Phenotypic plasticity along altitudinal gradient affects the feeding efficiency and development of the parthenium beetle, Zygogramma bicolorata(Coleoptera: Chrysomelidae)
2020-11-12DayaRamBHUSALKishorChandraGHIMIRERekhaUPADHYAYMahadevBISTABhupendraKUMAR
Daya Ram BHUSAL, Kishor Chandra GHIMIRE, Rekha UPADHYAY,Mahadev BISTA,Bhupendra KUMAR
(1.Central Department of Zoology, Tribhuvan University, Kirtipur, Kathmandu, Nepal; 2.Primary School, Nuawn,Varanasi, India; 3.Department of Zoology, Siddha Nath Science Campus(TU), Mahendranagar, Nepal;4.Department of Zoology, Banaras Hindu University, Varanasi, Uttar Pradesh, India)
Abstract: 【Aim】 The present study was designed to find out whether eco-climatic factors affect the morphometric parameters and basic biology of the progeny of the parthenium beetle, Zygogramma bicolorata adults inhabiting the Parthenium hysterophorus abundant regions of Nepal.【Methods】 Z.bicolorata adults were collected from Kathmandu(24℃, 1 400 m above sea level, warm-temperate climate), Chitwan(25℃, 415 m above sea level, upper-tropical/sub-tropical climate)and Mahendranagar(34℃, 229 m above sea level, humid-subtropical climate)regions of Nepal, and the 4th instar larvae and adult females of the first generation(F1)per eco-climatic zone were assessed for their feeding attributes under optimal laboratory conditions.We hypothesized that rearing the progeny under ad libitum food and optimal abiotic conditions would nullify the indirect effect of food and temperature; and regardless of the eco-climatic regions to which parents belong, the progeny would utilize the food uniformly.【Results】 We found that despite being reared under optimal conditions, the progeny of Z.bicolorata displayed body size pattern and feeding attributes similar to their parents.Offspring obtained from larger parents(Kathmandu)were large, but had lower food utilization efficiency than the progeny from smaller parents(Chitwan and Mahendranagar).Irrespective of the three eco-climatic regions, developing female adults were heavier and had reduced food utilization efficiency than the larvae.【Conclusion】 Present findings suggest that immediate effect of food and optimal abiotic conditions may not affect the phenotypic plasticity of Z.bicolorata progeny, and the progeny display body size pattern and feeding attributes similar to the parents.Possibly the heritable changes might be owing to genetic diversity within the species.We anticipate that such findings may be utilized to understand phenotypic plasticity, distribution pattern and feeding behaviour of Z.bicolorata adults under changing climate scenario.
Key words: Zygogramma bicolorata; Parthenium hysterophorus; phenotypic plasticity; biological control; feeding attributes; climate
1 INTRODUCTION
Phenotypic plasticity is defined as the potential of a single genotype to express different phenotypes in response to different environmental cues(Priceetal., 2003; Paenkeetal., 2007; Tederetal., 2008).It is universally found in all living organisms.These environment-governed phenotypic variations may be seen in behaviour, physiology, morphology and life-history traits of the affected organisms, and are heritable(Blanckenhorn, 2009).The phenotypic variations may even be seen due to genetic diversity within species.Phenotypic plasticity in body size is generally induced by numerous ecological and environmental factors(Stillwelletal., 2007; Tederetal., 2008), but food and temperature are the crucial ones(Davidowitzetal., 2004).Studies in ectotherms, especially in insects have shown that the adults have larger body size(=body biomass)when raised at lower temperature and/or high-quality diet.In contrast, the insects raised at high temperature or on lower-quality diet generally mature in a smaller size(Atkinson, 1994; Kingsolver and Huey, 2008).Insects also extend their latitudinal distributions by altering their feeding behaviour, development and survival(Bhusaletal., 2020).As host plants vary in their genetic origin with altitude, the response of phytophagous insects to these variations includes changes in their feeding efficiency, growth and survival rates, and reproduction(Hodkinson, 2005).While phytophagous insects are the links in the chain of matter and energy transmission in terrestrial ecosystem; knowledge of their food consumption, conversion and utilization towards various environmental cues is important(Fujikawaetal., 2009; Omkar and Afaq, 2011).Moreover, studying the adaptive strategies and phenotypic plasticity of phytophagous insects associated with food and temperature may be helpful in their mass multiplication and release as natural enemies for biological control of invasive weeds.
In the present study, adults of the parthenium beetle,Zygogrammabicolorata(Coleoptera: Chrysomelidae), the most effective biocontrol agents ofPartheniumhysterophorus(Family: Asteraceae), were collected from Kathmandu, Chitwan, and Mahendranagar regions of Nepal, and the progeny of the beetle were assessed for variations in their feeding efficiency and developmental rate underinvitroconditions.Partheniumhysterophorusis an alien invasive herbaceous weed with pan-tropical distribution that adversely affects the grazing land productivity, native biodiversity and causes naso-branchial allergy in humans.The weed is thought to have entered Nepal from India and is currently found in Tarai, Siwalik and hilly regions of Nepal.Rapid expansion ofP.hysterophorushas become a new agricultural and environmental problem for Nepal.From the initially invaded roadside vegetation, the weed has now spread into the cropping land and the forests.
Feeding byZ.bicoloratahas a negative effect onP.hysterophorusunder field conditions(Dhileepanetal., 2000)and has attracted a great interest of researchers in the use of this beetle for biological control of the weed.We hypothesized that rearing the first generation progeny of the field-caught beetles under optimal abiotic conditions would nullify the effect of environmental factors(food and temperature), and regardless of the eco-climatic regions to which the parents belong, the progeny would utilize the food biomass to their own biomass uniformly.It is expected that the results will not only enhance our understanding on the phenotypic plasticity in phytophagous insects, but will also be helpful in developing strategies for augmentative biocontrol of the weed.
2 MATERIALS AND METHODS
2.1 Study sites and duration
P.hysterophorusabundant regions of Nepal(Kathmandu, Chitwan and Mahendranagar)were surveyed from 18 June, 2019 till July 10, 2019.Kathmandu(27.7172°N, 85.3240°E; 1 400 m above sea level)is situated in warm temperate zone of Nepal, having summer(April-July)and winter(November-February)temperatures between 16-28℃ and 3-18℃, respectively.The rainfall is mostly monsoon based(June to September).Chitwan(27.5291°N, 84.3542°E; 415 m above sea level)lies in upper tropical and sub-tropical zone of Nepal, and experiences temperature between 20-30℃ in summers(April-July)and 8-24℃ in winters(December-February).The monsoon rain takes place between July-September.However, Mahendranagar(28.9873°N, 80.1652°E; 229 m above sea level)has a humid subtropical climate.The temperature of the region ranges between 22-46℃ in summers and 5-20℃ in winters(http:∥mfd.gov.np/; Bhusaletal., 2020).
Data on daily minimum and maximum temperatures of the three eco-climatic regions were recorded from Department of Hydrology and Meteorology(Kathmandu, Nepal)(http:∥mfd.gov.np/), throughout the study period to calculate the average temperature(Kathmandu: 24℃; Chitwan: 25℃; Mahendranagar: 34℃).
2.2 Methodology
2.2.1Feeding attributes and developmental duration of parents: Eggs ofZ.bicoloratawere identified within the weed abundant agricultural fields of Kathmandu(Tribhuvan University campus), Chitwan(private agricultural farms)and Mahendranagar(Siddha Nath Science Campus).They were observed for hatching daily and the pre-weighed 1st instar larvae were transferred individually to pre-weighed plants ofP.hysterophorus(using analytical balance; 0.01 mg precision)that were superficially planted within the agricultural fields of Kathmandu, Chitwan and Mahendranagar.After 24 h, the 1st instar larvae as well as the host plants were weighed, and thereafter the larvae were transferred to fresh pre-weighed host plants that were placed superficially within the agricultural fields of the respective eco-climatic regions.The experiments were conducted daily until the larvae emerged out as adults.Daily temperature and photoperiod of the agricultural fields were recorded throughout the study period(http:∥mfd.gov.np/).The feeding attributes of the 4th instar larvae and 10-day-old adult females(voracious feeding stages of the weed)and the developmental duration of each larval instar were quantified under the natural conditions.The feeding attributes(consumption rate, conversion efficiency and growth rate)were calculated using the following formulae(Pateletal., 2018):
2.2.2Feeding attributes and development of progeny: The adults ofZ.bicoloratafrom the field conditions were brought to the laboratory of Central Department of Zoology, Tribhuvan University, Kathmandu, Nepal.The adults were allowed to mate in plastic Petri dishes(9.0 cm×1.5 cm).They were provided with daily replenishedadlibitumsupply ofP.hysterophoruscollected from agricultural farms of the university campus so as to nullify the confounding effect of variations in food nutrient availability due to different eco-climatic zones.The intermixing of adults from different eco-climatic regions was avoided.Eggs laid by females were collected region-wise and kept under optimal abiotic conditions(temperature: 27±1℃; photoperiod: 14L∶10D; relative humidity: 65%±5%)in Biochemical Oxygen Demand(BOD)incubators.The eggs were observed for hatching and the 1st instar larvae were reared individually in plastic Petri dishes(9.0 cm×1.5 cm)till adult emergence on daily replenishedadlibitumsupply ofP.hysterophorusunder temperature: 27±1℃; photoperiod: 14L∶10D; relative humidity: 65%±5%.The incubation period of eggs and the developmental duration of each immature stage(n=25 per eco-climatic region)were recorded to calculate the total developmental period.Body biomass of each developmental stage(n=20 per eco-climatic region)was also quantified.
Being the voracious feeders ofP.hysterophorus, the 4th instar larvae and 10-day-old adult females of F1generation in per eco-climatic region were further assessed for their feeding attributes.Prior to experiments, the 4th instar larvae(n=15)and adult females(n=15)per eco-climatic region were starved for 12 h.Thereafter, a pre-weighed(using analytical balance; 0.01 mg precision)4th instar larva/adult female was placed in a Petri dish for the next 24 h.During this period, the 4th instar larva/adult female was allowed to feed on a pre-weighed twig ofP.hysterophorus(containing fresh young leaves with 2-3 branches; biomass 750 mg)collected from Tribhuvan University campus, and kept in BOD incubator under the controlled abiotic conditions(temperature: 27±1℃; photoperiod: 14L∶10D; relative humidity: 65%±5%).After 24 h, the 4th instar larva/adult female was shifted to another Petri dish, and simultaneously the biomass(=wet weight)of the 4th instar larva/adult female along with the biomass of leftover weed twig was recorded using analytical balance(0.01 mg precision).Each experiment was replicated 15 times per stage per eco-climatic region, and the feeding attributes(consumption rate, conversion efficiency and growth rate)were calculated.
2.3 Data analysis
Distributions of data sets obtained in the study were checked for normality using Kolmogorov-Smirnoff test.Means were separated using Tukey’s test when data were normally distributed and variances were homogeneous(Bartlett’s test for equal variances).The obtained data on feeding attributes and mean body biomass of the 4th instar larvae/adult females were subjected to three-way repeated measures of ANOVA(three-way rANOVA)with consumption rate, conversion efficiency, growth rate and mean body biomass as dependent variables; and eco-climatic regions(Chitwan, Kathmandu and Mahendranagar), generation(parent or progeny), developmental stage(larva or adult)and their interaction as independent variables, followed by Tukey’spost-hoccomparison of means.
Data on the developmental duration ofZ.bicoloratalarvae were subjected to two-way repeated measures of ANOVA(two-way rANOVA)with developmental duration as dependent variable; and eco-climatic regions(Chitwan, Kathmandu and Mahendranagar), generation(parent or progeny)and their interaction as independent variables, followed by Tukey’spost-hoccomparison of means.All statistical analyses were performed using MINITAB 16(Minitab Inc., State College, Pennsylvania, USA).
3 RESULTS
Results of two-way repeated measures of ANOVA revealed significant effect of eco-climatic regions(F=32.00;df=2, 149;P<0.0001)on the total developmental period of immature stages.However the effects of generation(F=0.01;df=1, 149;P=0.909)and the interaction between eco-climatic regions and generation(F=0.13;df=2, 149;P=0.874)on the total developmental period of immature stages were insignificant.In addition, three-way rANOVA values revealed significant effects of eco-climatic regions and developmental stage on mean body biomass of the 4th instar larvae and adult females ofZ.bicolorata.The mean body biomasses of the 4th instar larvae and adult females did not differ significantly by the generation and the interaction between the three independent factors.Tukey’sposthoccomparison of means revealed that despite provided with optimal abiotic conditions and abundant food, developing stages having parents from Kathmandu region were the heaviest in biomass as similar to their parents(Table 1).However, parents and their progeny from Mahendranagar region completed their development faster than those from Kathmandu and Chitwan regions(Fig.1).
Results of three-way rANOVA also revealed significant effects of eco-climatic regions and developmental stage on consumption rate, conversion efficiency and growth rate of the 4th instar larvae and adult females ofZ.bicolorata, both in parental and F1generations.However, the feeding attributes and mean body biomasses of the 4th instar larvae and adult females did not differ significantly by the generation and the interaction between the three independent factors.Comparison of means revealed that while the 4th instar larvae and adult females having parents from Kathmandu region had higher food consumption rates, those from Chitwan and Mahendranagar regions had higher conversion efficiency and growth rates(Table 1).Results of the present study have also exposed that amongst the developmental stages, early larval instars had faster development than the later instars.And, irrespective of the three eco-climatic regions, the developing females were heavier, but had lower conversion efficiency and growth rates than the developing larvae(Table 1; Fig.1).
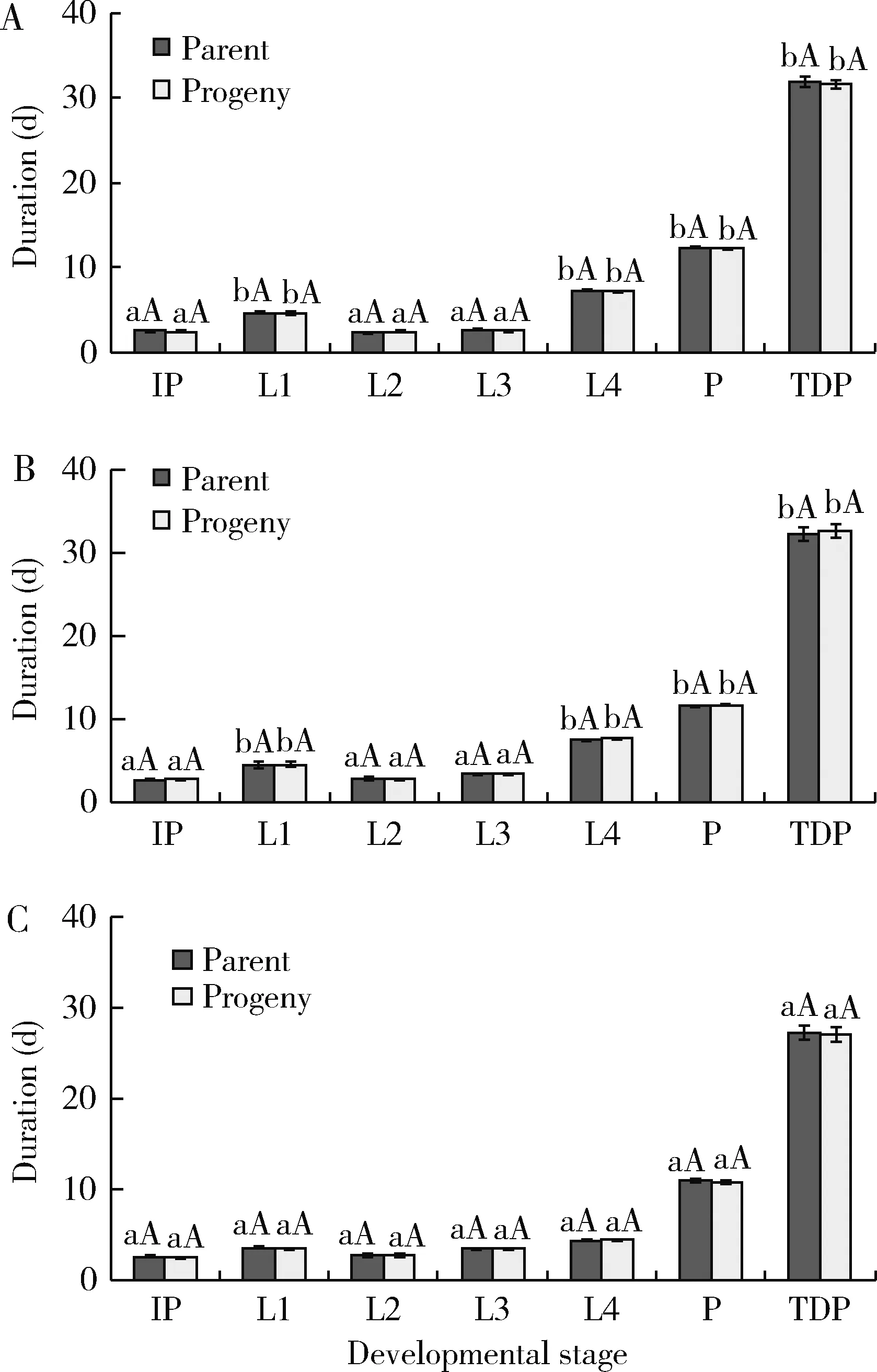
Fig.1 Variations in the developmental duration and total developmental period of parents and their progeny of Zygogramma bicolorata in Kathmandu(A),Chitwan(B), and Mahendranagar(C)regions IP, L1, L2, L3, L4, P and TDP represent incubation period, 1st, 2nd, 3rd and 4th larval instars, pupal stage and total developmental period, respectively.Small and large letters represent comparison of means amongst eco-climatic regions and between the generations, respectively, at the 0.05 level by Tukey’s post-hoc comparison.
4 DISCUSSION
In the present study, despite provided with optimal abiotic conditions and abundant food, the developing stages having parents from Kathmandu region were the heaviest in biomass as similar to their parents.Such results may possibly be owing to the fact that Kathmandu is situated at a high altitude, and the average temperature of Kathmandu is lower than the other two eco-climatic regions.Therefore, the size(=body biomass)of beetles in wild populations of such a high altitudinal region with low temperature may be large.Previous studies have also shown that decreasing temperature slows down the developmental rate and insects have a larger/heavier body at a lower temperature, conforming to the temperature-size rule(Atkinson, 1994; Klok and Harrison, 2013; Chenetal., 2019).
The ‘food resources versus body size’ hypothesis also suggests that animals grow larger and heavier in resource-rich environments, but remain smaller when resources are limited(Atkinson and Sibly, 1997).Similarly, ‘plant vigour’ hypothesis proposes that more nutritious plants enhance the feeding and reproductive attributes of insect herbivores which increase their body sizes(Awmack and Leather, 2002; Stiling and Moon, 2005).Since the quality ofP.hysterophorusvaries from one eco-climatic region to another(Cowieetal., 2019), there are huge possibilities that the weed grown in colder regions and at high altitudes may be enormously rich in nutrients that results in larger size ofZ.bicolorataadults and their progeny.In addition, there are possibilities that reduced predation pressure may have allowed adults ofZ.bicoloratato evolve larger body size in Kathmandu region, as predation pressure decreases along the elevational gradient(Presleyetal., 2012; Roslinetal., 2017).Our earlier published data(Bhusaletal., 2019)have also revealed thatZ.bicolorataadults collected from wild populations of Kathmandu are the largest, followed by Chitwan, while those of Mahendranagar are the smallest in size.
The faster developmental rate in parents and progeny of Mahendranagar region may be explained on the basis of ‘voltinism/generation number versus body size’ hypothesis.The hypothesis emphasizes that in multivoltine terrestrial insects, body size decreases but the number of generations per year increases with increasing temperature or decreasing latitude(Horneetal., 2015; Zeussetal., 2017).This is because the smaller insects have shorter developmental duration and they complete their reproductive cycles earlier than the larger insects(Mishraetal., 2011).Such a trade-off between temperature-dependent body size and generation number may possibly be a survival strategy of insects to successfully overcome the harsh climatic conditions created by high temperature, as recorded from Mahendranagar region(~34℃).SinceZ.bicoloratais a multivoltine chrysomelid beetle, the adults inhabiting high temperature and low altitude regions(Mahendranagar)are small with possibly more generation numbers than those inhabiting the low temperature and high altitude regions(Kathmandu and Chitwan)(Bhusaletal., 2019).
The higher consumption rates of parents and progeny from Kathmandu region may possibly be owing to their higher body biomasses.However, the higher food conversion efficiency and growth rates of the 4th instar larvae/adult females having parents from Chitwan or Mahendranagar region than those from Kathmandu region may possibly be owing to the fact that small-sized beetles consume and utilize more prey per unit biomass per unit time during their feeding periods.They, therefore, show higher food conversion efficiency and growth rates and complete their development earlier than the large-sized beetles.Present findings are in accordance with those reported earlier(Mishraetal., 2011, 2012).
In addition, the early larval stages have shown faster development than the later stages in the present study.Possibly the small body size, low energy requirements and reduced metabolic costs of the former may be responsible for their faster development than the latter.At later stages of their development, the larvae need more time and sufficient energy to attain the threshold biomass and to internally prepare them for pupation, thereby increasing their body biomass and prolonging their developmental duration(Kogan and Cope, 1974; Mishraetal., 2012).Irrespective of the three eco-climatic regions, the developing females were heavier, but had lower conversion efficiency and growth rates than the developing larvae.The large size of females may be ascribed to their high energy needs for gonadal development and reproduction.However, the higher conversion efficiency and growth rate of the 4th instar larvae over the adult females, as recorded in the present may be due to their small size.Our present findings are in compliance with the results of Kohleretal.(1987)in the nymphs of Central European grasshoppers and Mishraetal.(2011)in coccinellid beetles.
In brief, our study contradicts our hypothesis and states that inZ.bicoloratathe immediate effects of food and temperature may not affect the phenotypic plasticity of progeny and they may display the body size pattern and feeding behaviour similar to their parents.There are also possibilities that the heritable changes might be owing to genetic diversity within the species.High altitude and cold climatic conditions(Kathmandu)may provide the adults ofZ.bicoloratato evolve large size and higher consumption rates, as the food is abundant and risk of predation is less.In contrast, the low altitude and hot climatic conditions(Mahendranagar)may promote the development of small adults, possibly because of the availability of limited food resources.But the small-sized adults are possibly equipped with the advantages of having shorter developmental duration, and higher food conversion and utilization efficiency to compensate for their lower consumption rates and to overcome the food stress conditions.The present results may be helpful in developing future strategies for rapid mass multiplication ofZ.bicolorataadults in laboratories for augmentative biocontrol of the weed.
ACKNOWLEDGEMENTSThe authors are thankful to Indian National Science Academy(INSA)and Nepal Academy of Science and Technology(NAST)for NAST-INSA bilateral exchange program for financial assistance to carry out this research work.
