Histo-blood group antigens in Crassostrea gigas and binding pro files with GII.4 Norovirus*
2018-08-02MALiping马丽萍LIUHui刘慧SULaijin苏来金ZHAOFeng赵峰ZHOUDeqing周德庆DUANDelin段德麟
MA Liping (马丽萍) LIU Hui (刘慧) SU Laijin (苏来金) ZHAO Feng (赵峰) ZHOU Deqing (周德庆) DUAN Delin (段德麟)
1 Key Laboratory of Experimental Marine Biology, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071,China
2 Key Laboratory for Sustainable Utilization of Marine Fisheries Resources, Ministry of Agriculture, Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Qingdao 266071, China
3 Laboratory for Marine Biology and Biotechnology, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266071, China
Abstract Noroviruses (NoVs) are the main cause of viral gastroenteritis outbreaks worldwide, and oysters are the most common carriers of NoV contamination and transmission. NoVs bind speci fically to oyster tissues through histo-blood group antigens (HBGAs), and this facilitates virus accumulation and increases virus persistence in oysters. To investigate the interaction of HBGAs in Paci fic oysters with GII.4 NoV, we examined HBGAs with ELISAs and investigated binding patterns with oligosaccharide-binding assays using P particles as a model of five GII.4 NoV capsids. The HBGAs in the gut and gills exhibited polymorphisms. In the gut, type A was detected (100%), whereas type Leb (91.67%) and type A (61.11%)were both observed in the gills. Moreover, we found that seasonal NoV gastroenteritis outbreaks were not signi ficantly associated with the speci fic HBGAs detected in the oyster gut and gills. In the gut, we found that strain-2006b and strain-96/96US bound to type A and H1 but only weakly bound to type Leb; in contrast, the Camberwell and Hunter strains exhibited weak binding to types H1 and Ley, and strain-Sakai exhibited no binding to any HBGA type. In the gills, strain-96/96US and strain-2006b bound to type Leb but only weakly bound to type H1; strains Camberwell, Hunter, and Sakai did not bind to oyster HBGAs. Assays for oligosaccharide binding to GII.4 NoV P particles showed that strain-95/96US and strain-2006b strongly bound to type A, B, H1, Leb, and Ley oligosaccharides, while strains Camberwell and Hunter showed weak binding ability to type H1 and Ley oligosaccharides and strain-Sakai showed weak binding ability to type Leb and Ley oligosaccharides. Our study presents new information and enhances understanding about the mechanism for NoV accumulation in oysters. Further studies of multiple NoV-tissue interactions might assist in identifying new or improved strategies for minimizing contamination, including HBGA-based attachment inhibition or depuration.
Key word: Crassostrea gigas; norovirus; histo-blood group antigen; binding
1 INTRODUCTION
Norovirus (NoV) is the most prevalent pathogen associated with food-borne acute non-bacterial gastroenteritis. Outbreaks usually occur suddenly in early spring and winter. It is transmitted by consumption of contaminated oysters, which are known to accumulate viruses. Oyster-related gastroenteritis is now regarded as a major seafood safety risk to humans. NoV has been widely detected in oysters around the world (Nishida et al., 2007;DePaola et al., 2010; Lowther et al., 2012; Ma et al.,2013; Schaeffer et al., 2013). Depuration is not effective for the complete elimination of NoV in shell fish (Savini et al., 2009; Provost et al., 2011;Rodriguez-Manzano et al., 2014). Although NoVs cannot propagate without their host, they can survive in a favorable micro-environment for a long time(Chung and Sobsey, 1993).
NoVs belong to the human-infecting Caliciviridae family (Anbazhagi and Kamatchiammal, 2010), and they are classi fied into five genogroups (GI, GII, GIII,GIV, and GV) based on the viral capsid peptide sequences of the virus whole genome. GI and GII NoVs are the main groups involved in human infection, with genotype GII.4 being the most prevalent and infectious form (Fankhauser et al.,2002; Siebenga et al., 2009).
Structurally, NoVs have a 7.7-kb single-stranded RNA genome with three open reading frames (ORFs),of which ORF2 encodes both the major (VP1) and minor (VP2) capsid proteins. VP1 consists of the shell(S) (1–225 AA) and the protruding (P) domains (226–530 AA) (Prasad et al., 1999). The P domain is regarded as domain that binds to histo-blood group antigens (HBGAs) (Tan et al., 2004; Tan and Jiang,2007; Tan and Jiang, 2005a, b). Saliva-based HBGA-binding assays indicate that P particles retain binding capability to human HBGAs (Tan and Jiang, 2005b).Given their advantage of easy and stable in vitro expression in the E scherichia coli system, P particles are the protein typically used for NoV-HBGA interaction analyses and vaccine production.
In oysters, the oligosaccharide residues of HBGA are regarded as the site that binds to the NoV P domain; this interaction is similar to that of ABH blood group antigens in the human digestive tract(Maalouf et al., 2011). Tian et al. (2006) veri fied that NoV can bind to A-like antigens in oyster gastrointestinal cells, and Maalouf et al. (2011)convincingly showed that the binding site in oysters is the same as that during human infection.Nonetheless, little information is available about the various types of HBGA and their distributions in oysters, and the binding patterns of GII.4 NoV to HBGA in oyster tissues, in particular, are not clearly understood. It is therefore important to study HBGA binding patterns, especially those of GII.4 NoV to oyster tissue HBGAs.
The objective of our study was to enhance understanding of the binding abilities and forms of HBGAs in the Paci fic oyster ( Crassostrea gigas) to GII.4 NoV P particles; this knowledge may help to decrease NoV contamination in oysters and subsequently increase the safety of human seafood consumption.

Table 1 The five strains of GII.4 NoV used in this study
2 MATERIAL AND METHOD
2.1 GII.4 NoV strains, monoclonal antibodies, and oligosaccharide preparation
Five GII.4 NoV strains (Table 1) were obtained from the Chinese Center for Disease Control and Prevention (CDC); the P proteins were expressed in E. coli (BL21) and then puri fied as previously described (Ma et al., 2015). The antisera for binding the HBGAs coating P particles were supplied by Dr.Jin Miao.
Monoclonal antibodies (MAbs; Covance, USA)were applied for the detection of HBGAs in oyster tissues. The applied MAbs were speci fic to blood antigen precursor (BG-1), type A (BG-2), type B(BG-3), type H1 (BG-4), Lea (BG-5), Leb (BG-6),Lex (BG-7), and Ley (BG-8).
Arti ficial synthesis of oligosaccharides was applied to our investigation of the binding patterns and abilities of GII.4 NoV. These oligosaccharides were polyacrylamide (PAA)-biotin conjugated type A, B,H1, Lea, and Lex trisaccharides and Leb and Ley tetrasaccharides (GlycoTech Corporation, Rockville,MD, USA).
2.2 Oyster tissue collection and HBGA preparation
Fresh Paci fic oysters ( C. gigas) were collected from the Qingdao Langya oyster farm in 2016.Collection was carried out three times each month(six oysters on average). After collection, the oyster tissues were scrubbed and rinsed, and the gut and gills were immediately dissected out. About 3 g (fresh weight) of gut or gill tissue was homogenized in 9 mL of phosphate-buffered saline (PBS) (pH 7.4) with a homogenizer (PB100, Prima, London, UK), kept for 10 min at 95°C, and then centrifuged at 3 000× g for 15 min at 4°C. The supernatant was recovered and was transferred into a new 50-mL tube; the protein concentration of the extracted solution was assayed with a bicinchoninic acid (BCA) protein assay kit(Thermo Fisher Scienti fic, Waltham, MA, USA)according to the manufacturer’s instructions, and the resultant solution was examined using a spectrophotometer at optical density (OD)260/280wavelength absorption. Last, the extracted protein solution was diluted with PBS (pH 7.4) to a final concentration of 20 μg/mL for the subsequent characterization of HBGA and the binding detection of NoV P particles.
2.3 ELISA detection of HBGA in oyster gut and gill tissue
Detection of HBGA was performed according to the method of Tian et al. (2008) with minor modi fications. Samples of tissue supernatant (100 μL/well) were incubated overnight in 96-well microplates(Corning, NY, USA) at 4°C. Each experiment was conducted in triplicate. The reaction wells of the microplate were washed three times with 300 μL of PBS per wash to remove unbound agents, and the microplate was then blocked with 250 μL of 10%skim milk per well (Yili, Beijing, China). The blocked microplates were subsequently sealed separately in plastic bags and incubated at 37°C for 2 h. After the incubation, the wells were washed three times with PBS and dried at room temperature (25°C). The MAbs (Covance) (anti-type A, -type B, -H1, -Lex,and -Ley) were diluted with ratios of 1:1 000, anti-Lea MAb was diluted 1:800, and anti-Leb MAb was diluted 1:600. The diluted MAb solutions (100 μL per well) were then added into the appropriate wells and incubated at 37°C for 1 h. After the incubation, the wells were washed three times with 200 μL per well of PBS containing 0.5% Tween 20 to remove the unbound MAbs. Goat anti-mouse IgG antibody for MAbs BG-2, BG-4, or BG-5 and goat anti-mouse IgM antibody for MAbs BG-1, BG-3, BG-6, BG-7, or BG-8 were diluted with PBS at a ratio of 1:2 000, and 100 μL per well of appropriate antibody were added to each well for incubation at 37°C for 1 h. After incubation, the wells were washed three times with 200 μL per well of PBS containing 0.5% Tween 20.Soluble TMB substrate solution (100 μL; Tiangen,Beijing, China) was added into each well for incubation in the dark for 15 min, and 100 μL per well of 1 mol/L sulfuric acid was then added to terminate the reaction. The reaction solution was assayed at OD450nmusing a VICTOR™ X3 Multilabel Plate Reader (PerkinElmer, Waltham, MA, USA). The cutoff point for the ELISA data that were considered to be above the background level was calculated using positive-to-negative (P/N) ratios as described previously (Tian et al., 2008). The OD450nmof the negative control was less than 0.1, and P/N ratios ≥2.0 were considered positive.
2.4 Immunohistochemical examination for HBGAs.
About 100 μg (fresh weight) each of gut and gill tissue were selected and fixed in a 4% paraformaldehyde solution (Yuanmu Ltd. Co., Shanghai, China) for 24 h. The fixed tissues were then embedded in paraffin wax and sectioned into 5-μm thick slices. After that,these sectioned tissues were dried at 68°C for 20 min,incubated in 3% H2O2at 37°C for 10 min, washed with PBS solution three times for 5 min each, boiled in 0.01 mol/L citrate buffer (pH 6.0) (95°C, 15–20 min), and then rewashed with PBS solution three times for 5 min each. Goat serum was used for blocking for 10 min at 37°C. The supernatant was removed, and the primary antibody was added for 2 h,according to the method described in Section 2.2. The prepared slides were washed and stained with a Streptavidin-Biotin Complex (SABC) Kit (Boster Ltd. Co, Wuhan, China) according to the manufacturer’s instructions. For the control tests, the exposed sections without added primary antibody were used in a parallel examination. Hematoxylin dying agents were applied for 15 s at room temperature.Finally, the dyed slides were observed, and the results were recorded under a microscope (COIC Industrial Co., Ltd., Chongqing, China).
2.5 Detection of the binding patterns of NoV to oyster gut and gills
The supernatants from gut and gill samples were used detect the binding patterns of NoV according to the method described above for HBGA. After the removal of unbound HBGA, 10% skim milk (Yili Ltd. Co, Beijing, China) was added for blocking for 2 h. P particles (0.5–1.0 μg/mL) were added into wells containing diluted 2% skim milk (Yili Ltd. Co). GST protein (0.5–1.0 μg/mL) was added as the negative control group. The bound P particles were detected using a pooled rabbit antiserum resulting from immunization with strain 95/96US P particles; this antiserum was used at dilutions from 1:2 000 to 1:3 000. HRP-conjugated goat anti-rabbit antibody was then added as the secondary antibody (Sanjian Ltd. Co, Tianjin, China) (dilution, 1:3 000).
2.6 Binding patterns of P particles to synthetic oligosaccharides
Microtiter plates were coated with NoV P particles(2–10 μg/mL) overnight at 4°C, and the same concentration of GST protein was added as the negative control. After adding 5% skim milk, the oligosaccharide-PAA-biotin conjugate solution(2 μg/mL) was added, and the samples were incubated at 4°C overnight. To remove unbound oligosaccharides,the reaction solution in each well was washed five times with 250 μL of PBS-T solution. Afterward,100 μL of HRP-streptavidin (diluted 1:1 000 in PBS)was added into the reaction solution, and the plate was incubated for 1 h at 37°C and rewashed three times with 200 μL per well of PBS-T solution. The captured HRP-Streptavidin was assayed according to the procedures described in Section 2.2.
3 RESULT
3.1 HBGA content and types detected in the oyster gut and gills
To assess the HBGA types and their distribution in the gut and gills of Paci fic oysters, ELISAs were performed on samples from over 200 oysters. The HBGA types and content in the gut and gills showed diverse reactions to the eight tested types of MAb(Tables 2, 3). Interestingly, both secretory types (A,B, H1, Leb, and Ley) and non-secretory types (Lea and Lex) of HBGAs were simultaneously detected in the oysters. Of the eight assayed HBGA types, seven types (A, B, H1, Lea, Leb, Lex and Ley) were detected in the gut and six types (A, B, H1, Lea, Leb, and Lex)were detected in the gills. More speci fically, type A(100%) was the type most commonly detected in the gut (Fig.1), followed by types Lea (44.44%), H1(33.33%), Lex (30.56%), Leb (25%), B (22.22%),and Ley (16.67). In contrast, in the gills, types Leb(91.67%) and A (61.11%) were detected (Fig.1), but no type Ley was found. Generally, no type precursor HBGA was detected in the oyster gut and gills.

Fig.1 Detection of the rates of different types of HBGAs in oyster gut and gills

Table 2 HBGA patterns detected in oyster guts

Table 3 HBGA patterns detected in oyster gills
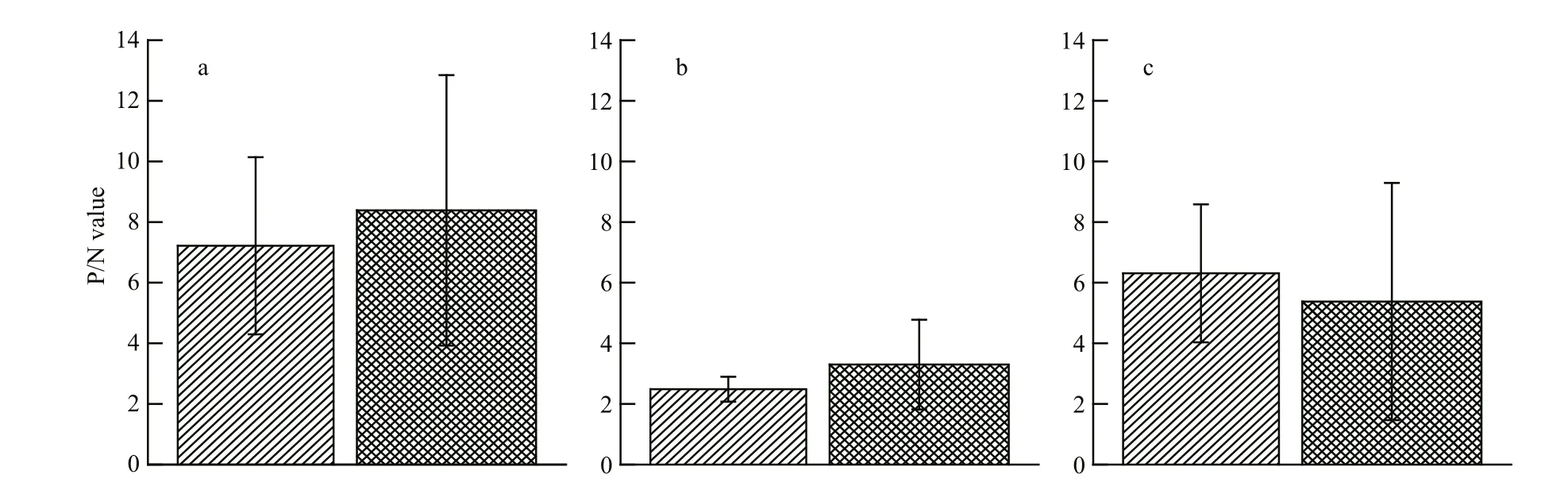
Fig.2 Seasonal variations of HBGA in the oyster gut and gill
3.2 HBGA content detected in oysters collected during different seasons
We next investigated if there was a seasonal effect on the HBGA content in oysters by performing ELISAs to detect HBGA types in the gut and gills of oysters collected during different seasons. The results indicate that the amounts of type A HBGA trended higher in April–October, during which gastroenteritis cases are not typically due to NoV, compared with those in November–March, which are typical NoV gastroenteritis outbreak months (Fig.2). There was also a trend toward higher amounts of type Leb HBGA in November–March in the gills (Fig.2), but this difference did not reach statistical signi ficance either. Based on these data, seasonal outbreaks of NoV gastroenteritis are not signi ficantly correlated with the amount of HBGA in the oyster gut or gills.
3.3 Immunohistochemical examination of HBGAs in oyster tissues
To con firm our ELISA results, we further examined the HBGA types in oyster tissues by performing immunohistochemistry. In these experiments, types A, H1, and Lea were examined. Type A was more strongly stained than types H1 and Lea in both the oyster gut (Fig.3f–h) and oyster gills (Fig.3b–d).
3.4 Characterization of GII.4 NoV binding patterns to HBGA
The speci fic HBGA types with the maximum P/N ratios in oyster gut and gills were further studied to characterize their GII.4 NoV-binding properties. In the oyster gut, P particles from strain-2006b could strongly bind to types A and H1 and weakly bind to types Leb, Ley, and B, but they could not bind to types Lea or Lex (Fig.4a). Similarly, strain-96/96US P particles in the oyster gut could bind to types A and H1 and weakly bind to type Leb, but they could not bind to types B, Lea, Lex, or Ley. Furthermore, P particles from strains Camberwell and Hunter bound to type H1 and Ley only weakly, and those from strain-Sakai did not bind to any HBGA type. In contrast, in the gills, most GII.4 NoV P particles exhibited weak binding abilities to HBGA; both strain-96/96US and strain-2006b could strongly bind to type Leb and weakly bind to type H1, but strains Camberwell, Hunter, and Sakai did not exhibit any HBGA-binding abilities (Fig.4b).

Fig.3 Immunohistochemical detection of HBGA in oyster gills and gut
3.5 Binding of GII.4 NoV particles to synthesized oligosaccharide
We next used synthesized oligosaccharide to perform a binding pattern analysis of five GII.4 NoV strains. During the assay optimization process, we observed that the applied oligosaccharide residues exhibited speci fic binding abilities to their corresponding monoclonal antibodies without any cross-reactions. The results show that different NoV strains each exhibit a different binding ability to oligosaccharides (Fig.5). Strain-95/96US and strain-2006b strongly bound to types A, B, H1, Leb, and Ley and strain-Camberwell and strain-Hunter weakly bound to types H1 and Ley, while strain-Sakai weakly bound to types Leb and Ley. None of the five tested NoV strains bound to types Lea and Lex.
4 DISCUSSION
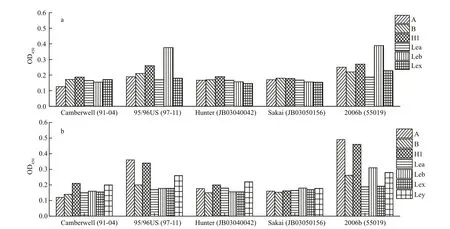
Fig.4 Binding abilities of GII.4 NoV P particles to HBGAs in oyster gut or gills

Fig.5 Binding patterns of GII.4 NoV P particles to synthetic oligosaccharides
The HBGA types and the contents we detected in samples from different seasons are consistent with the findings of our previous study (Ma et al., 2013); NoV was found to be present in Paci fic oyster throughout the year. In May and July, seven HBGA types were detected in the gut, while in February and May, six HBGA types were observed in the gill. Thus, oysters are more easily contaminated by NoV in these months.Type A HBGA was detected throughout the gut,con firming the findings of previous work (Le Guyader et al., 2006; Tian et al., 2006; Tian and Jiang, 2007).Since types Leb and A were detected in the gills of most tested oysters, the gills could be considered as a major target tissue for NoV veri fication and isolation(Wang et al., 2008).
Usually, outbreaks of NoV occur more frequently in the winter (Lindesmith et al., 2003) and less frequently in the summer (Myrmel et al., 2004). From analyzing HBGAs, we found that HBGA expression and NoV-binding were similarly high in both the non-NoV gastroenteritis season (April–October) and the NoV gastroenteritis season (November–March). This suggests that the seasonal NoV gastroenteritis outbreaks are not closely related to any particular HBGA expression pattern. It has been reported that NoV outbreaks are triggered not only by the seasons but also by other environmental factors, such as precipitation and temperature (Sinton et al., 1994;Lipp et al., 2001; Love et al., 2010; Maalouf et al.,2010).
NoVs are mainly located in oyster pancreatic tissue(digestive diverticula) through their speci fic binding to HBGA; this binding facilitates their bioaccumulation and persistence in oysters. In the present study, we detected seven HBGA types in the gut and six HBGA types in the gills of Paci fic oysters, and these HBGAs exhibited polymorphisms. In support of these findings,the results from our immunohistochemical analyses showed that NoV recognizes types H1, A, and Lea epitopes in the gut, and types A, H1, and Leb in the gills, which reveals that HBGA distributions are tissue-speci fic in Paci fic oysters. Compared with the study by Le Guyader et al. (2006), which was conducted only in the oyster gut, we found that more HBGA types exist in the gut and gills of the Paci fic oyster. Furthermore, investigations of gastrointestinal tissue from different species, Crassostrea virginica,C. gigas, and Crassostrea sikamea (Tian and Jiang,2007; Tian et al., 2008), found the occurrence of only type A and type H HBGA. Maalouf et al. (2010)demonstrated that the binding to the gills and mantle tissue sections involves a sialic acid in an α2,3 linkage,whereas, in digestive tissues, the interaction involves both this sialic acid and an A-like carbohydrate ligand.Combined with our findings, these differential ligand recognitions by NoV strains may lead to distinct outcomes in terms of the persistence of viral particles within the different organs.
HBGA-binding patterns are diverse and strainspeci fic, and eight distinct binding pro files have been described previously (Huang et al., 2005). Because the HBGA structure in oysters is not clear, we simulated oligosaccharides with different types of human HBGA epitopes for testing the binding pattern of NoV-HBGA, and we applied P particles to studying the binding pro files of oyster HBGA with NoV. These experiments revealed that strain-95/96US and strain-2006b both bound strongly to synthesized oligosaccharide derived from types A, B, H1, Leb,and Ley. Speci fically, they mainly bound to types A and H1 HBGA in the gut and to type Leb in the gills.We speculate that the existence of types A, H1, or Leb HBGA in the digestive tissue and gills are the main reasons for GII.4 NoV distribution in the Paci fic oyster. For other GII.4 NoV variants, no obvious binding pro files were obtained, probably due to their weak binding ability with HBGA and the low content of HBGA in oysters.
5 CONCLUSION
Several different HBGA types exist in the Paci fic oyster gut and gills, but their content is not closely related with NoV gastroenteritis outbreaks. There are multiple GII.4 NoV P particle-binding epitopes in the oyster gills and gut. Our results support further exploration of the mechanism of NoV accumulation and provide new insight that may be useful for NoV depuration from the Paci fic oyster.
6 DATA AVAILABILITY STATEMENT
Huang P W, Farkas T, Zhong W M, Tan M, Thornton S,Morrow A L, Jiang X. 2005. Norovirus and histo-blood group antigens: demonstration of a wide spectrum of strain speci ficities and classi fication of two major binding groups among multiple binding patterns. J. Virol., 79(11):6 714-6 722.
Le Guyader F S, Loisy F, Atmar R L, Hutson A M, Estes M K,Ruvoën-Clouet N, Pommepuy M, Le Pendu J. 2006.Norwalk virus-speci fic binding to oyster digestive tissues.Emerg. Infect. Dis., 12(6): 931-936.
Lindesmith L, Moe C, Marionneau S, Ruvoen N, Jiang X,Lindblad L, Stewart P, LePendu J, Baric R. 2003. Human susceptibility and resistance to Norwalk virus infection.Nat. Med., 9(5): 548-553.
Lipp E K, Kurz R, Vincent R, Rodriguez-Palacios C, Farrah S R, Rose J B. 2001. The effects of seasonal variability and weather on microbial fecal pollution and enteric pathogens in a subtropical estuary. E stuaries, 24(2): 266-276.
Love D C, Lovelace G L, Sobsey M D. 2010. Removal of Escherichia coli, Enterococcus fecalis, coliphage MS2,poliovirus, and hepatitis A virus from oysters ( Crassostrea virginica) and hard shell clams (M ercinaria mercinaria)by depuration. Int. J. Food Microbiol., 143(3): 211-217.
Lowther J A, Gustar N E, Hartnell R E, Lees D N. 2012.Comparison of norovirus RNA levels in outbreak-related oysters with background environmental levels. J. Food Protect., 75(2): 389-393.
Ma L P, Su L J, Zhao F, Zhou D Q. 2015. Type-like of HBGAs in Crassostrea gigas and its binding properties with norovirus P particles. J. Food Saf. Qual., 6(10): 3 970-3 975. (in Chinese with English abstract)
Ma L P, Zhao F, Yao L, Li X G, Zhou D Q, Zhang R L. 2013.The presence of genogroup II norovirus in retail shell fish from seven coastal cities in China. Food Environ. Virol.,5(2): 81-86.
Maalouf H, Schaeffer J, Parnaudeau S, Le Pendu J, Atmar R L,Crawford S E, Le Guyader F S. 2011. Strain-dependent norovirus bioaccumulation in oysters. Appl. Environ.Microbiol., 77(10): 3 189-3 196.
Maalouf H, Zakhour M, Le Pendu J, Le Saux J C, Atmar R L,Le Guyader F S. 2010. Distribution in tissue and seasonal variation of norovirus genogroup I and II ligands in oysters. Appl. Environ. Microbiol., 76(16): 5 621-5 630.
Myrmel M, Berg E M M, Rimstad E, Grinde B. 2004. Detection of enteric viruses in shell fish from the Norwegian coast.Appl. Environ. Microbiol., 70(5): 2 678-2 684.
Nishida T, Nishio O, Kato M, Chuma T, Kato H, Iwata H,Kimura H. 2007. Genotyping and quantitation of noroviruses in oysters from two distinct sea areas in Japan. Microbiol. Immunol., 51(2): 177-184.
Prasad B V V, Hardy M E, Dokland T, Bella J, Rossmann M G,Estes M K. 1999. X-ray crystallographic structure of the Norwalk virus capsid. Science, 286(5438): 287-290.
Provost K, Dancho B A, Ozbay G, Anderson R S, Richards G P, Kingsley D H. 2011. Hemocytes are sites of enteric virus persistence within oysters. Appl. Environ. Microbiol.,77(23): 8 360-8 369.
Data sharing is not applicable to this article, as no datasets were generated or analyzed during the present study.
7 ACKNOWLEDGEMENT
We thank Dr. JIN Miao at the CDC, China, for providing the GII.4 norovirus variants and antisera,Dr. Ian R. Jenkinson for performing English revision.We also thank Katie Oakley, PhD, from Liwen Bianji,Edanz Editing China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
猜你喜欢
杂志排行
Journal of Oceanology and Limnology的其它文章
- Editorial Statement
- Recent insights into physiological responses to nutrients by the cylindrospermopsin producing cyanobacterium,Cylindrospermopsis raciborskii*
- Response of Microcystis aeruginosa FACHB-905 to different nutrient ratios and changes in phosphorus chemistry*
- In fluence of light availability on the speci fic density, size and sinking loss of Anabaena flos- aquae and Scenedesmus obliquus*
- Application of first order rate kinetics to explain changes in bloom toxicity—the importance of understanding cell toxin quotas*
- Regime shift in Lake Dianchi (China) during the last 50 years*
