸收污染物到自身体内的过程称为蚯蚓生物积累[5]。蚯蚓通过肠道消化吸收或表皮接触吸附等途径从环境中吸收污染物。在肠道消化吸收中,含污染物的土壤经E. fetida取食后被肠道消化,从而被其他组织吸附(摄食作用);在表皮接触吸收中,污染物的电化学势降低而被蚯蚓体壁吸收,随后在蚯蚓体内各器官组织间迁移(扩散作用)[12]。
污染物类型是决定蚯蚓生物积累效率的关键因素[8]。Sizmur 等调查了Lumbricus terrestris对土壤中As、Cu、Pb和Zn迁移性和形态的影响,结果表明蚯蚓对重金属含量、存在形态的影响与土壤性质和重金属类型相关[74]。Sizmur等调查了表栖类E. veneta、深栖类L. terrestris、内栖类A. chlorotica对金属迁移率和生物有效性的影响。结果表明,与无蚯蚓对照组相比,蚯蚓显著增加了渗滤液中重金属含量及离子活性[75]。其中,L. terrestris处理效果最为显著,分别将渗滤液中As、Cu、Pb、Zn浓度提高了267%、393%、190%、429%[75]。A. rosea、E. fetida、L. mauritii、N. caliginosus通过体内金属硫蛋白(MT)结合吸收重金属污染物[76]。金属硫蛋白是调节蚯蚓肠道内必需及非必需金属离子动态的一类小分子蛋白,其分子量约6~7 ku[77],可以与Cu2+、Zn2+、Mn2+等重金属离子结合[78]。此外,土壤理化性质是影响重金属吸附-解吸附行为及生物有效性的重要因素。Huang等报道土壤pH值与重金属生物有效性显著负相关[79]。施用粪肥增加土壤可溶性有机碳(DOC)含量,可促进根围土壤的重金属生物有效性增加[80]。Wen等发现蚯蚓活动通过提高土壤pH值、水溶性重金属组分和可溶性有机碳(DOC)含量,进而增加了重金属的迁移性和生物有效性;同时蚯蚓活动还促进了微生物种群增加、小麦生长,增加了小麦中重金属含量[81]。Liu等发现,随着暴露时间增加溴敌隆逐渐被蚯蚓富集,且生物富集因子(BSAFs)随着土壤中溴敌隆浓度的增加而减小;造成这种现象的原因可能是当疏水性有机物在土壤和沉积物中浓度较高时,生物体内可利用的结合位点达到了饱和,并且产生了不可逆解吸现象[82]。
2.2 蚯蚓转化降解污染物
蚯蚓转化是蚯蚓修复的重要机理之一,指通过蚯蚓和微生物的联合作用将可生物降解的固体废物转化为肥料的过程[5]。蚯蚓通过肠道酶和微生物转化污染物,并将其以蚓粪形式排泄到土壤中[83]。Yang等报道蚯蚓和微生物消除土壤中687%菲污染,同时通过氧合作用将菲转化为原儿茶酸[84]。Gu等发现M. guillelmi可以将四溴双酚A(TBBPA)转化为代谢产物或固定为残留物。TBBPA进入蚯蚓体内后经循环系统转移至其他组织,在环带组织中被迅速转化为毒性较低的二甲基四溴双酚A醚,随后以蚓粪排出体外。M.guillelmi对TBBPA的解毒作用主要是通过在消化道产生 O-甲基化代谢产物及形成结合态产物实现的[85]。蚯蚓对污染物的转化需体内多种酶共同参与[86]。蚯蚓体内转化代谢污染物的酶主要有羧酸酯酶、谷胱甘肽硫转移酶、细胞色素P450等[83]。Sanchez-Hernandez等发现L. terrestris肠道微环境是土壤羧酸酯酶活性的主要来源,且蚓粪能增加土壤中酯酶活性。蚯蚓处理12周后,土壤羧酸酯酶活性相比对照组高2~4倍,表明蚯蚓通过增加土壤酶活性,从而促进农药污染土壤的修复[87]。
蚯蚓降解指蚯蚓利用肠道微生物或体内酶系统,如细胞色素酶(CYP450)、过氧化物酶(POD)、羧酸酯酶(CarE)、谷胱甘肽硫转移酶(GST)降解不同的污染物[5]。研究表明CYP450能够通过芳香环断裂的方式降解PAHs、PCBs、农药等有机污染物[88]。Sanchez-Hernandez等报道A. caliginosa通过Ⅰ相酶CYP450和Ⅱ相酶GST解毒酶共同降解土壤毒死蜱污染[89]。体内酶的氧化与水解是典型的相Ⅰ反应,反应产物与内源分子结合进行相Ⅱ反应[83]。E. fetida可以将PAHs代谢为相Ⅱ共轭物,代谢产物通常具有更高的水溶性和生物有效性,因此更易被蚯蚓和植物吸收消除[90]。
蚯蚓堆肥主要通过蚯蚓和微生物间的互作固定并氧化有机质,是降解有机污染物的有效方法之一[91]。添加蚯蚓后增加了污染物的异化分解代谢,且蚯蚓分解的小分子碳、分泌的黏液、排泄的蚓粪等均有助于土壤微生物的生长繁殖[55]。在土壤和堆肥的混合物中添加蚯蚓后,菲的矿化程度从68%提高到86%,表明蚯蚓+堆肥处理显著增加了污染物的异化代谢,从而降低菲污染[92]。Dendrobaena veneta+堆肥也增加了土壤中微生物对柴油污染的消解[93]。E. fetida+堆肥处理60 d后亚甲蓝消除率高达98%[94]。
2.3 与蚯蚓相关的微生物
蚯蚓肠道微生物与蚯蚓是互利共生關系,两者间的互作可以共同促进土壤有机质及污染物的降解,也会影响彼此种群、群落的变化及分泌的酶[95]。微生物降解污染物主要利用微生物分泌的胞外酶降解以及污染物被微生物吸收到细胞内由胞内酶降解[96]。如肠道细菌Bacillus licheniformis菌株KX657843分泌的胞外聚合物(EPS)可以吸附Cu(Ⅱ)、 Zn(Ⅱ),且最大吸附能力分别为58.82、5245 mg/g[97]。近年来,E. fetida肠道中需氧和厌氧细菌群落相继被研究发现,需氧菌为Aeromonas、Bacillus、Photobacterium、Pseudomonas、Shewanella等菌属,厌氧菌为Aeromonas、Bacillus、Shewanella、Paenibacillus、Clostridium、 Cellulosimicrobium、Streptomyces、Chloroflexi等菌属[98]。其中,Bacillus、Clostridium、Pseudomonas、Streptomyces和Shewanella等可以降解有机污染物[99-100];Pseudomonas、Alcaligenes和Acidobacterium等可以降解烃类及其他有机污染物[101-102];Pseudomonas、Acidobacterium、Penicillium、Mucor、Aspergillus等可提高PAHs的消除率[103-104];肠道细菌Hydrogenophaga和Streptomyces可以降解DDT[73]。Zhang等从Pheretima tschiliensis肠道中分离出真菌Trichoderma brevicompactum QYCD-6,并发现该微生物对多种重金属胁迫具有耐受性和良好的消除效果,其中对Pb(Ⅱ)的消除效率最高,达到97.5%[105]。
添加蚯蚓能够提高土壤微生物的活性和分解能力,进而促进污染物的降解[69]。E. fetida+A. caliginosa+稻秸秆联合处理后,Pseudomonas、Luteimonas、Rhodanobacter、Sphingomonas、Gemmatimonas、Flavobacterium、Leifsonia等细菌群落生物量显著增加,而菲浓度显著下降,表明蚯蚓+秸秆通过增加微生物群落多样性及生物量促进菲的降解[106]。Lin等报道蚯蚓堆肥处理42 d后微生物群落结构发生显著变化,PCP被微生物显著降解。采用系统进化及序列分析鉴定其属于六大细菌家族:鞘脂杆菌(Sphingobacteriaceae)、黄杆菌(Flavobacteriaceae)、假单胞菌(Pseudomonadaceae)、细菌TM7、黄单胞菌(Xanthomonadaceae)、Opitutaceae及四大真菌家族[毛霉菌(Mucoraceae)、Tremellaceae、Trichocomaceae、Hypocreaceae][69]。Liu等报道蚯蚓能够刺激除草剂2-甲基-4-氯苯氧乙酸(MCPA)降解菌的生长和活性,细菌Xanthomonadaceae在该过程中起主要降解作用[4]。添加蚯蚓显著刺激了变形菌门微生物的生长,且不同类型的变形菌门均能将萘、蒽、菲等多环芳烃污染物完全矿化降解[107-108]。
蚯蚓可以通过取食直接调节细菌群落[109],也可以将土壤中高分子有机物分解为小分子化合物,继而被污染物降解菌作为碳源利用[110]。Tiunov等在L. terrestris的洞穴中发现红球菌(Rhodococcus)与固氮菌(Azotobacter)[111],且Rhodococcus可以将蒽、菲、芘、荧蒽等化学污染物作为碳和能量的唯一来源[112]。
综上所述,蚯蚓自身的生命活动及肠道内功能微生物可共同促进外源污染物的降解转化,但两者在该过程中的贡献难以衡量[83]。因此,未来研究应侧重解析污染物消解过程所涉及的机理。代谢组学、毒理基因组学、转录组学等有助于阐明蚯蚓-微生物对污染物降解的作用机理[8]。
3 小结与展望
蚯蚓修复作为一种环境友好的技术近年来发展迅速,本文综述了蚯蚓对不同类型土壤污染物的修复潜力及机理。笔者认为以下方面将成为该领域研究的重点:(1)不同类型污染物对蚯蚓的毒性風险研究;(2)蚯蚓响应污染物胁迫的机理研究;(3)利用分子生物及基因工程技术改造蚯蚓基因,筛选培育超累积高耐性的蚯蚓品种;(4)采用强化策略提高污染物的生物有效性;(5)采用微生物+动物或微生物+动物+植物的协同修复策略;(6)利用不同生态群的蚯蚓品种来消除不同类型的污染物。现阶段蚯蚓修复技术仍处于实验室模拟阶段,如何将这项技术切实有效地应用在实际污染场地,在大田进行推广、应用、示范,亟需开展大量的研究。
参考文献:
[1]Liang L C,Liu W T,Sun Y B,et al. Phytoremediation of heavy metal contaminated saline soils using halophytes:current progress and future perspectives[J]. Environmental Reviews,2017,25(3):269-281.
[2]Lian J P,Zhao L F,Wu J N,et al. Foliar spray of TiO2 nanoparticles prevails over root application in reducing Cd accumulation and mitigating Cd-induced phytotoxicity in maize (Zea mays L.)[J]. Chemosphere,2020,239:124794.
[3]Zeng S Y,Ma J,Yang Y J,et al. Spatial assessment of farmland soil pollution and its potential human health risks in China[J]. Science of the Total Environment,2019,687:642-653.
[4]Liu W T,Zhou Q X,Zhang Z,et al. Evaluation of Cadmium phytoremediation potential in Chinese cabbage cultivars[J]. Journal of agricultural and food chemistry,2011,59(15):8324-8330.
[5]Shi Z M,Liu J H,Tang Z W,et al. Vermiremediation of organically contaminated soils:concepts,current status,and future perspectives[J]. Applied Soil Ecology,2020,147:103377.
[6]Naik M M,Dubey S K. Lead resistant bacteria:lead resistance mechanisms,their applications in lead bioremediation and biomonitoring[J]. Ecotoxicology and Environmental Safety,2013,98:1-7.
[7]Sutherland D L,Ralph P J. Microalgal bioremediation of emerging contaminants-Opportunities and challenges[J]. Water Research,2019,164:114921.
[8]Rodriguez-Campos J,Dendooven L,Alvarez-Bernal D,et al. Potential of earthworms to accelerate removal of organic contaminants from soil:a review[J]. Applied Soil Ecology,2014,79:10-25.
[9]Rajpal A,Arora S,Bhatia A,et al. Co-treatment of organic fraction of municipal solid waste (OFMSW) and sewage by vermireactor[J]. Ecological engineering,2014,73:154-161.
[10]Ravindran B,Contreras-Ramos S M,Sekaran G. Changes in earthworm gut associated enzymes and microbial diversity on the treatment of fermented tannery waste using epigeic earthworm Eudrilus eugeniae[J]. Ecological Engineering,2015,74:394-401.
[11]Curry J P,Schmidt O. The feeding ecology of earthworms-a review[J]. Pedobiologia,2007,50(6):463-477.
[12]Shi Z M,Xu L,Hu F. A hierarchic method for studying the distribution of phenanthrene in Eisenia fetida[J]. Pedosphere,2014,24(6):743-752.
[13]Santana N A,Ferreira P A A,Tarouco C P,et al. Earthworms and mycorrhization increase copper phytoextraction by Canavalia ensiformis in sandy soil[J]. Ecotoxicology and Environmental Safety,2019,182:109383.
[14]Swati A,Hait S. Fate and bioavailability of heavy metals during vermicomposting of various organic wastes——a review[J]. Process Safety and Environmental Protection,2017,109:30-45.
[15]Dendooven L,Alvarez-Bernal D,Contreras-Ramos S M. Earthworms,a means to accelerate removal of hydrocarbons (PAHs) from soil? A mini-review[J]. Pedobiologia,2011,54:S187-S192.
[16]Datta S,Singh J,Singh S,et al. Earthworms,pesticides and sustainable agriculture:a review[J]. Environmental Science and Pollution Research,2016,23(9):8227-8243.
[17]Yuvaraj A,Karmegam N,Tripathi S,et al. Environment-friendly management of textile mill wastewater sludge using epigeic earthworms:bioaccumulation of heavy metals and metallothionein production[J]. Journal of Environmental Management,2020,254:109813.
[18]Liang S H,Chen S C,Chen C Y,et al. Cadmium-induced earthworm metallothionein-2 is associated with metal accumulation and counteracts oxidative stress[J]. Pedobiologia,2011,54(5/6):333-340.
[19]Liu X L,Hu C X,Zhang S Z. Effects of earthworm activity on fertility and heavy metal bioavailability in sewage sludge[J]. Environment International,2005,31(6):874-879.
[20]Lavelle P. Earthworm activities and the soil system[J]. Biology and Fertility of Soils,1988,6(3):237-251.
[21]Suthar S,Sajwan P,Kumar K. Vermiremediation of heavy metals in wastewater sludge from paper and pulp industry using earthworm Eisenia fetida[J]. Ecotoxicology and Environmental Safety,2014,109:177-184.
[22]Suthar S. Metal remediation from partially composted distillery sludge using composting earthworm Eisenia fetida[J]. Journal of Environmental Monitoring,2008,10(9):1099-1106.
[23]Wu Y,Chen C,Wang G,et al. Mechanism underlying earthworm on the remediation of cadmium-contaminated soil[J]. Science of The Total Environment,2020,728(1):138904.
[24]Richardson J B,Grres J H,Jackson B P,et al. Trace metals and metalloids in forest soils and exotic earthworms in northern New England,USA[J]. Soil Biology and Biochemistry,2015,85:190-198.
[25]Nannoni F,Protano G,Riccobono F. Uptake and bioaccumulation of heavy elements by two earthworm species from a smelter contaminated area in northern Kosovo[J]. Soil Biology and Biochemistry,2011,43(12):2359-2367.
[26]lvarez C R,Moreno M J,Bernardo F J G,et al. Mercury methylation,uptake and bioaccumulation by the earthworm Lumbricus terrestris (Oligochaeta)[J]. Applied Soil Ecology,2014,84:45-53.
[27]Zhang Z S,Zheng D M,Wang Q C,et al. Bioaccumulation of total and methyl mercury in three earthworm species (Drawida sp.,Allolobophora sp.,and Limnodrilus sp.)[J]. Bulletin of Environmental Contamination and Toxicology,2009,83(6):937-942.
[28]Li L X Y,Xu Z L,Wu J Y,et al. Bioaccumulation of heavy metals in the earthworm Eisenia fetida in relation to bioavailable metal concentrations in pig manure[J]. Bioresource Technology,2010,101(10):3430-3436.
[29]Liu Y Q,Du Q Y,Wang Q,et al. Causal inference between bioavailability of heavy metals and environmental factors in a large-scale region[J]. Environmental Pollution,2017,226:370-378.
[30]Davies N A,Hodson M E,Black S. The influence of time on lead toxicity and bioaccumulation determined by the OECD earthworm toxicity test[J]. Environmental Pollution,2003,121(1):55-61.
[31]Nannoni F,Rossi S,Protano G. Soil properties and metal accumulation by earthworms in the Siena urban area (Italy)[J]. Applied Soil Ecology,2014,77:9-17.
[32]Wang K,Qiao Y H,Zhang H Q,et al. Bioaccumulation of heavy metals in earthworms from field contaminated soil in a subtropical area of China[J]. Ecotoxicology and Environmental Safety,2018,148:876-883.
[33]Sivakumar S. Effects of metals on earthworm life cycles:a review[J]. Environmental Monitoring and Assessment,2015,187(8):530.
[34]Nirola R,Megharaj M,Saint C,et al. Metal bioavailability to Eisenia fetida through copper mine dwelling animal and plant litter,a new challenge on contaminated environment remediation[J]. International Biodeterioration & Biodegradation,2016,113:208-216.
[35]Ernst G,Zimmermann S,Christie P,et al. Mercury,cadmium and lead concentrations in different ecophysiological groups of earthworms in forest soils[J]. Environmental Pollution,2008,156(3):1304-1313.
[36]Mondal A,Goswami L,Hussain N,et al. Detoxification and eco-friendly recycling of brick kiln coal ash using Eisenia fetida:a clean approach through vermitechnology[J]. Chemosphere,2020,244:125470.
[37]Panday R,Basnet B B,Bhatt P S,et al. Bioconcentration of heavy metals in vermicomposting earthworms (Eisenia fetida,Perionyx excavatus and Lampito mauritii) in Nepal[J]. Journal of Microbiology,Biotechnology and Food Sciences,2020,9(5):416-418.
[38]潘 政,郝月崎,趙丽霞,等. 蚯蚓在有机污染土壤生物修复中的作用机理与应用[J]. 生态学杂志,2020,39(9):3108-3117.
[39]Hoeffner K,Monard C,Cluzeau D,et al. Response of temperate anecic earthworm individual biomass to species interactions[J]. Applied Soil Ecology,2019,144:8-11.
[40]Tharakan J,Tomlinson D,Addagada A,et al. Biotransformation of PCBs in contaminated sludge:potential for novel biological technologies[J]. Engineering in Life Sciences,2006,6(1):43-50.
[41]Luepromchai E,Singer A C,Yang C H,et al. Interactions of earthworms with indigenous and bioaugmented PCB-degrading bacteria[J]. FEMS Microbiology Ecology,2002,41(3):191-197.
[42]Huang L,Wang W,Zhang S F,et al. Bioaccumulation and bound-residue formation of 14C-decabromodiphenyl ether in an earthworm-soil system[J]. Journal of Hazardous Materials,2017,321:591-599.
[43]Liang X W,Zhu S Z,Chen P,et al. Bioaccumulation and bioavailability of polybrominated diphynel ethers (PBDEs) in soil[J]. Environmental pollution,2010,158(7):2387-2392.
[44]Li M,Liu Z T,Xu Y,et al. Comparative effects of Cd and Pb on biochemical response and DNA damage in the earthworm Eisenia fetida (Annelida,Oligochaeta)[J]. Chemosphere,2009,74(5):621-625.
[45]Zhang W,Chen L,Liu K,et al. Bioaccumulation of decabromodiphenyl ether (BDE209) in earthworms in the presence of lead (Pb)[J]. Chemosphere,2014,106:57-64.
[46]Sinha R K,Bharambe G,Ryan D. Converting wasteland into wonderland by earthworms-a low-cost natures technology for soil remediation:a case study of vermiremediation of PAHs contaminated soil[J]. The Environmentalist,2008,28(4):466-475.
[47]Sivaram A K,Logeshwaran P,Lockington R,et al. Phytoremediation efficacy assessment of polycyclic aromatic hydrocarbons contaminated soils using garden pea (Pisum sativum) and earthworms (Eisenia fetida)[J]. Chemosphere,2019,229:227-235.
[48]Coutio-González E,Hernández-Carlos B,Gutiérrez-Ortiz R,et al. The earthworm Eisenia fetida accelerates the removal of anthracene and 9,10-anthraquinone,the most abundant degradation product,in soil[J]. International Biodeterioration & Biodegradation,2010,64(6):525-529.
[49]Sun H W,Li J M,Wang C P,et al. Enhanced microbial removal of pyrene in soils in the presence of earthworms[J]. Soil and Sediment Contamination:An International Journal,2011,20(6):617-630.
[50]Hernández-Castellanos B,Ortíz-Ceballos A,Martínez-Hernández S,et al. Removal of benzo (a) pyrene from soil using an endogeic earthworm Pontoscolex corethrurus (Müller,1857)[J]. Applied Soil Ecology,2013,70:62-69.
[51]Gomez-Eyles J L,Sizmur T,Collins C D,et al. Effects of biochar and the earthworm Eisenia fetida on the bioavailability of polycyclic aromatic hydrocarbons and potentially toxic elements[J]. Environmental Pollution,2011,159(2):616-622.
[52]Parrish Z D,White J C,Isleyen M,et al. Accumulation of weathered polycyclic aromatic hydrocarbons (PAHs) by plant and earthworm species[J]. Chemosphere,2006,64(4):609-618.
[53]Sun Y,Zhao L X,Li X J,et al. Stimulation of earthworms (Eisenia fetida) on soil microbial communities to promote metolachlor degradation[J]. Environmental Pollution,2019,248:219-228.
[54]Singh S I,Singh S,Vig A P. Earthworm-assisted bioremediation of agrochemicals[M]//Prasad M N V.Agrochemicals detection,treatment and remediation. Oxford:Butterworth-Heinemann,2020:307-327.
[55]Shan J,Xu J,Zhou W Q,et al. Enhancement of chlorophenol sorption on soil by geophagous earthworms (Metaphire guillelmi)[J]. Chemosphere,2011,82(2):156-162.
[56]Schreck E,Geret F,Gontier L,et al. Neurotoxic effect and metabolic responses induced by a mixture of six pesticides on the earthworm Aporrectodea caliginosa nocturna[J]. Chemosphere,2008,71(10):1832-1839.
[57]Farenhorst A,Topp E,Bowman B T,et al. Earthworm burrowing and feeding activity and the potential for atrazine transport by preferential flow[J]. Soil Biology and Biochemistry,2000,32(4):479-488.
[58]Binet F,Kersanté A,Munier-Lamy C,et al. Lumbricid macrofauna alter atrazine mineralization and sorption in a silt loam soil[J]. Soil Biology and Biochemistry,2006,38(6):1255-1263.
[59]Wang F,Ji R,Jiang Z W,et al. Species-dependent effects of biochar amendment on bioaccumulation of atrazine in earthworms[J]. Environmental Pollution,2014,186C:241-247.
[60]Lin Z,Li X M,Li Y T,et al. Enhancement effect of two ecological earthworm species (Eisenia foetida and Amynthas robustus E. Perrier) on removal and degradation processes of soil DDT[J]. Journal of Environmental Monitoring,2012,14(6):1551-1558.
[61]Schaefer M,Juliane F. The influence of earthworms and organic additives on the biodegradation of oil contaminated soil[J]. Applied Soil Ecology,2007,36(1):53-62.
[62]Chachina S B,Voronkova N A,Baklanova O N. Biological remediation of the engine lubricant oil-contaminated soil with three kinds of earthworms,Eisenia fetida,Eisenia andrei,Dendrobena veneta,and a mixture of microorganisms[J]. Procedia Engineering,2015,113:113-123.
[63]Fernández M D,Pro J,Alonso C,et al. Terrestrial microcosms in a feasibility study on the remediation of diesel-contaminated soils[J]. Ecotoxicology and Environmental Safety,2011,74(8):2133-2140.
[64]Martinkosky L,Barkley J,Sabadell G,et al. Earthworms (Eisenia fetida) demonstrate potential for use in soil bioremediation by increasing the degradation rates of heavy crude oil hydrocarbons[J]. Science of The Total Environment,2017,580:734-743.
[65]Hanna S H S,Weaver R W. Earthworm survival in oil contaminated soil[J]. Plant and Soil,2002,240(1):127-132.
[66]Rorat A,Wloka D,Grobelak A,et al. Vermiremediation of polycyclic aromatic hydrocarbons and heavy metals in sewage sludge composting process[J]. Journal of Environmental Management,2017,187:347-353..
[67]Tejada M,Masciandaro G. Application of organic wastes on a benzo (a) pyrene polluted soil. Response of soil biochemical properties and role of Eisenia fetida[J]. Ecotoxicology and Environmental Safety,2011,74(4):668-674.
[68]Lin Z,Zhen Z,Liang Y Q,et al. Changes in atrazine speciation and the degradation pathway in red soil during the vermiremediation process[J]. Journal of hazardous materials,2019,364:710-719.
[69]Lin Z,Bai J,Zhen Z,et al. Enhancing pentachlorophenol degradation by vermicomposting associated bioremediation[J]. Ecological Engineering,2016,87(3):288-294.
[70]Zhao S Y,Zhu L Y. Uptake and metabolism of 10:2 fluorotelomer alcohol in soil-earthworm (Eisenia fetida) and soil-wheat (Triticum aestivum L.) systems[J]. Environmental Pollution,2017,220:124-131.
[71]Ekperusi O A,Aigbodion F I. Bioremediation of petroleum hydrocarbons from crude oil-contaminated soil with the earthworm:Hyperiodrilus africanus[J]. 3 Biotech,2015,5(6):957-965.
[72]Zenteno-Rojas A , Martinez-Romero E , Rincón-Molina C I,et al. Removal of high concentrations decachlorobiphenyl of earthworm Eisenia fetida and its symbiotic bacteria in a vermicomposting system[J]. Water, Air, & Soil Pollution, 2019,230(6): 116.
[73]Xu H J,Bai J,Li W Y,et al. Removal of persistent DDT residues from soils by earthworms:a mechanistic study[J]. Journal of Hazardous Materials,2019,365:622-631.
[74]Sizmur T,Palumbo-Roe B,Watts M J,et al. Impact of the earthworm Lumbricus terrestris (L.) on As,Cu,Pb and Zn mobility and speciation in contaminated soils[J]. Environmental Pollution,2011,159(3):742-748.
[75]Sizmur T,Wingate J,Hutchings T,et al. Lumbricus terrestris L. does not impact on the remediation efficiency of compost and biochar amendments[J]. Pedobiologia,2011,54S:S211-S216.
[76]Stürzenbaum S R,Hckner M,Panneerselvam A,et al. Biosynthesis of luminescent quantum dots in an earthworm[J]. Nature Nanotechnology,2013,8(1):57-60.
[77]Babula P,Masarik M,Adam V,et al. Mammalian metallothioneins:properties and functions[J]. Metallomics,2012,4(8):739-750.
[78]Irvine G W,Summers K L,Stillman M J. Cysteine accessibility during As3+metalation of the α-and β-domains of recombinant human MT1a[J]. Biochemical and Biophysical Research Communications,2013,433(4):477-483.
[79]Huang C D,Wang W Y,Yue S Z,et al. Role of biochar and Eisenia fetida on metal bioavailability and biochar effects on earthworm fitness[J]. Environmental Pollution,2020,263:114586.
[80]Zhang C J,Clark G J,Patti A F,et al. Contrasting effects of organic amendments on phytoextraction of heavy metals in a contaminated sediment[J]. Plant and Soil,2015,397(1/2):331-345.
[81]Wen B,Hu X Y,Liu Y,et al. The role of earthworms (Eisenia fetida) in influencing bioavailability of heavy metals in soils[J]. Biology and Fertility of Soils,2004,40(3):181-187.
[82]Liu J , Xiong K , Ye X Q, et al. Toxicity and bioaccumulation of bromadiolone to earthworm Eisenia fetida[J]. Chemosphere, 2015, 135:250-256.
[83]Katagi T,Ose K. Toxicity,bioaccumulation and metabolism of pesticides in the earthworm[J]. Journal of Pesticide Science,2015,40(3):69-81.
[84]Yang W X,Hadibarata T,Mahmoud A H,et al. Biotransformation of pyrene in soil in the presence of earthworm Eisenia fetida[J]. Environmental Technology & Innovation,2020,18:100701.
[85]Gu J Q,Chen X,Wang Y F,et al. Bioaccumulation,physiological distribution,and biotransformation of tetrabromobisphenol a (TBBPA) in the geophagous earthworm Metaphire guillelmi-hint for detoxification strategy[J]. Journal of Hazardous Materials,2020,388:122027.
[86]Jakoby W B. The enzymes of detoxication[J]. Transactions of the New York Academy of Sciences,1983,41(1):71-75.
[87]Sanchez-Hernandez J C,Morcillo S M,del Pino J N,et al. Earthworm activity increases pesticide-sensitive esterases in soil[J]. Soil Biology and Biochemistry,2014,75:186-196.
[88]Lee R F. Annelid cytochrome P-450[J]. Comparative Biochemistry and Physiology Part C,Pharmacology,Toxicology and Endocrinology,1998,121(1/2/3):173-179.
[89]Sanchez-Hernandez J C,Narvaez C,Sabat P,et al. Integrated biomarker analysis of chlorpyrifos metabolism and toxicity in the earthworm Aporrectodea caliginosa[J]. Science of the total environment,2014,490C:445-455.
[90]Schmidt N,Boll E S,Malmquist L M V,et al. PAH metabolism in the earthworm Eisenia fetida-identification of phase II metabolites of phenanthrene and pyrene[J]. International Journal of Environmental Analytical Chemistry,2017,97(12):1151-1162.
[91]Bhat S A,Singh S,Singh J,et al. Bioremediation and detoxification of industrial wastes by earthworms:vermicompost as powerful crop nutrient in sustainable agriculture[J]. Bioresource Technology,2018,252:172-179.
[92]Hickman Z A,Reid B J. The co-application of earthworms (Dendrobaena veneta) and compost to increase hydrocarbon losses from diesel contaminated soils[J]. Environment International,2008,34(7):1016-1022.
[93]Hickman Z A,Reid B J. Increased microbial catabolic activity in diesel contaminated soil following addition of earthworms (Dendrobaena veneta) and compost[J]. Soil Biology and Biochemistry,2008,40(12):2970-2976.
[94]Dey M D,Das S,Kumar R,et al. Vermiremoval of methylene blue using Eisenia fetida:a potential strategy for bioremediation of synthetic dye-containing effluents[J]. Ecological Engineering,2017,106:200-208.
[95]Brown G G,Barois I,Lavelle P. Regulation of soil organic matter dynamics and microbial activityin the drilosphere and the role of interactionswith other edaphic functional domains[J]. European Journal of Soil Biology,2000,36(3/4):177-198.
[96]Das N,Chandran P. Microbial degradation of petroleum hydrocarbon contaminants:an overview[J]. Biotechnology Research International,2011:941810.
[97]Biswas J K,Banerjee A,Sarkar B,et al. Exploration of an extracellular polymeric substance from earthworm gut bacterium (Bacillus licheniformis) for bioflocculation and heavy metal removal potential[J]. Applied Sciences,2020,10(1):349.
[98]Hong S W,Kim I S,Lee J S,et al. Culture-based and denaturing gradient gel electrophoresis analysis of the bacterial community structure from the intestinal tracts of earthworms (Eisenia fetida)[J]. Journal of Microbiology and Biotechnology,2011,21(9):885-892.
[99]Tiquia S M. Salt‐adapted bacteria isolated from the Rouge River and potential for degradation of contaminants and biotechnological applications[J]. Environmental Technology,2010,31(8/9):967-978.
[100]Wu X,Monchy S,Taghavi S,et al. Comparative genomics and functional analysis of niche-specific adaptation in Pseudomonas putida[J]. FEMS Microbiology Reviews,2011,35(2):299-323.
[101]Johnsen A R,Wick L Y,Harms H. Principles of microbial PAH-degradation in soil[J]. Environmental Pollution,2005,133(1):71-84.
[102]Singleton D R,Hendrix P F,Coleman D C,et al. Identification of uncultured bacteria tightly associated with the intestine of the earthworm Lumbricus rubellus (Lumbricidae;Oligochaeta)[J]. Soil Biology and Biochemistry,2003,35(12):1547-1555.
[103]Contreras-Ramos S M,Alvarez-Bernal D,Dendooven L. Removal of polycyclic aromatic hydrocarbons from soil amended with biosolid or vermicompost in the presence of earthworms (Eisenia fetida)[J]. Soil Biology and Biochemistry,2008,40(7):1954-1959.
[104]Shi Z M,Wang C Y,Zhao Y H. Effects of surfactants on the fractionation,vermiaccumulation,and removal of fluoranthene by earthworms in soil[J]. Chemosphere,2020,250:126332.
[105]Zhang D,Yin C P,Abbas N,et al. Multiple heavy metal tolerance and removal by an earthworm gut fungus Trichoderma brevicompactum QYCD-6[J]. Scientific Reports,2020,10(1):6940.
[106]Elyamine A M,Hu C. Earthworms and rice straw enhanced soil bacterial diversity and promoted the degradation of phenanthrene[J]. Environmental Sciences Europe,2020,32(1):124.
[107]Ma L L,Xie Y W,Han Z H,et al. Responses of earthworms and microbial communities in their guts to Triclosan[J]. Chemosphere,2017,168:1194-1202.
[108]Crampon M,Cébron A,Portet-Koltalo F,et al. Low effect of phenanthrene bioaccessibility on its biodegradation in diffusely contaminated soil[J]. Environmental Pollution,2017,225:663-673.
[109]Drake H L,Horn M A. As the worm turns:the earthworm gut as a transient habitat for soil microbial biomes[J]. Annual Review of Microbiology,2007,61(1):169-189.
[110]Purnomo A S,Koyama F,Mori T,et al. DDT degradation potential of cattle manure compost[J]. Chemosphere,2010,80(6):619-624.
[111]Tiunov A V,Dobrovolskaya T G. Fungal and bacterial communities in Lumbricus terrestris burrow walls:a laboratory experiment[J]. Pedobiologia,2002,46(6):595-605.
[112]Dean-Ross D,Moody J D,Freeman J P,et al. Metabolism of anthracene by a Rhodococcus species[J]. FEMS Microbiology Letters,2001,204(1):205-211.
