Molecular characterization and expression analysis of Lilytype lectin ( Sm LTL) in turbot Scophthalmus maximus, and its response to Vibrio anguillarum*
2018-05-07XIADandan夏丹丹MAAijun马爱军HUANGZhihui黄智慧SHANGXiaomei商晓梅CUIWenxiao崔文晓YANGZhi杨志QUJiangbo曲江波
XIA Dandan (夏丹丹) MA Aijun (马爱军) HUANG Zhihui (黄智慧) SHANG Xiaomei (商晓梅) CUI Wenxiao (崔文晓) YANG Zhi (杨志) QU Jiangbo (曲江波)
1 Key Laboratory of Sustainable Development of Marine Fisheries, Ministry of Agriculture; Qingdao Key Laboratory for Marine Fish Breeding and Biotechnology; Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Qingdao 266071, China
2 Ocean University of Shanghai, Shanghai 201306, China
3 Laboratory for Marine Biology and Biotechnology, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266071, China
4 Yantai Tianyuan Aquatic Limited Corporation, Yantai 264006, China
1 INTRODUCTION
The skin of fishes is covered with mucus (Suzuki et al., 2003). The mucosal layer is the first line of defense against the invasion of foreign substances and pathogenic microorganisms, and serves as a main organ of defense (Suzuki et al., 2003). The main constituent of this barrier is a mucous gel that forms a layer which covers the epithelial cells (Van der Marel et al., 2010). The mucous layer is secreted by various epidermal or epithelial mucus cells, such as goblet cells (Shephard, 1994; Spitzer and Koch, 1998). The skin mucus layer is mainly composed of water and mucins, which are glycoproteins that contain high molecular weight oligosaccharides. Skin mucus is involved in fish respiration, osmoregulation,reproduction, locomotion, defense against microbial infection, disease resistance, excretion, and communication (Shephard, 1994; Khong et al., 2009).One of the most interesting functions of fish mucus is related to its role in the immune response and disease resistance (Guardiola et al., 2014); however, further characterization of this role is needed.
Lectins are not enzymes but able to bind to carbohydrate, are present in the mucus (Ingram, 1980;Alexander and Ingram, 1992), and are capable of defending against pathogens (Suzuki et al., 2003).The biological effects of lectins primarily occur via the binding of the active site of the lectin chain to carbohydrates (Ke et al., 2005). Fish lectins are mediators of non-self recognition in a variety of biological processes. Specifically, fish lectins are involved in the identification and stimulation of pathogen uptake by phagocytes, the facilitation of innate complement-mediated cell lysis, and the enhancement of natural killer cell activity (Hoff mann et al., 1999; Sharon and Lis, 2004; Kim et al., 2011).There are already several researches of lectin in different fishes, includingCongermyriaster(Kamiya et al., 1988; Shiomi et al., 1989),Repomucenus richardsonii(Shiomi et al., 1990),Misgurnus anguillicaudatus(Goto-Nance et al., 1995),Genypteruscapensis(Toda et al., 1996),Anguilla japonica(Tasumi et al., 2002), andChannastriata(Arasu et al., 2013).
Based on their distinct structures and functions,lectins have been classified as galectins, C-type lectins,lily-type lectins, and rhamnose-binding lectins (Kim et al., 2011; Suzuki et al., 2003). Pufflectin, a mannosespecific lectin purified from the skin mucus of puffish by Tsutsui (2003), was named lily-type lectin (LTL).While LTL shares no significant sequence similarity with any known animal lectins, it surprisingly shares sequence homology with mannose-binding lectins of monocotyledonous plants (Suzuki et al., 2003) such asGalanthusnivalis(Van Damme et al., 1991),Clivia miniata(Van Damme et al., 1994),Alliumprorrum(Van Damme et al., 1993), andAlliumsativum(Smeets et al., 1997). The lectins of these monocotyledonous plants all contain a specific mannose-binding domain(QxDxNxVxY). Interestingly, the amino acid sequence of pufflectin contains two of these characteristic motifs(Tsutsui et al., 2003).Channastriatalily-type lectin(CsLTL-1) was demonstrated to contain two QxDxNxVxY mannose binding sites (Abirami Arasu,2013). Other studies have also reported the existence of QxDxNxVxY mannose binding sites in fish lectins(Chandra, 1999; Tsutsui, 2003; Tsutsui et al., 2006).These previous reports provide an important basis for the study ofScophthalmusmaximuslily-type lectin(SmLTL).
Turbot (Scophthalmusmaximus) is an important farmed fish species with high commercial value in northern China and Europe. Since turbot was introduced to China in 1992, breeding scope has been constantly expanding (Huang et al., 2011). One of the most important factors influencing turbot growth is the variety of pathogens (fungi, bacteria, viruses, and parasites) which cause adverse developmental effects and high rates of mortality in this species. Vibriosis is one of the most disturbing bacterial diseases in turbot aquaculture, which have caused great damage and economic loss in aquaculture production among the world (Saulnier, 2000).Vibrioanguillarumis one of the major pathogens causing vibriosis (Toranzo,1997). The anti-V.anguillarumresponse deserves more attention in order to develop more effective methods of preventingV.anguillarum. In order to efficiently manage disease and provide theory support for the enhancement of aquaculture production,immune mechanism should be thoroughly studied in turbot. Previous proteomic studies in this laboratory revealed thatSmLTL protein expression was significantly changed following high temperature stress in turbot (Ma et al., 2013). In this study, the corresponding proteome maps were constructed by two-dimensional gel electrophoresis (2-DE), from which the peptide mass map with matrix-assisted laser desorption / ionization tandem time-of-flight(MALDI-TOF-TOF) was obtained, andSmLTL protein was identified by database retrieval. The partial protein sequence ofSmLTL was also identified by mass spectrometry analysis. The full-length sequence, expression, and structure prediction analysis ofSmLTL has not been previously published.In the current study,SmLTL was cloned, spatially analyzed, and its tertiary structure predicted for the first time. Furthermore, in turbot (Scophthalmus maximus) aquaculture, vibriosis is one of the most disturbing bacterial diseases which have caused great damage and economic loss in aquaculture production among the world (Saulnier, 2000).Vibrioanguillarumis one of the major pathogens causing vibriosis(Toranzo et al., 1997). Antibiotics are used as traditional strategy for fish disease, while it could due to the development and spread of antibiotic resistant pathogens which would have negative impacts on environment and human health (Chen et al., 2016). So we need an efficient and safe method to solve this problem. Recently many innate immune actors in turbot have been characterized, such as Stomatin-like protein2 (Chi, 2016), chemokines (Meng et al., 2013;Chen, 2015), MyD88 (Lin et al., 2015) and lysozyme(Gao et al., 2016).SmLTL was one kind of innate immune actors, could be one of the most important players on the mucus for host protection.

Table 1 Primers used in this study
2 MATERIAL AND METHOD
2.1 Fish
Healthy turbot ((90±10.2) g) were obtained from the Tianyuan Fisheries Co. Ltd. (Yantai, China).Tissue samples, including head-kidney, kidney, liver,spleen, intestine, muscle, gill, and skin, were dissected from euthanized fish and immediately frozen in RNA holder (Tiangen Biotech Co. Ltd., Beijing, China) and stored at -80°C until use.
2.2 Cloning and sequencing of Sm LTL
According to theSmLTL protein (Ma et al., 2013),we designed primers (L-R-S-5 and L-R-AS-297) for rapid-amplification of cDNA ends (RACE) (Table 1).Total RNA was isolated from fish skin using an RNAprep Tissue Kit (Tiangen). The first-strand cDNA was synthesized from total skin RNA using the SMART RACE cDNA amplification kit (Clontech,Mountain View, CA, USA) for 3′-RACE with primers L-R-S-5 and L-R-AS-297. The cDNA was stored at-20°C prior to further analysis. The polymerase chain reactions (PCR) consisted of denaturation at 94°C for 2 min, 35 cycles of amplification (94°C for 30s, 63°C for 30 s, 72°C for 30 s), and a final extension at 72°C for 2 min. The same procedures were followed for cDNA production for 5′-RACE. All samples were analyzed in triplicate. Cloning and sequencing was performed by Sangon Biotech Co. Ltd. (Shanghai,China) after agarose gel electrophoresis separation and recovery of products by TIANgel Midi Purification Kit (Tiangen). Lasergene Seqman software (DNASTAR, Madison, WI, USA) was used for sequence assembly of the full-length sequence from the 5′ and 3′ terminals. With primer L-QC-S and L-QC-AS (Table 1) to PCR, after agarose gel electrophoresis detection and recovery agarose gel(Tiangen), cloned and sequenced by Sangon Biotech Co., Ltd. of China to confirm the full-length sequence.
2.3 Bioinformatic analysis of Sm LTL
The full-lengthSmLTL sequence was compared with other sequences available in the NCBI database(http://blast.ncbi.nlm.nih.ov/Blast) and the similarities were analyzed. The open reading frame (ORF) and amino acid sequence ofSmLTL was obtained using NCBI. The hydrophilicity of theSmLTL protein was analyzed using ProtScale (http://web.expasy.org/protscale/). Sequence identity, similarity and gap percentages were calculated using the FASTA program (http://fasta.bioch.virginia.edu/fastawww2/fastawww.cgi). Signal peptide analysis was performed using SignalP (http://www.cbs.dtu.dk). The domains and motifs were analyzed using ProtScale (http://web.expasy.org/protscale/). Secondary structure was predicted and analyzed using Jpred4 (http://www.compbio.dundee.ac.uk/jpred/). The deduced amino acid sequences were submitted to multiple alignment using DNAman version 8.0 (Lynnon Biosoft, San Ramon, CA, USA). A phylogenetic tree was constructed using the Neighbor Joining method,considering 1 000 bootstrap hits in DNAman. Protein tertiary structure was predicted and inspected using PDBsum Generate (http://www.ebi.ac.uk/thorntonsrv/databases/cgi-bin/pdbsum/GetPage.pl?pdbcode=index.html). The predicted protein model was checked using PROCHECK (http://www.ebi.ac.uk/thorntonsrv/software/PROCHECK/).
2.4 Tissue-specific expression of Sm LTL

Fig.1 The complete cDNA and deduced amino acid sequence of Sm LTL
Total RNA was isolated using an RNAprep Tissue Kit followed by cDNA synthesis using 0.05–5 μg of total RNA. Total RNA was mixed with random primers and RNase-free d H2O, heated to 65°C for 5 min, placed on ice for 5 min, followed by the addition of 2x TS Reaction Mix and RI Enzyme Mix(TransGen Biotech). The mixture was then incubated at 42°C for 30 min and then heated to 85°C for 5 min.The cDNA was stored at -20°C prior to further analysis. Expression analysis ofSmLTL was conducted using qPCR) with the L-YGDL-S-117 /L-YGDL-AS-117 primers using a SYBR Premix Ex Taq Kit (TaKaRa, Dalian, China) according to the manufacturer’s instructions. β-actin was amplified with specific F and R primers (Table 1) for use as a reference gene. The PCR consisted of denaturation at 94°C for 2 min, 35 cycles of amplification (94°C for 30 s, 63°C for 30 s, 72°C for 30 s), and a final extension at 72°C for 2 min. The reactions were performed using an ABI 7500 Real-time Detection System (Applied Biosystems, Foster City, CA, USA).All samples were analyzed in triplicate.
2.5 Bacterial challenge
V.anguillarumwas conserved in our laboratory in Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences.V.anguillarumchallenge was carried out as previously reported (Ma et al., 2014).V.anguillarumwere inoculated on the sterilized TSB, 28°C incubated for about 24 h and were harvested in the logarithmic phase of growth,which was monitored by the optical density assay.V.anguillarumcells were washed, resuspended, and diluted to 109CFU/mL in sterile PBS. Fish were challenged by intraperitoneal injection with nine concentrations liveV. anguillarum(dose=experimental fish body weight (g)×1 μL/g bacteria) per fish, and PBS alone was used as a control. Each group had five fish in each of three replicates (i.e. five per tank and 15 in total). At 0, 2, 4, 6, 8, 16, 24 and 24 h post infection (hpi), the gill, intestine and skin were collected and preserved at -80°C until subsequent use.The method of tissue-specific expression ofV.anguillarumchallenge reference 2.4.
2.6 Statistical analysis
The data were expressed as mean±SD and subjected to ANOVA (two-way analysis of variance) to determine differences among treatments. The differences were considered as significant atP<0.05.All the Statistical analysis was performed using SPSS V 19.0 for windows.
3 RESULT
3.1 Cloning and sequencing of turbot Sm LTL cDNA
The full-lengthSmLTL cDNA was 569 bp long,included 506 bp coding sequence (CDS) (GenBank accession No. KU199003) and contained an ORF of 339 bp that encoded 112 amino acids (Fig.1). The predictedSmLTL peptide has a theoretical molecular mass of 12.652 3 kDa, an isoelectric point (pI) of 7.86, fat factor of 71.34, and an average hydrophilicity of -0.517. (Fig.2).
3.2 Structure prediction and analysis of Sm LTL protein
3.2.1 Secondary Structure

Fig.2 Hydrophilicity analysis of Sm LTL using the method of Kyte and Doolittle (1982)

Fig.3 Multiple sequence alignment of Sm LTL with other homologous genes: lily-type lectin from Channa striata, Epinephelus coioides, Esox lucius, Larimichthys crocea, Leiognathus nuchalis, Lophiomus setigerus,Oncorhynchus mykiss, Oplegnathus fasciatus, and Platycephalus indicus

Fig.4 The three-dimensional structure of Sm LTL
The secondary structure analysis ofSmLTL revealed that the protein contains 5.36% alpha helices, 39.29%extended strands, 16.07% beta turns, and 39.29%random coils. The SignalP analysis of theSmLTL amino acid sequence did not reveal the presence of a signal peptide or transmembrane region. TheSmLTL protein contains a bulb-type mannose binding lectin(β-lectin) domain between amino acids 3 and 112 (a total of 109 amino acids). Within the β-lectin domain,two mannose binding sites were found between amino acids 30 and 99 with the specific motif of QxDxNxVxY in a three-fold reversed internal repeat (β-prism architecture) (Fig.1). The first repeat was located at Gln30-Asp32-Asn34-Val36-Tyr38 and the second repeat was located at Gln59-Asp61-Asn63-Val65-Tyr67. The third repeat (TxTxGxRxV) was located at Thr91-Thr93-Gly95-Arg97-Val99. differences in the amino acid sequence between species were observed within the third repeat region (Fig.3).
The sequence identity ofSmLTL was compared with other lectin superfamily members, including lily-type lectin, skin mucus lectin, and mannose binding lectin, from different fishes. TheSmLTL was found to have highest identity with the lily-type lectin fromC.striata(60%),Lophiomussetigerus(53%),Platycephalusindicus(52%), andLarimichthys crocea(50%). The length of theSmLTL sequence was similar to other species, and conserved motifs were observed among the sequences used in the analysis,thus confirming the identity of the gene asSmLTL(Fig.3).
3.2.2 Tertiary structure
Protein tertiary structure was predicted and inspected by PDBsum Generate (Fig.4). The overall folding ofSmLTL consisted of three anti-folding β-sheets, comprised of 10 β-strands, 14 β-turns, and 5 β-hairpins (Figs.5, 6), and contained four protein binding sites (Fig.7). The mannose-binding sites were located in the clefts formed by the three bundles of β-sheets.

Fig.5 Secondary structure of Sm LTL contained 10 β-strands, 14 β-turns, and 5 β-hairpins

Fig.6 Secondary topology of Sm LTL
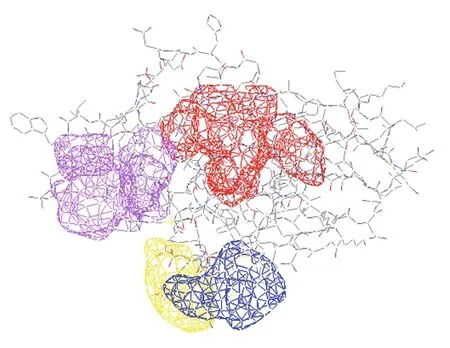
Fig.7 Schematic of the four predicted carbohydrate bindingsites of Sm LTL
3.3 Tissue distribution of Sm LTL mRNA
Transcripts forSmLTL were abundant (P<0.01) in the skin, intestine, and gill. Lower levels ofSmLTL transcripts were observed in the liver, head-kidney,spleen, and muscle (Fig.9).
3.4 Tissue-specific expression of V. anguillarum challenge
Choosing the gill, intestine, and skin, which was abundant (P<0.01) in tissue distribution ofSmLTL mRNA to challengeV.anguillarum. As shown in Fig.10 the mRNA expression ofSmLTL were upregulated afterV.anguillarumchallenge, and reached the highest level at 6 h.
4 DISCUSSION
In this study, a novel lectin was isolated from turbot(S.maximus) using RACE techniques and was classified asS.maximuslily-type lectin (SmLTL) based on its structural and functional characteristics. Lilytype lectin is able to bind to specific carbohydrates and plays key roles in non-self-recognition and clearance of pathogen (Dodd and Drickamer, 2001; Vasta et al.,2004). The study ofSmLTL could be valuable for further identification of mucus lecin in fish.
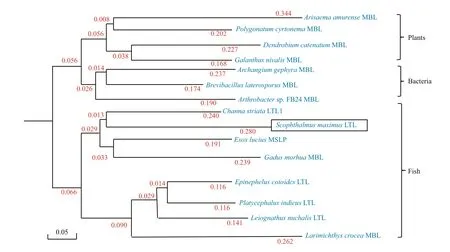
Fig.8 Phylogenetic tree of lily-type lectins constructed using the Neighbor-Joining method
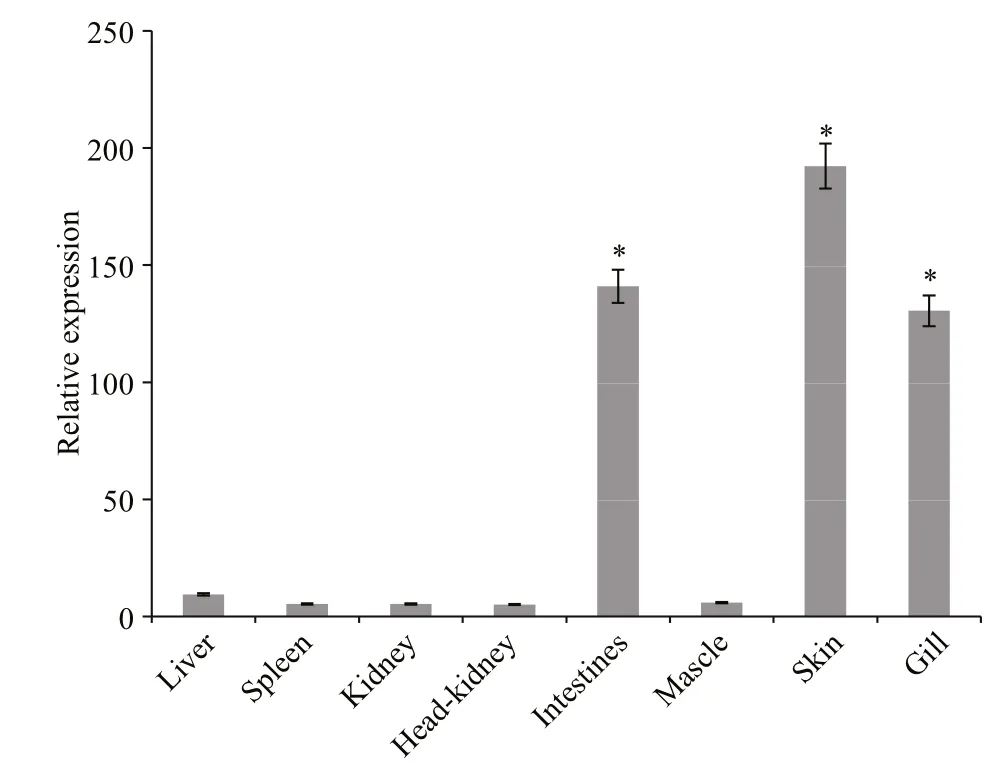
Fig.9 Tissue distribution of Sm LTL mRNA detected by quantitative real time reverse transcriptase PCR
The full-lengthSmLTL cDNA that was obtained in this study has a length of 569 bp and contains a 336-bp ORF that encodes a 112-residue protein that lacks a signal peptide. Sequence alignment and phylogenetic analysis (Figs.3, 8) revealed thatSmLTL had high similarity with lily-type lectin-1 fromC.striata(60%),L.setigerus(53%),P.indicus(52%),Esox lucius(52%),L.crocea(50%),Oncorhynchusmykiss(42%), and other known lectin sequences from bacteria and plants. These data, together with the structural features ofSmLTL, indicate that it belongs to the lily-type lectin family. The tertiary structure model of theSmLTL protein also indicated a high similarity with a variety of β-prism lectins from other species, includingGalanthusnivalis(30%),Narcissus pseudonarcissus(27%),Galanthusnivalis(26%),and other monocot plant lectin proteins. The similarities betweenSmLTL and monocot lectins suggest new areas of research regarding the structure and function ofSmLTL.
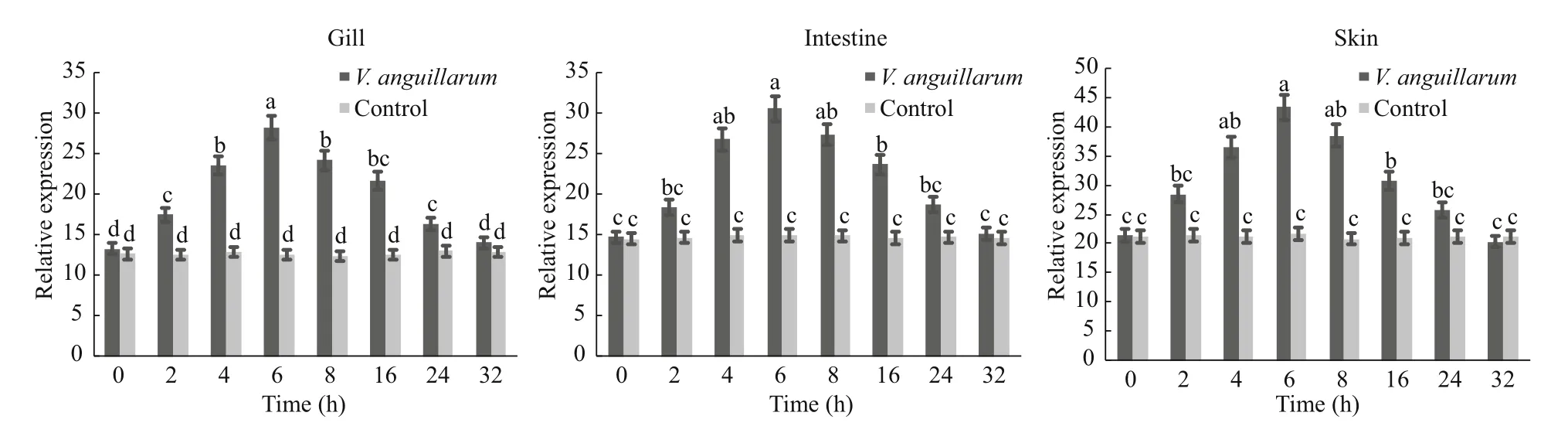
Fig.10 Sm LTL gene expression in turbot gill, intestine and skin at 0–32 h after V. anguillarum challenge
The alignment ofSmLTL with other lily-type lectins revealed that the mannose-binding motifs of some lectins differ slightly from the standard form(QDNVY); however, these amino acid differences are expected to affect their ability to bind mannose(Afolabi-Balogun et al., 2012; Arasu et al., 2013). In particular, repeat three was found to exhibit slight changes in fish based on current reports. For example,repeat three was reported as TxNxDxQxV inC.striata(Arasu et al., 2013, Genbank: CCQ25776),TxTxDxHxV inE.lucius(Leong et al., 2010,GenBank: ACO14169), and YxRxDxNxV inO.mykiss(Berthelot et al., 2014, GenBank:CDQ78238). However, the other two repeats (one and two) exist in all LTL. These results suggest that these lectin protein motifs are evolutionarily conserved and play an important role in their biological function (Kai et al., 2004). The modification of Arg, Lys, and Ser residues did not modify binding activity, whereas the loss of function following changes in Trp (W) or Asp/Glu (D/E) and Tyr (Y) residues indicated their crucial role in the binding activity ofColocasiaesculentalectin (Pereira, 2014). The reason for the presence of amino acid residues that a lack of interaction of lectin with mannose may be due to the substitution, deletion,or insertion of key amino acid residues during evolution (Luo et al., 2007). It was speculated that repeat three (TxTxGxRxV) may lead to changes in binding activity based on species-specific and the standard form (QDNVY) determined them combined with mannose. Further studies are required to investigate the binding of carbohydrates bySmLTL.
Three mannose recognition sites were identified inSmLTL, in addition to four identical subunits and three anti-folding β-sheets, which were comprised of 10 β-strands, 14 β-turns, and 5 β-hairpins. The secondary structure analysis ofSmLTL revealed that the protein contains 5.36% alpha helices, 39.29%extended strands, 16.07% beta turns, and 39.29%random coils. The whole folding ofSmLTL, which typically consists of β-sheets connected by turns and loops, creates a very tight structural scaff old. This is very similar to the 3D structure of other mannosebinding lectins (Barre et al., 2001; Zhao et al., 2003).The monocot β-prism lectin structure also contains three mannose recognition sites and is a homotetrameric protein that is folded in a classic pattern (beta-prism II fold) to form its advanced structure (Hester and Wright, 1996). Molecular characterization ofSmLTL, such as mannose-binding site analysis, signal cleavage site prediction, and analyses of secondary and 3D structures, indicated that it shares many Exemplary features with monocot mannose-binding lectins. These similarities signify thatSmLTL might have similar functions as many other mannose-binding lectins, such as binding to parasites, viruses, and fungi. For example, lectins have been observed to bindMeloidogyneincognita(Bhat et al., 2010), HIV (Ding et al., 2008), HSV-II(Luo et al., 2007), andRhizoctoniasolani(Tian et al.,2008). The cloning ofSmLTL performed in the current study will enable further research into its potential functions in disease resistance.
The abundance ofSmLTL transcripts was highest in the skin, intestine, and gill, but was weak in the liver, head-kidney, spleen, and muscle. The gills in fish are involved in gas exchange and are in continuous contact with the aquatic environment and are,therefore, more susceptible to pathogen infection.The expression ofCsLTL-1 mRNA was significantly higher in the gills, liver, intestine, and skin ofC.striata(Abirami Arasu, 2013). Similarly, pufflectin mRNA was also widely expressed in the gills,followed by the oral cavity wall, esophagus, and skin ofTakifugurubripes(Suzuki, 2003). Park et al. (2016)also reported thatRbLTL transcript was abundant in gill and intestinal tissue in rock bream (Oplegnathus fasciatus). In addition, fish can absorb environmental antigens into the body via the skin (Moore et al.,1998). The lactose-binding lectin in Japanese eel(AJL-2) was demonstrated to be produced only in the skin (Tasumi et al., 2002). According to Suzuki(2003), the intestinal isoform of pufflectin was identified in intestine ofT.rubripes. In teleosts, the gut, skin and gill are the main mucosal surfaces and immune barriers (Goel et al., 2015). The high abundance of SmLTL transcripts in the skin, intestine,and gills may reflect the role of this protein in the immune response.
AsV.anguillarumis one of the important pathogens responsible for major mortalities in turbot fish, the ability ofSmLTL to inhibit it was of significant importance. Here,SmLTL showed the direct activity of facilitating the clearance ofV.anguillarumin vivo in turbot. The mRNA expression of gill, skin and intestine inSmLTL were up-regulated afterV.anguillarumchallenge, and reached the highest level at 6 h. Recently many similar results have showed, such as, a novel C-type lectin (FcLec4) in Chinese white shrimp (Wang et al., 2009), a C-type lectin (AiCTL-3) in bay scallop (Huang et al., 2013),pathogen recognition receptors TLR2 in turbot (Liu et al., 2016). Obviously, mucosal immune stress response was produced afterV.anguillaruminjection in turbot, and stimulate the secretion ofSmLTL. Our results suggest that lily-type lectins serve as the first line of defense against microbial infections and play a pivotal role in the innate mucosal immune system. We intend to further investigate the functions ofSmLTL in the mucosal immune system through comparative pathogens studies.
5 CONCLUSION
TheSmLTL from turbot was identified and characterized in this study. The general characteristics of have been reported here, including protein and cDNA sequences, tissue expression profile, domain architectures andV.anguillarumstimulate. The most important result of the present study is thatSmLTL not only shares similarity with monocotyledonous plant lectins, but also contains identical mannosebinding sites. However, the function of binding site three ofSmLTL requires further study. The information reported here will be useful for the investigation into the multifaceted functions ofSmLTL.
Afolabi-Balogun N B, Inuwa H M, Ishiyaku M F,Bakareodunoola M T, Nok A J. 2012. Isolation and characterization of a mannose-binding insecticidal lectin gene fromAlliumsativum(garlic) and its putative role in insect resistance using bioinformatics tools.Infect.Genet.Evol.,12(7): 1 508-1 512.
Alexander J B, Ingram G A. 1992. Noncellular nonspecific defense mechanism of fish.AnnualReviewofFish Diseases,2: 249-279.
Arasu A, Kumaresan V, Sathyamoorthi A, Palanisamy R,Prabha N, Bhatt P, Roy A, Thirumalai M K, Gnaname A J,Pasupuleti M, Marimuthu K, Arockiaraj J. 2013. Fish lily type lectin-1 contains β-prism architecture: immunological characterization.Mol.Immunol.,56(4): 497-506.
Barre A, Bourne Y, Van Damme E J M, Peumans W J, Rougé P. 2001. Mannose-binding plant lectins: different structural scaff olds for a common sugar-recognition process.Biochimie,83(7): 645-651.
Berthelot C, Brunet F, Chalopin D, Juanchich A, Bernard M,Noël B, Bento P, Da Silva C, Labadie K, Alberti A, Aury J M, Louis A, Dehais P, Bardou P, Montfort J, Klopp C,Cabau C, Gaspin C, Thorgaard G H, Boussaha M, Quillet E, Guyomard R, Galiana D, Bobe J, Volff J N, Genêt C,Wincker P, Jaillon O, Crollius H R, Guiguen Y. 2014. The rainbow trout genome provides novel insights into evolution after whole-genome duplication in vertebrates.Nat.Commun.,5: 3 657.
Bhat G G, Shetty K N, Nagre N N, Neekhra V V, Lingaraju S,Bhat R S, Inamdar S R, Suguna K, Swamy B M. 2010.Purification, characterization and molecular cloning of a monocot mannose-binding lectin fromRemusatia viviparawith nematicidal activity.Glycoconj.J.,27(3):309-320.
Chandra N R, Ramachandraiah G, Bachhawat K, Dam T K,Surolia A, Vijayan M. 1999. Crystal structure of a dimeric mannose-specific agglutinin from garlic: quaternary association and carbohydrate specificity.J.Mol.Biol.,285(3): 1 157-1 168.
Chen S R., Tang J X, Cheng J M, Li J, Jin C, Li X Y, Deng S L,Zhang Y, Wang X X, Liu Y X. 2015. Loss of gata4 in sertoli cells impairs the spermatogonial stem cell niche and causes germ cell exhaustion by attenuating chemokine signaling.Oncotarget,6(35): 37 012-37 027.
Chen Y D, Zhou S H, Jiang Z Q, Wang X L, Liu Y. 2016.Chemokine receptor CXCR3 in turbot (Scophthalmus maximus): cloning, characterization and its responses to lipopolysaccharide.FishPhysiol.Biochem.,42(2): 659-671.
Chi H, Hu Y H. 2016. Stomatin-like protein 2 of turbotScopthalmusmaximus: gene cloning, expression profiling and immunoregulatory properties.Fish&Shellfish Immun.,49: 436.
Ding J J, Bao J K, Zhu D Y, Zhang Y, Wang D C. 2008.Crystallization and preliminary x-ray diffraction analysis of a novel mannose-binding lectin with antiretroviral properties fromPolygonatumcyrtonemahua.Protein Pept.Lett.,15(4): 411-414.
Dodd R B, Drickamer K. 2001. Lectin-like proteins in model organisms: implications for evolution of carbohydratebinding activity.Glycobiology,11(5): 71R-79R.
Gao C B, Fu Q, Zhou S, Song L, RenY C, Dong X Y, Su B F,Li C. 2016. The mucosal expression signatures of g-type lysozyme in turbot (Scophthalmusmaximus) following bacterial challenge.FishShellfishImmunol.,54: 612-619.
Goel C, Barat A, Pande V, Sahoo P K. 2015. Molecular cloning and characterization of mannose binding lectin homologue from snow trout (Schizothoraxrichardsonii).Theprotein J.,34(1): 1-8.
Goto-Nance R, Watanabe Y, Kamiya H, Ida H. 1995.Characterization of lectins feom the skin mucus of the LonachMisgurnusanguillicaudatus.Fish.Sci.,61(1):137-140.
Guardiola F A, Cuesta A, Arizcun M, Meseguer J, Esteban M A. 2014. Comparative skin mucus and serum humoral defence mechanisms in the teleost gilthead seabream(Sparusaurata).FishShellfishImmunol.,36(2): 545-551.
Hester G, Wright C S. 1996. The mannose-specific bulb lectin fromGalanthusnivalis(snowdrop) binds mono- and dimannosides at distinct sites. Structure analysis of refined complexes at 2.3 Å and 3.0 Å resolution.J.Mol.Biol.,262(4): 516-531.
Hoff mann J A, Kafatos F C, Janeway C A, Ezekowitz R A B.1999. Phylogenetic perspectives in innate immunity.Science,284(5418): 1 313-1 318.
Huang M M, Song X Y, Zhao J M, Mu C K, Wang L L, Zhang H, Zhou Z, Liu X L, Song L S. 2013. A C-type lectin(AiCTL-3) from bay scallopArgopectenirradianswith mannose/galactose binding ability to bind various bacteria.Gene,531(1): 31-38.
Huang Z H, Ma A J, Wang X A. 2011. The immune response of turbot,Scophthalmusmaximus(L.), skin to high water temperature.J.FishDis.,34(8): 619-627.
Ingram G A. 1980. Substances involved in the natural resistance of fish to infection—a review.J.FishBiol.,16(1): 23-60.
Kai G Y, Zhao L X, Zheng J G, Zhang L, Miao Z Q, Sun X F,Tang K X. 2004. Isolation and characterization of a new mannose-binding lectin gene fromTaxusmedia.J.Biosci.,29(4): 399-407.
Kamiya H, Muramoto K, Goto R. 1988. Purification and properties of agglutinins from conger eel,Conger myriaster(Brevoort), skin mucus.Dev.Comp.Immunol.,12(2): 309-318.
Ke J Y, Chen Y S, Rao X Z. 2005. Lectins and its biological function.J.NingdeTeach.Coll.Nat.Sci.,17(1): 19-22.(in Chinese with English abstract)
Khong H K, Kuah M K, Jaya-Ram A, Shu-Chien A C. 2009.Prolactin receptor mRNA is upregulated in discus fish(Symphysodonaequifasciata)skin during parental phase.Comp.Biochem.Physiol.B:Biochem.Mol.Biol.,153:18-28.
Kim B S, Nam B H, Kim J W, Park H J, Song J H, Park C I.2011. Molecular characterisation and expression analysis of a fish-egg lectin in rock bream, and its response to bacterial or viral infection.Fish&ShellfishImmun.,31(31): 1 201-1 207.
Kyte J, Doolittle R F. 1982. A simple method for displaying the hydropathic character of a protein.J.Mol.Biol.,157(1):105-132.
Leong J S, Jantzen S G, von Schalburg K R, Cooper G A, Messmer A M, Liao N Y, Munro s, Moore R, Holt R A,Jones S J M, Davidson W S, Koop B F. 2010.SalmosalarandEsoxluciusfull-length cDNA sequences reveal changes in evolutionary pressures on a posttetraploidization genome.BMCGenomics,11(1): 279.
Lin J Y, Hu G B, Yu C H, Li S, Liu Q M, Zhang S C. 2015.Molecular cloning and expression studies of the adapter molecule myeloid differentiation factor 88 (MyD88) in turbot (Scophthalmusmaximus).Dev.Comp.Immunol.,52(2): 166-171.
Liu F Q, Su B F, Gao C B, Zhou S, Song L, Tan F H, Dong X Y, Ren Y C, Li C. 2016. Identification and expression analysis of TLR2 in mucosal tissues of turbot(ScophthalmusmaximusL.) following bacterial challenge.FishShellfishImmunol.,55: 654-661.
Luo Y, Xu X, Liu J, Li J, Sun Y, Liu Z, Liu J Z, Van Damme E,Balzarini J, Bao J. 2007. A novel mannose-binding tuber lectin fromTyphonium divaricatum(L.) Decne (family Araceae) with antiviral activity against HSV-II and antiproliferative effect on human cancer cell lines. BMB Rep.,40(3): 358-367.
Ma A J, Guo J L, Wang X A, Huang Z H. Wang T, Shang X M.2014. Family selection and estimation of disease resistance in turbot,Scophthalmusmaximus.J.Fish.Sci.China,21(3): 484-493. (in Chinese with English abstract)
Ma A J, Huang Z H, Wang X A. 2013. Changes in protein composition of epidermal mucus in turbotScophthalmus maximus(L.) under high water temperature.FishPhysiol.Biochem.,39(6): 1 411-1 418.
Meng Y Q, Liu X F, Liu Y, Chang X Q, Wang X L, Jiang Z Q.2013. Chemokine receptor genesCCR3andCCR9in turbot (Scophthalmusmaximus): cloning and tissue distribution.J.Fish.Sci.China,20(5): 918-930. (in Chinese with English abstract)
Moore J D, Ototake M, Nakanishi T. 1998. Particulate antigen uptake during immersion immunisation of fish: the eff ectiveness of prolonged exposure and the roles of skin and gill.FishShellfishImmunol.,8(6): 393-408.
Park H J, Jeong J M, Bae J S, Kim J W, An C M, Min B H, Kim S Y, Myeong J I, Hwang H K, Park C I. 2016. Molecular cloning and expression analysis of a new lily-type lectin in the rock bream,Oplegnathusfasciatus.Dev.Comp.Immunol.,65: 25-30.
Pereira P R, Winter H C, Verícimo M A, Meagher J L, Stuckey J A., Goldstein I J, Paschoalin V M F, Silva J T. 2014.Structural analysis and binding properties of isoforms of tarin, the gna-related lectin fromColocasiaesculenta.BBA-Proteinsproteome.,1854(1): 20-30.
Sharon N, Lis H. 2004. History of lectins: from hemagglutinins to biological recognition molecules.Glycobiology,14(11): 53R-62R.
Shephard K L. 1994. Functions for fish mucus.Rev.FishBiol.Fish.,4(4): 401-429.
Shiomi K, Uematsu H, Ito H, Yamanaka H, Kikuchi T. 1990.Purification and properties of a lectin in the skin mucus of the dragonetRepomucenusrichardsonii.NipponSuisan Gakk.,56(1): 119-123.
Shiomi K, Uematsu H, Yamanaka H, Kikuchi T. 1989.Purificatioin and characterization of a galactose-binding lectin from the skin mucus of the conger eelConger myriaster.Comp.Biochem.Physiol.BComp.Biochem.,92(2): 255-261.
Smeets K, Van Damme E J M, Verhaert P, Barre A, Rougé P,Van Leuven F, Peumans W J. 1997. Isolation,characterization and molecular cloning of the mannose-binding lectins from leaves and roots of garlic (Allium sativumL.).PlantMol.Biol.,33(2): 223-234.
Spitzer R H, Koch E A. 1998. Hagfish skin and slime glands.In: Jørgensen J M, Lomholt J P, Weber R E, Malte H eds.The Biology of Hagfishes. Springer, Netherlands. p.109-132.
Suzuki Y, Tasumi S, Tsutsui S, Okamoto M, Suetake H. 2003.Molecular diversity of skin mucus lectins in fish.Comp.Biochem.Physiol.BBiochem.Mol.Biol.,136(4): 723-730.
Tasumi S, Ohira T, Kawazoe I, Suetake H, Suzuki Y, Aida K.2002. Primary structure and characteristics of a lectin from skin mucus of the Japanese eelAnguillajaponica.J.Biol.Chem.,277(30): 27 305-27 311.
Tian Q, Wang W, Miao C, Peng H, Liu B, Leng F, Dai L, Chen F, Bao J. 2008. Purification, characterization and molecular cloning of a novel mannose-binding lectin from rhizomes ofOphiopogonjaponicus, with antiviral and antifungal activities.PlantSci.,175(6): 877-884.
Toda M, Goto-Nance R, Muramoto K, Kamiya H. 1996.Characterization of the lectin from the skin mucus of the KingklipGenypteruscapensis.Fish.Sci.,62(1): 138-141.
Toranzo A E, Santos Y, Barja J L. 1997. Immunization with bacterial antigens: vibrio infections.Dev.Biol.Stand.,90:93-105.
Tsutsui S, Tasumi S, Suetake H, Suzuki Y. 2003. Lectins homologous to those of monocotyledonous plants in the skin mucus and intestine of puff erfish,Fugurubripes.J.Biol.Chem.,278(23): 20 882-20 889.
Tsutsui S, Tasumi S, Suetake H, Kikuchi K, Suzuki Y. 2006.Carbohydrate-binding site of a novel mannose-specific lectin from fugu (Takifugurubripes) skin mucus.Comp.Biochem.Physiol.BBiochem.Mol.Biol.,143(4): 514-519.
van Damme E J M, De Clercq N, Claessens F, Hemschoote K,Peeters B, Peumans W J. 1991. Molecular cloning and characterization of multiple isoforms of the snowdrop(GalanthusnivalisL.) lectin.Planta,186(1): 35-43.
van Damme E J M, Smeets K, Engelborghs I, Aelbers H,Balzarini J, Pusztai A, van Leuven F, Goldstein I J,Peumans W J. 1993. Cloning and characterization of the lectin cDNA clones from onion, shallot and leek.Plant Mol.Biol.,23(2): 365-376.
van Damme E J M, Smeets K, Van Leuven F, Peumans W J.1994. Molecular cloning of mannose-binding lectins fromCliviaminiata.PlantMol.Boil.,24(5): 825-830.
van der Marel M, Caspari N, Neuhaus H, Meyer W, Enss M L,Steinhagen D. 2010. Changes in skin mucus of common carp,CyprinuscarpioL., after exposure to water with a high bacterial load.J.FishDis.,33(5): 431-439.
Vasta G R, Ahmed H, Odom E W. 2004. Structural and functional diversity of lectin repertoires in invertebrates,protochordates and ectothermic vertebrates.Curr.Opin.Struct.Biol.,14(5): 617-630.
Wang X W, Zhang X W, Xu W T, Zhao X F, Wang J X. 2009.A novel C-type lectin (FcLec4) facilitates the clearance ofVibrioanguillaruminvivoin Chinese white shrimp.Dev.Comp.Immunol.,33(9): 1 039-1 047.
Zhao X Y, Yao J H, Liao Z H, Zhang H Y, Chen F, Wang L, Lu Y Q, Sun X F, Yu S Q, Tang K X. 2003. Molecular cloning of a novel mannose-binding lectin gene fromArisaema heterophyllum.PlantSci.,165(1): 55-60.
猜你喜欢
杂志排行
Journal of Oceanology and Limnology的其它文章
- An aftereffect of global warming on tropical Pacific decadal variability*
- Analysis of monthly variability of thermocline in the South China Sea*
- A numerical study of the South China Sea Warm Current during winter monsoon relaxation*
- Predicting the sinkage of a moving tracked mining vehicle using a new rheological formulation for soft deep-sea sediment*
- Chemical characterization of fractions of dissolved humic substances from a marginal sea—a case from the Southern Yellow Sea*
- The morphological and molecular detection for the presence of toxic Cylindrospermopsis (Nostocales, Cyanobacteria) in Beijing city, China*
