植物硝酸盐转运蛋白功能及表达调控研究进展
2017-11-27宋田丽周建建徐晨曦蔡晓锋戴绍军王全华王小丽
宋田丽, 周建建, 徐晨曦, 蔡晓锋, 戴绍军, 王全华, 王小丽
(上海师范大学 生命与环境科学学院 植物种质资源开发协同创新中心,上海 200234)
植物硝酸盐转运蛋白功能及表达调控研究进展
宋田丽, 周建建, 徐晨曦, 蔡晓锋, 戴绍军, 王全华, 王小丽*
(上海师范大学 生命与环境科学学院 植物种质资源开发协同创新中心,上海 200234)
植物硝酸盐转运蛋白不仅能够吸收、运转硝态氮,而且在植物其他生理过程中也发挥重要作用.重点介绍了硝酸盐转运蛋白在氮素吸收运转、硝酸盐积累、侧根发育、激素运转以及逆境响应调控等方面的最新研究进展,并概括了硝酸盐转运蛋白的表达调控模式.
硝酸盐转运蛋白; 硝酸盐积累; 根系发育; 激素转运; 逆境响应
0 引 言
硝态氮(NO3--N)是植物最重要的氮素营养之一,与植物的生长发育、形态建成、产量品质密切相关.因此研究硝态氮的吸收运转对于增强作物的氮素吸收和转运能力、提高氮素利用效率有重要指导意义.植物根系以主动运输方式吸收土壤或环境介质中的硝态氮,并运输到其他组织器官中.为适应环境硝态氮供应浓度的时空变化,植物进化出两种硝态氮吸收系统,低亲和转运系统(LATS)和高亲和转运系统(HATS),分别在外源NO3-物质的量浓度为0.001~1 mmol·L-1和高于1 mmol·L-1时,由硝酸盐转运蛋白(NRT)的NRT1/ PTR和NRT2两大转运蛋白家族负责执行[1].根据最新命名规则,NRT1/PTR家族用NPF(NRT1/PTR family)表示[2].在拟南芥中预测到53个NPF和7个NRT2成员,在其他植物中也陆续预测出大量的NRT家族成员(表1).随着拟南芥NRT研究的深入,研究者发现NRT除具有硝态氮转运功能外,还在植物信号感受、形态建成、发育调控及与环境相互作用等过程中发挥不可忽视的作用.因此,有必要对NRT最新研究进展进行综述,以充分认识NRT的功能特征,解析复杂氮代谢相关生物现象,并为植物基因改良提供基础.

表1 不同物种NRT成员数目比较
注:表中数据来源于已发表文章、基因组数据库及部分综述[8]
1 蛋白结构
不同物种NRT序列高度同源,具有相似的保守序列.拟南芥NPF属于major facilitator super(MFS)家族的小肽转运体(PTR)家族,一般含450~600个氨基酸,12个跨膜区,在第6、7跨膜区之间,有一个大的亲水环相连[3],在第5跨膜区有一个PTR家族的保守基序(F-Y-x-x-I-N-x--S-L)[9].NRT2属于MFS家族的nitrate /nitrite porter(NNP)家族,其典型特征为具有12个跨膜结构域,每6个跨膜区分成一组,中间由一个大的亲水环连接[10],在第2和第3个跨膜螺旋结构之间有一个广泛的MFS序列(G-x-xx-D-x-x-G-x-R)[9,11],在第5个跨膜螺旋结构上有一个NNP标志序列(G-W/L-G-N-M/A-G)[12].
2 生理功能
2.1氮素吸收运转
拟南芥中几个NRT成员功能较清楚.AtNPF6.3(AtNRT1.1)是一个双亲和性的诱导型硝酸盐转运蛋白,能感知外源NO3-的浓度而进行高低亲和性的转换,通过该蛋白质序列N端的第101位的苏氨酸磷酸化开关控制硝酸盐吸收[13].AtNPF4.6(AtNRT1.2)在根的表皮细胞中呈组成型表达,AtNRT1.2突变拟南芥株系的LATS下降50%~70%[14].AtNRT1.3在拟南芥叶中的表达受NO3-诱导,但在根中的表达受NO3-抑制[15].AtNPF6.2(AtNRT1.4)主要在叶柄细胞的液泡膜及叶脉表达,其功能是调控NO3-在叶片的分配,将NO3-储存到液泡中,在调节叶柄和叶片的NO3-稳态平衡中发挥重要作用[16].AtNPF7.3(AtNRT1.5)在拟南芥根中柱鞘细胞膜表达,其功能是将NO3-装载到木质部,在蒸腾拉力的作用下通过长距离运输将NO3-运送到地上部分[17].相反,定位在木质部薄壁细胞的AtNPF7.2(AtNRT1.8)和定位于韧皮部伴胞的AtNPF2.9(NRT1.9)的功能是将NO3-从木质部卸载,三者共同参与调节NO3-在根系和地上部分的分配[18-20].AtNPF2.12(AtNRT1.6)仅表达于角果的维管束和胚珠的珠柄处,主要通过调控NO3-从母体向胚的运输,进而影响胚的早期发育[21].AtNPF2.13(NRT1.7)主要在叶片韧皮部的薄壁细胞表达,其表达丰度受氮饥饿的诱导[22],负责将NO3-向韧皮部运输,从而调控衰老叶片中NO3-的再利用[23].AtNRT2.1、AtNRT2.2和AtNRT2.4定位于根表皮细胞,需与AtNAR2.1结合才能激活高亲和转运活性[24].AtNRT2.1是诱导型HATS中的主要转运蛋白,其突变体中的HATS活性下降了50%~72%.Atnrt2.2突变体HATS活性仅下降19%,说明Atnrt2.2是对AtNRT2.1高亲和转运活性功能的补充[25].AtNRT2.4和AtNRT2.5是定位于质膜的高亲和性转运蛋白,主要在侧根的表皮和叶韧皮部薄壁细胞表达,参与根系NO3-高亲和性转运和韧皮部NO3-的分配,并均受氮饥饿的强烈诱导[23,26];AtNRT2.5突变后,拟南芥高亲和性NO3-吸收显著受抑制,敲除AtNRT2.1、AtNRT2.2、AtNRT2.4和AtNRT2.5中任意3个基因均会显著抑制植株的生长[26].
除拟南芥外,其他植物中部分NRT的NO3-转运功能也得到验证.水稻(Oryzasativa)OsNRT1.1a和OsNRT1.1b为低亲和硝酸盐转运蛋白,主要在根中表达,缺氮下表达受抑制[27].OsNPF2.4为pH依赖的低亲和硝酸盐转运蛋白,主要定位于表皮细胞、木质部薄壁组织、韧皮部伴胞,能间接调控钾在地上地下的再分配[28].OsNPF2.2为pH依赖的受NO3-诱导的低亲和硝酸盐转运蛋白,主要表达于木质部薄壁组织,其功能是将硝酸盐从木质部卸载,参与调节NO3-在根系和地上部分的分配,并在水稻的生长发育中起重要作用[29].OsNRT2.3a是高亲和转运蛋白,定位于质膜,主要在木质部薄壁组织中表达,受NO3-诱导,在NO3-由根向地上部分转运的长距离运输中发挥重要作用[30].白菜(Brassicarapa)BraNRT2.1为诱导型高亲和硝酸盐转运蛋白,主要表达于根细胞质膜,能互补拟南芥atnrt2.1突变体的高亲和硝酸盐转运功能[31].油菜(Brassicacampestris)BcNRT1在拟南芥的根尖和地上部分表达,原生质体亚细胞定位于质膜,根部表达受25 mmol·L-1NO3-诱导,BcNRT1 mRNA注入爪蟾卵母细胞能诱导NO3-转运,且BcNRT1能恢复拟南芥chl1-5突变体植株的硝酸盐吸收功能,这些结果证明BcNRT1是一个低亲和的硝酸盐转运蛋白[32].Gu等[33]对菊花(Chrysanthemum)CmNRT1和CmNRT2进行了功能验证,发现CmNRT1能被10 mmol·L-1NO3-诱导,转化拟南芥发现其编码一个组成型的低亲和转运蛋白;CmNRT2表达水平受NO3-诱导,定位于质膜,与CmNAR2有相互作用,共同负责NO3-的高亲和吸收[34].通过爪蟾卵母细胞表达系统及膜片钳技术发现葡萄(Vitisvinifera)VvNPF3.2是一个低亲和转运蛋白,能转运NO3-和NO2-[35].蒺藜状苜蓿(Medicagotruncatula)MtNPF6.8为诱导型低亲和转运蛋白,同时参与ABA由根向地上部分的转载[36].
2.2硝酸盐积累
硝酸盐是一类极易在蔬菜特别是叶菜类蔬菜中高量累积的物质,过量摄入硝酸盐对人体健康存在极大的潜在危害[37].研究发现一些NRT确实与植物硝酸盐积累性状密切相关,可应用于控制蔬菜硝酸盐积累.拟南芥nrt1.4突变体植株叶柄中的硝酸盐含量只有野生型拟南芥的50%~64%,而叶片中硝酸盐含量则稍高于野生型叶片[16].盐胁迫下拟南芥NPF2.3(一个参与根系溢泌的硝酸盐转运蛋白编码基因,又称NAXT1)缺失突变体中硝态氮由根系向地上部分转运减少,地上部分硝酸盐含量明显下降[38].黄瓜(Cucumissativus)中发现与AtNRT1.7同源的CsNRT1.7基因,将其在拟南芥nrt1.7-2的T-DNA插入突变体中表达,发现叶片面积增加的同时硝酸盐含量显著降低[39].通过比较硝酸盐积累差异的两个大白菜品种的BnNRT1.1和BnNRT2.1基因表达量发现,高(2 mmol·L-1)、低(0.2 mmol·L-1)硝酸盐处理下,高硝酸盐积累品种根、茎、叶中BnNRT2基因表达量均显著高于低硝酸盐品种,且其吸收速率也明显高于低硝酸盐品种,NRT1.1基因表达仅在根中有差异,推测NRT2可能是与品种间硝酸盐积累差异相关,而NRT1可能仅对根部硝酸盐含量起部分作用[24].Quaggiotti等发现硝酸盐积累差异的两个玉米(Zeamays)杂交种,其根和叶中ZmNrt2.1基因时间表达趋势与硝酸盐积累量变化趋势相符,可能与玉米硝酸盐积累存在一定的相关性[40].对NRT功能的研究有助于从分子水平解释蔬菜硝酸盐积累机理,可为通过生物技术手段降低植物硝酸盐积累提供理论和技术支撑.
2.3信号转导
目前比较明确的参与NO3-信号途径的NPF是AtNRT1.1,参与硝酸根信号途径在基因表达、根系发育、种子休眠以及逆境响应等过程的调控[41].例如,AtNRT1.1作为NO3-受体,通过CIPK23改变其第101位苏氨酸位点(T101)的磷酸化状态(低NO3-浓度下Thr101位点磷酸化,反之,去磷酸化),从而改变AtNRT1.1的低亲/高亲活性以及硝酸盐初级响应.NRT1.1感应的信号通过一些激酶(如CIPK8)和转录因子(NLP7、NLP6、SPL9和LBD37/38/39)传递给下游基因,影响下游基因(如NIR、NRT2.1和NRT2.2)的表达,调控硝态氮信号的初级响应过程[42].NRT1.1传递的信号还可以磷酯酶C(PLC)、Ca2+作为第二信使,调控转录因子(TGA1/4)、硝酸盐转运蛋白基因(NRT2.1、NRT2.2和NRT3.1)及硝态氮同化(NIA1和NiR)等基因的表达;还可以在不依赖PLC、Ca2+的条件下,将信号传递给生长素受体AFB3,并进一步调控转录因子NAC4和OBP4的转录活性,调控根系形态建成[43].
2.4侧根发育
硝态氮对根系发育起着重要的调控作用.低氮(0.5~10 mmol·L-1)促进拟南芥侧根生长,而高氮(>10 mmol·L-1)抑制侧根生长;增加拟南芥根系局部区域的硝酸根浓度,可以显著促进该区域侧根的生长[30,44].根系局部供应硝态氮条件下,低氮一侧由于NO3-供应不足以及NRT1.1将侧根生长素(IAA)转移,导致侧根生长素浓度下降,进而抑制了侧根发育;相反,高硝态氮浓度一侧NRT1.1对生长素输出功能受抑制,生长素大量积累,促进侧根生长[45-46].在此过程中,NRT1.1还作为NO3-信号受体参与NO3-信号对根系形态的调节,首先NRT1.1将感受到的局部高浓度硝酸根信号传导给ANR1,促进ANR1的表达,然后调控未知的下游基因,促进侧根的伸长[44,47].还有可能的途径是生长素经NRT1.1运输后,其浓度变化信号传递给生长素受体AFB3,进而调控一些对生长素敏感的基因(如ARF、NAC4和OBP4)表达,导致植物主根伸长受抑制并诱导侧根的生长[43,48].此外,NO3-被根系吸收还原后的含氮代谢产物,可以诱导miR393表达,而上调后的miR393抑制AFB3的表达[49],二者协同参与了植物根系发育的调控.除NRT1.1外,NRT2.1对根系形态建成也具有重要作用,NRT1.1传递的信号通过PLC、Ca2+传递给转录因子(TGA1/4),进而调控NRT2.1的表达[43].在极低NO3-浓度(0.01 mmol·L-1)条件下,NRT2.1抑制侧根起始[50],而在0.5 mmol·L-1NO3-浓度条件下NRT2.1促进侧根原基发育,并且通过调节NO3-的吸收量来决定侧根发育状况[51].
2.5激素转运
硝酸盐转运蛋白还参与IAA、脱落酸(ABA)、赤霉素(GAs)、茉莉酸异亮氨酸(JA-Ile)等多种激素的转运,并进而直接或间接参与这些激素参与的生理调控过程.NPF6.3(NRT1.1/CHL1)参与IAA的运输,并受高硝态氮浓度的抑制,在局部供应硝态氮根系的形态建成中起重要作用.根系局部供应硝态氮条件下,由于NPF6.3(NRT1.1/CHL1)将更多IAA从侧根运出,降低了侧根中生长素浓度,从而抑制侧根发育,相反,高硝态氮浓度一侧生长素输出受抑制,生长素大量积累,促进侧根生长[45].NRT1.1的生长素转运能力依赖于Thr101位点的磷酸化水平,在表达磷酸化形式NTT1.1-T101D的卵母细胞中生长素的转运能力远高于未磷酸化形式的NTT1.1-T101A[52].利用酵母双杂交系统和LC-MS/MS激素含量检测,Kanno等[53]发现,拟南芥NPF4.6、NPF4.1、NPF4.2和NPF4.5可能参与酵母中ABA的转运;NPF4.1除转运ABA外,还参与转运GAs和JA-Ile[54];NPF2.12(NRT1.6)和NPF5.6参与不同类型GAs的转运[54].此外,Tal等[55]在拟南芥GAs转运突变体中发现,定位于根内皮层细胞的NPF3过表达能极大促进GAs的跨膜运输,且其表达受GAs抑制,说明拟南芥NPF3作为输入载体可能也参与了GAs体内的分配和活性调控.与拟南芥ABA转运蛋白AtNPF4.6相似,Pellizzaro等[36]发现,在爪蟾卵母细胞中注射蒺藜苜蓿MtNPF6.8(MtNRT1.3)和AtNPF4.6 cRNA,均能使爪蟾卵母细胞ABA吸收增加,说明MtNPF6.8也具有ABA转运功能,同时还发现MtNPF6.8和ABA共同了参与NO3-抑制蒺藜苜蓿主根生长的调控作用.
2.6胁迫防御
NRT还在植物胁迫防御方面发挥重要作用,如在气孔保卫细胞中表达的AtNRT1.1,通过增加保卫细胞中NO3-含量引起保卫细胞去极化,促进气孔张开,从而负调控植物的干旱耐受性[56].NRT1.8参与的硝酸盐分配在耐镉胁迫中起着重要的作用.镉处理下AtNRT1.8显著上调能使更多的NO3-从地上部分运输到根部,根部较高浓度的NO3-有助于根系适应镉胁迫.相反,atnrt1.8突变体的根中NO3-比例较低,其根的伸长和植株生长也受抑制[18].硝态氮供应条件下,AtNRT1.5的功能下调或缺失能增强植株盐胁迫、干旱胁迫以及镉胁迫等许多胁迫的耐受能力.与野生型植株相比,盐处理下atnrt1.5突变体木质部汁液中的Na+含量显著下降,植株地上部的Na+含量降低而根中的显著增加,使得atnrt1.5突变体耐盐能力显著增强[20].渗透胁迫下,atnrt1.5突变体根和地上部分抗旱相关功能基因P5CS1和RD29A的表达丰度显著高于野生型植株,且抗旱能力也明显强于野生型植株[20];当把硝态氮换成铵态氮,atnrt1.5突变体对于这些胁迫的耐受能力不复存在.研究表明,逆境条件下NRT1.5和NRT1.8对植物胁迫耐受能力的调控与其介导的NO3-由地上向根部大量积累有关,ET/JA(乙烯/茉莉酸)-NRT介导的信号途径通过促进NRT1.8表达而抑制NRT1.5表达参与了逆境下NO3-的向根分配[57].
2.7提高氮素利用效率及其他
转基因试验证明NRT与作物产量和氮素利用效率密切相关.Fan等[58]发现不同硝态氮供应水平下,OsPTR6过表达都能够增加水稻生物量,并提高了水稻氮积累量和谷氨酰胺合成酶活性;OsNRT1.1a和OsNRT1.1b过表达水稻植株地上部分生物量明显增加,且OsNRT1.1b过表达植株的总氮含量更高,在低氮条件(0.125 mmol·L-1NH4NO3)下仍明显高于野生型,说明OsNRT1.1b较OsNRT1.1a更能够促进低氮下水稻对氮的吸收,提高作物的产量和氮素利用效率[27,59].Fan等[60]还发现OsNRT2.3b高表达水稻有着更强的pH缓冲能力,可以增强氮素、铁、磷吸收能力,OsNRT2.3b过表达水稻根和地上部分的总磷含量在300 μmol·L-1无机磷(Pi)处理下比野生型分别增加了102%和75%,10 μmol·L-1Pi条件下增加了63%和62%.因为氮和磷是水稻生长最重要的两个大量元素,因此过表达OsNRT2.3b能够显著提高水稻籽粒产量和氮素利用效率,比野生型粮食产量和稻草产量分别增加了44%和25%[61].
此外,NRT在植物次生代谢产物运输中也发挥重要作用.Payne等[62]发现长春花(Catharanthusroseus)CrNPF2.9基因经病毒诱导的基因沉默(VIGS)后,异胡豆苷在液泡中大量积累,同时减少了下游生物碱的合成,且过表达CrNPF2.9导致异胡豆苷积累量提高10倍,说明定位于液泡膜的CrNPF2.9在异胡豆苷从液泡向细胞质转运的过程中发挥关键作用.
3 表达调控
NRT的表达及其功能的发挥受外界和内部许多因素调控,如氮素浓度、pH、激素、非生物胁迫、光合产物[21,63]及翻译后调控等[64](表2).
3.1氮素调控
氮素对NRT表达调控已有大量报道[65].根据NRT对NO3-供应的响应差异,通常将NRT分为诱导型和组成型.不同物种NRT对NO3-的响应与拟南芥不完全一致,且表达部位也不尽相同,如AtNRT1.1受低浓度NO3-诱导表达,而低浓度NO3-对于黄瓜CsNRT1.1无显著诱导效果,且高NO3-供应时表达下调[6];AtNRT1.9主要在根部组成型表达,而CsNRT1.9在生长到第1、4、8周的黄瓜根中均未检测到其表达,而在子叶和老叶中的表达极为丰富,且能被高NO3-或短时的NO3-供应强烈地诱导[6].不同物种NRT同源序列对氮素调控响应的差异,可能与其不同的生理功能有关.此外,NO3-对NRT表达的调控具有明显的时间动态变化特点.1 mmol·L-1NO3-处理3~6 h后,大麦根中HvNRT2.1、HvNRT2.2和HvNRT2.3转录水平达到最高,12~24 h后表达量已降低到无法检测,而处理3~48 h内HvNRT2.4的表达水平能一直保持较高水平[66].Sorgonà等[67]对玉米幼苗供给50 μmol·L-1NO3-,前4 h内ZmNRT2.1的转录水平呈明显增加趋势,24 h后逐渐降低.
3.2激素调控
AtNRT1.1主要在根和茎的新生部位表达,其表达受到IAA的调节.施加外源IAA对NO3-吸收速率以及AtNRT1.1基因表达量有明显促进作用[68],对成熟根系施加IAA能促进侧根发育[45].镉与钠胁迫产生的ET/JA信号通过EIL1调节了ERF的表达,并由此上调了NRT1.8的表达,而ET/JA信号通过EIN3/EIL1等下调了NRT1.5的表达,促进硝酸盐向根部的转运[57].
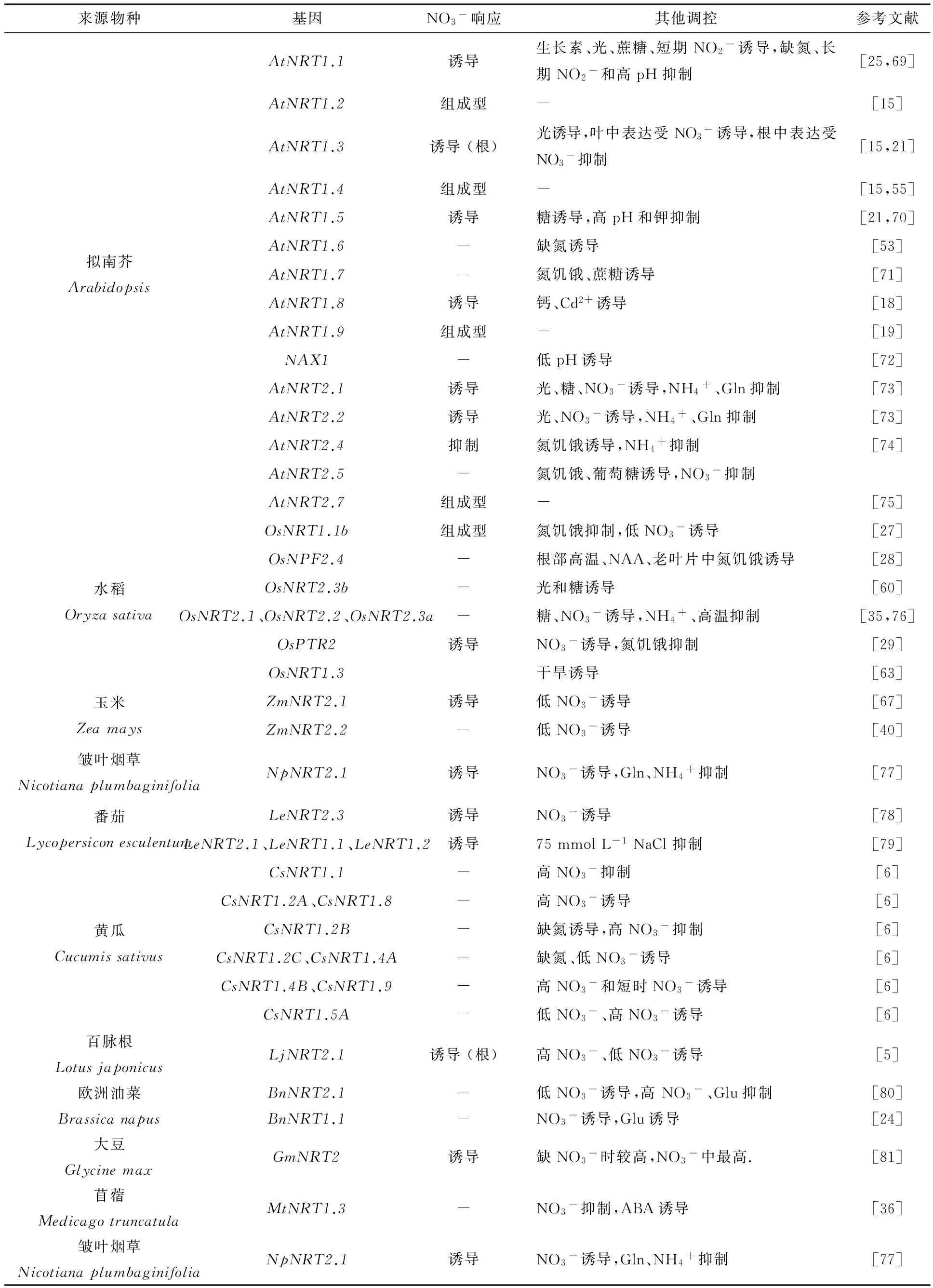
表2 部分植物硝酸盐转运蛋白的硝酸盐及其他调控汇总
3.3环境胁迫调控及其他
环境胁迫能够显著影响植物NRT的表达水平.芥菜(Brassicajuncea)7个NPF编码基因(BjNRT1.1,BjNRT1.2,BjNRT1.3,BjNRT1.4,BjNRT1.5,BjNRT1.7,BjNRT1.8)中,BjNRT1.1和BjNRT1.5在低温、热、盐和渗透胁迫1 h时均上调,可能与逆境适应有关,而BjNRT1.1和BjNRT2.1的表达水平在以上非生物胁迫处理24 h时均下调,推测两者可能在逆境导致的芥菜生长发育抑制中起关键作用[82].干旱土壤中小麦NRT编码基因的表达,取决于小麦基因型、生长阶段及氮素供应状况.不同小麦基因型的TaNRT1.1和TaNRT1.2表达不同,低亲和转运蛋白TaNRT1.1和TaNRT1.2的转录水平在XY6基因型中受干旱诱导,却在XY107基因型中受干旱抑制;高亲和性NRT基因TaNRT2.1的表达在干旱胁迫时下调,而TaNRT2.2和TaNRT2.3的表达与小麦生长阶段和氮素供应状况有关;小麦XY107基因型中TaNRT2.1的表达显著高于XY6基因型[83].镉胁迫下根部AtNRT1.1活性受抑制,但同时抑制了根对镉的吸收[84].钼是硝酸还原酶的金属辅酶,是NR活性所依赖的元素,外施钼元素草莓NRT1.1和NRT2.1表达水平上调,NO3-向地上部分运输及还原能力增强,氮素利用效率提高[85].此外,不同发育阶段NRT表达不同,黄瓜CsNRT1.7是一种衰老诱导型基因,其在黄瓜老叶中的表达要明显高于成年叶与幼叶,CsNRT1.7也能被低氮诱导表达[39].
4 总结与展望
随着对NRT研究的深入,发现其不仅参与NO3-的吸收和转运,还参与众多的植物生理过程,在植物生长发育中发挥重要的作用.对NRT的功能研究正引起越来越多研究者的重视.然而到目前为止,对NRT的研究主要集中在模式植物如拟南芥和水稻上,在其他作物如叶菜中的研究还很少.叶菜是重要的经济作物,也是产量、品质严重依赖硝态氮的作物,明确NRT在叶菜硝态氮吸收利用及生长发育过程中的作用可能对实现叶菜减肥增效的可持续生产具有重要指导意义.此外,虽然已从不同植物中克隆出了多个NRT编码基因,并鉴定了一些NRT的功能,但由于NRT通常存在多层次调控模式及多个NRT之间的协同合作,并受外界环境变化和自身发育水平的调控,全面认识NRT的生理功能还比较困难.此外,NRT还参与硝酸根信号通路,能够与信号途径中激素、转录因子及一些下游基因发生相互作用,加上氮代谢与碳代谢的偶联关系,使得NRT功能可能更为多样,研究也更为复杂.传统单一的生理学、分子生物学方法在NRT研究上存在明显的局限性,综合利用各种生理生化及组学方法,从系统生物学水平上解析硝酸盐转运蛋白的功能可能是以后研究的一个热点和难点.
[1] Wang Y Y,Hsu P K,Tsay Y F.Uptake,allocation and signaling of nitrate [J].Trends in Plant Science,2012,17(8):458-67.
[2] Léran S,Varala K,Boyer J C,et al.A unified nomenclature of NITRATE TRANSPORTER 1/PEPTIDE TRANSPORTER family members in plants [J].Trends in Plant Science,2014,19(1):5-9.
[3] Tsay Y F,Chiu C C,Tsai C B,et al.Nitrate transporters and peptide transporters [J].Febs Letters,2007,581(12):2290-300.
[4] Bai H,Euring D,Volmer K,et al.The nitrate transporter (NRT) gene family in poplar [J].Plos One,2013,8(8):e72126.
[5] Criscuolo G,Valkov V T,Parlati A,et al.Molecular characterization of theLotusjaponicusNRT1(PTR) and NRT2 families [J].Plant Cell and Environment,2012,35(9):1567-1581.
[6] Migocka M,Warzybok A,Kibus G.The genomic organization and transcriptional pattern of genes encoding nitrate transporters 1 (NRT1) in cucumber [J].Plant and Soil,2013,364(1-2):245-260.
[7] Santos T B D,Lima J E,Felicio M S,et al.Genome-wide identification,classification and transcriptional analysis of nitrate and ammonium transporters inCoffea[J].Genetics & Molecular Biology,2017,40(1,Suppl):346-359.
[8] von Wittgenstein N J,Le C H,Hawkins B J,et al.Evolutionary classification of ammonium,nitrate,and peptide transporters in land plants [J].BMC Evolutionary Biology,2014,14(1):11.
[9] Forde B G.Nitrate transporters in plants:structure,function and regulation [J].Biochimica Et Biophysica Acta,2000,1465(1-2):219-235.
[10] Galvan A,Fernández E.Eukaryotic nitrate and nitrite transporters [J].Cellularand Molecular Life Sciences,2001,58(2):225-233.
[11] 钱瑜,察倩倩,孔敏,等.植物NRT2家族的分子进化 [J].江苏农业学报,2015,31(1):45-54.
Qian Y,Cha Q Q,Kong M,et al.Molecular evolution of NRT2 gene family in plant [J].Jiangsu Journal of Agricultural Science,2015,31(1):45-54.
[12] Jargeat P,Rekangalt D,Verner M C,et al.Characterisation and expression analysis of a nitrate transporter and nitrite reductase genes,two members of a gene cluster for nitrate assimilation from the symbiotic basidiomyceteHebelomacylindrosporum[J].Current Genetics,2003,43(3):199-205.
[13] Martín Y,Navarro F J,Siverio J M.Functional characterization of theArabidopsisthaliananitrate transporter CHL1 in the yeastHansenulapolymorpha[J].Plant Molecular Biology,2008,68(3):215-224.
[14] Huang N C,Liu K H,Lo H J,et al.Cloning and functional characterization of anArabidopsisnitrate transporter gene that encodes a constitutive component of low-affinity uptake [J].Plant Cell,1999,11(8):1381-1392.
[15] Okamoto M,Vidmar J J,Glass A D M.Regulation ofNRT1 andNRT2 gene families ofArabidopsisthaliana:responses to nitrate provision [J].Plant and Cell Physiology,2003,44(3):304-317.
[16] Chiu C C,Lin C S,Hsia A P,et al.Mutation of a nitrate transporter,AtNRT1:4,results in a reduced petiole nitrate content and altered leaf development [J].Plant and Cell Physiology,2004,45(9):1139-1148.
[17] Dechorgnat J,Nguyen C T,Armengaud P,et al.From the soil to the seeds:The long journey of nitrate in plants [J].Journal of Experimental Botany,2011,62(4):1349-1359.
[18] Li J Y,Fu Y L,Pike S M,et al.TheArabidopsisnitrate transporter NRT1.8 functions in nitrate removal from the xylem sap and mediates cadmium tolerance [J].Plant Cell,2010,22(5):1633-1646.
[19] Wang Y Y,Tsay Y F.Arabidopsisnitrate transporter NRT1.9 is important in phloem nitrate transport [J].Plant Cell,2011,23(5):1945-1957.
[20] Chen C Z,Lyu X F,Li J Y,et al.ArabidopsisNRT1.5 is another essential component in the regulation of nitrate reallocation and stress tolerance [J].Plant Physiology,2012,159(4):1582-1950.
[21] Almagro A,Lin S H,Tsay Y F.Characterization of theArabidopsisnitrate transporter NRT1.6 reveals a role of nitrate in early embryo development [J].Plant Cell,2008,20(12):3289-3299.
[22] Liu W,Sun Q,Wang K,et al.Nitrogen limitation adaptation (NLA) is involved in source-to-sink remobilization of nitrate by mediating the degradation of NRT1.7 in Arabidopsis [J].New Phytologist,2016,214(2):734-744.
[23] Barbier-Brygoo H,De A A,Filleur S,et al.Anion channels/transporters in plants:From molecular bases to regulatory networks [J].Annual Review of Plant Biology,2011,62(62):25-51.
[24] Zhao S P,Ye X Z,Zhang Y Z,et al.The contribution of bnnrt1 and bnnrt2 to nitrate accumulation varied according to genotypes in Chinese cabbage [J].African Journal of Biotechnology,2013,9(31):4910-4917.
[25] Li W,Wang Y,Okamoto M,et al.Dissection of theAtNRT2.1:AtNRT2.1 inducible high-affinity nitrate transporter gene cluster [J].Plant Physiology,2007,143(1):425-433.
[26] Lezhneva L,Kiba T,Feriabourrellier A B,et al.TheArabidopsisnitrate transporter NRT2.5 plays a role in nitrate acquisition and remobilization in nitrogen-starved plants [J].Plant Journal for Cell and Molecular Biology,2014,80(2):230-241.
[27] Fan X,Feng H,Tan Y,et al.A putative 6-transmembrane nitrate transporter OsNRT1.1b plays a key role in rice under low nitrogen [J].Journal of Integrative Plant Biology,2016,58(6):590-599.
[28] Xia X,Fan X,Wei J,et al.Rice nitrate transporter OsNPF2.4 functions in low-affinity acquisition and long-distance transport [J].Journal of Experimental Botany,2015,66(1):317-331.
[29] Li Y,Ouyang J,Wang Y Y,et al.Disruption of the rice nitrate transporter OsNPF2.2 hinders root-to-shoot nitrate transport and vascular development [J].Scientific Reports,2015,5:e9635.
[30] Tang Z,Fan X,Li Q,et al.Knockdown of a rice stelar nitrate transporter alters long-distance translocation but not root influx [J].Plant Physiology,2012,160(4):2052-2063.
[31] Liu T,Wei D,Sun F,et al.Cloning and characterization of the nitrate transporter gene BraNRT2.1,in non-heading Chinese cabbage [J].Acta Physiologiae Plantarum,2014,36(4):815-823.
[32] Yang X,Sun F,Xiong A,et al.BcNRT1,a plasma membrane-localized nitrate transporter from non-heading Chinese cabbage [J].Molecular Biology Reports,2012,39(8):7997-8006.
[33] Gu C S,Zhang X X,Chen S M,et al.Isolation and characterization of theChrysanthemumnitrate transporter CmNRT1 [J].Genetics and Molecular Research,2016,15(1):15017148.
[34] Gu C,Zhang X,Jiang J,et al.ChrysanthemumCmNAR2 interacts with CmNRT2 in the control of nitrate uptake [J].Scientific Reports,2014,4:e5833.
[35] Pike S,Gao F,Kim M J,et al.Members of the NPF3 transporter subfamily encode pathogen-inducible nitrate/nitrite transporters in grapevine and Arabidopsis [J].Plant and Cell Physiology,2014,55(1):162-170.
[36] Pellizzaro A,Clochard T,Cukier C,et al.The nitrate transporter MtNPF6.8 (MtNRT1.3) transports abscisic acid and mediates nitrate regulation of primary root growth inMedicagotruncatula[J].Plant Physiology,2014,166(4):2152-2165.
[37] Anjana S U,Iqbal M.Nitrate accumulation in plants,factors affecting the process,and human health implications.A review [J].Agronomy for Sustainable Development,2007,27(1):45-57.
[38] Taochy C,Gaillard I,Ipotesi E,et al.TheArabidopsisroot stele transporter NPF2.3 contributes to nitrate translocation to shoots under salt stress [J].Plant Journal for Cell and Molecular Biology,2015,83(3):466-479.
[39] Wu T,Qin Z,Fan L,et al.Involvement ofCsNRT1.7 in nitrate recycling during senescence in cucumber [J].Journal of Plant Nutrition and Soil Science,2015,177(5):714-721.
[40] Quaggiotti S,Ruperti B,Borsa P,et al.Expression of a putative high-affinity NO3-transporter and of an H+-ATPase in relation to whole plant nitrate transport physiology in two maize genotypes differently responsive to low nitrogen availability [J].Journal of Experimental Botany,2003,54(384):1023-1031.
[41] 李建勇,龚继明.植物硝酸根信号感受与传导途径[J].植物生理学报,2011,47(2):111-118.
Li J Y,Gong J M.Nitrate signal sensing and transduction in higher plants [J].Plant Physiology Journal,2011,47(2):111-118.
[42] Krouk G,Crawford N M,Coruzzi G M,et al.Nitrate signaling:adaptation to fluctuating environments [J].Current Opinion in Plant Biology,2010,13(3):265-272.
[43] Undurraga S F,Ibarrahenríquez C,Fredes I,et al.Nitrate signaling and early responses inArabidopsisroots [J].Journal of Experimental Botany,2017,68(10):2541-2551.
[44] Zhou J J,Fernández E,Galván A,et al.A high affinity nitrate transport system fromChlamydomonas,requires two gene products [J].Febs Letters,2000,481(1):225-227.
[45] Krouk G,Lacombe B,Bielach A,et al.Nitrate-regulated auxin transport by NRT1.1 defines a mechanism for nutrient sensing in plants [J].Developmental Cell,2010,18(6):927-937.
[46] Bouguyon E,Gojon A,Nacry P.Nitrate sensing and signaling in plants [J].Seminars in Cell and Developmental Biology,2012,23(6):648-654.
[47] Walch-Liu,Pia,Forde B G.Nitrate signalling mediated by the NRT1.1 nitrate transporter antagonises l-glutamate-induced changes in root architecture [J].Plant Journal for Cell and Molecular Biology,2008,54(5):820-828.
[48] Vidal E A,Moyano T C,Gabriel K,et al.Integrated RNA-seq and sRNA-seq analysis identifies novel nitrate-responsive genes inArabidopsisthalianaroots [J].BMC Genomics,2013,14(1):e701.
[49] Vidal E A,Araus V,Lu C,et al.Nitrate-responsive miR393/AFB3 regulatory module controls root system architecture inArabidopsisthaliana[J].Proceedings of the National Academy of Sciences of the United States of America,2010,107(9):4477-4482.
[50] Little D Y,Rao H,Oliva S,et al.The putative high-affinity nitrate transporter NRT2.1 represses lateral root initiation in response to nutritional cues [J].Proceedings of the National Academy of Sciences of the United States of America,2005,102(38):13693-13698.
[51] Remans T,Nacry P,Pervent M,et al.A central role for the nitrate transporter NRT2.1 in the integrated morphological and physiological responses of the root system to nitrogen limitation inArabidopsis[J].Plant Physiology,2006,140(3):909-921.
[52] Jacquot A,Li Z,Gojon A,et al.Post-translational regulation of nitrogen transporters in plants and microorganisms [J].Journal of Experimental Botany,2017,68:2567-2580.
[53] Kanno Y,Seo M.Identification of an abscisic acid transporter by functional screening using the receptor complex as a sensor [J].Proceedings of the National Academy of Sciences of the United States of America,2012,109(24):9653-9658.
[54] Chiba Y,Shimizu T,Miyakawa S,et al.Identification ofArabidopsisthaliana,NRT1/PTR FAMILY (NPF) proteins capable of transporting plant hormones [J].Journal of Plant Research,2015,128(4):679-686.
[55] Tal I,Zhang Y,Jørgensen M E,et al.TheArabidopsisNPF3 protein is a GA transporter [J].Nature Communications,2016,7:e11486.
[56] Guo F Q,Young J,Crawford N M.The nitrate transporter AtNRT1.1 (CHL1) functions in stomatal opening and contributes to drought susceptibility inArabidopsis[J].Plant Cell,2003,15(1):107-117.
[57] Zhang G B,Gong J M.TheArabidopsisethylene/jasmonic acid-NRT signaling module coordinates nitrate reallocation and the trade-off between growth and environmental adaptation [J].Plant Cell,2014,26(10):3984-3998.
[58] Fan X,Xie D,Chen J,et al.Over-expression ofOsPTR6,in rice increased plant growth at different nitrogen supplies but decreased nitrogen use efficiency at high ammonium supply [J].Plant Science,2014,227(10):1-11.
[59] Duan D,Zhang H.A single SNP inNRT1.1Bhas a major impact on nitrogen use efficiency in rice [J].Science China-Life Sciences,2015,58(8):827-828.
[60] Fan X,Tang Z,Tan Y,et al.Overexpression of a pH-sensitive nitrate transporter in rice increases crop yields [J].Proceedings of the National Academy of Sciences of the United States of America,2016,113(26):7118-7123.
[61] Feng H,Li B,Zhi Y,et al.Overexpression of the nitrate transporter,OsNRT2.3b,improves rice phosphorus uptake and translocation [J].Plant Cell Reports,2017:1287-1296.
[62] Payne R M,Xu D,Foureau E,et al.An NPF transporter exports a central monoterpene indole alkaloid intermediate from the vacuole [J].Nature Plants,2017,3(16208):e16208.
[63] Hu T Z,Cao K M,Xia M,et al.Functional characterization of a putative nitrate transporter gene promoter from rice [J].Functional characterization of a putative nitrate,2006,38(11):795-802.
[64] Ruffel S,Gojon A,Lejay L.Signal interactions in the regulation of root nitrate uptake [J].Journal of Experimental Botany,2014,65(19):507-515.
[65] 尹辉,牟书勇,李冠.植物硝酸盐转运体的功能及其调控 [J].南方农业学报,2012,43(4):425-430.
Yin H,Mu S Y,Li G.Function and regulation of nitrate transporters in plants [J].Journal of Southern Agriculture,2012,43(4):425-430.
[66] Vidmar J J,Zhuo D,Siddiqi M Y,et al.Isolation and characterization ofHvNRT2.3 andHvNRT2.4,cDNAs encoding high-affinity nitrate transporters from roots of barley [J].Plant Physiology,2000,122(3):783-792.
[67] Sorgonà A,Lupini A,Mercati F,et al.Nitrate uptake along the maize primary root:an integrated physiological and molecular approach [J].Plant Cell and Environment,2011,34(7):1127-1140.
[68] 郑冬超,夏新莉,尹伟伦.生长素促进拟南芥AtNRT1.1基因表达增强硝酸盐吸收 [J].北京林业大学学报,2013,35(2):80-85.
Zheng D C,Xia X L,Yin W L.Auxin promotes nitrate uptake by up-regulatingAtNRT1.1 gene transcript level inArobidopsisthaliana[J].Journal of Beijing Forestry University,2013,35(2):80-85.
[69] Zhou J J,Theodoulou F L,Muldin I,et al.Cloning and functional characterization of aBrassicanapustransporter that is able to transport nitrate and histidine [J].Journal of Biological Chemistry,1998,273(20):12017-12023.
[70] Lin S H,Tsay Y F.Mutation of theArabidopsisNRT1.5 nitrate transporter causes defective root-to-shoot nitrate transport [J].Plant Cell,2008,20(9):2514-2528.
[71] Fan S C,Lin C S,Hsu P K,et al.TheArabidopsisnitrate transporter NRT1.7,expressed in phloem,is responsible for source-to-sink remobilization of nitrate [J].Plant Cell,2009,21(9):2750-2761.
[72] Segonzac C,Boyer J C,Ipotesi E,et al.Nitrate efflux at the root plasma membrane:Identification of anArabidopsisexcretion transporter [J].Plant Cell,2007,19(11):3760-3777.
[73] Cerezo M,Tillard P,Muos S,et al.Major alterations of the regulation of root NO3-uptake are associated with the mutation ofnrt2.1 andnrt2.2 genes inArabidopsis[J].Plant Physiology,2001,127(1):262-271.
[74] Kiba T,Feria-Bourrellier A B,Lafouge F,et al.TheArabidopsisnitrate transporter NRT2.4 plays a double role in roots and shoots of nitrogen-starved plants [J].Plant Cell,2012,24(1):245-258.
[75] Chopin F,Orsel M,Dorbe M F,et al.TheArabidopsisATNRT2.7 nitrate transporter controls citrate content in seeds [J].Plant Cell,2007,19(5):1590-1602.
[76] Araújo O J L,Pinto M S,Sperandio M V L,et al.Expression of the genesOsNRT1.1,OsNRT2.1,OsNRT2.2,and kinetics of nitrate uptake in genetically contrasting rice varieties [J].American Journal of Plant Sciences,2015,6(2):306-313.
[77] Quesada A,Krapp A,Trueman L J,et al.PCR-identification of aNicotianaplumbaginifoliacDNA homologous to the high-affinity nitrate transporters of thecrnAfamily [J].Plant Molecular Biology,1997,34(2):265-274.
[78] Fu Y,Yi H,Bao J,et al.LeNRT2.3 functions in nitrate acquisition and long-distance transport in tomato [J].Febs Letters,2015,589(10):1072-1079.
[79] Yao J,Shi W M,Xu W F.Effects of salt stress on expression of nitrate transporter and assimilation-related genes in tomato roots [J].Russian Journal of Plant Physiology,2008,55(2):232-240.
[80] Leblanc A,Segura R,Deleu C,et al.In low transpiring conditions,uncoupling theBnNrt2.1 andBnNrt1.1 NO3-transporters by glutamate treatment reveals the essential role ofBnNRT2.1 for nitrate uptake and the nitrate-signaling cascade during growth [J].Plant Signaling and Behavior,2013,8(2):e22904.
[81] Amarasinghe B H,de Bruxelles G L,Braddon M,et al.Regulation ofGmNRT2 expression and nitrate transport activity in roots of soybean (Glycinemax) [J].Planta,1998,206(1):44-52.
[82] Parul G,Kumar S A.Abiotic stresses downregulate key genes involved in nitrogen uptake and assimilation inBrassicajunceaL.[J].Plos One,2015,10(11):e0143645.
[83] Duan J F,Hui T,Yajun G.Expression of nitrogen transporter genes in roots of winter wheat (TriticumaestivumL.) in response to soil drought with contrasting nitrogen supplies [J].Crop and Pasture Science,2016,67(2):128-136.
[84] Mao Q Q,Guan M Y,Lu K X,et al.Inhibition of nitrate transporter 1.1-controlled nitrate uptake reduces cadmium uptake inArabidopsis[J].Plant Physiology,2014,166(2):934-944.
[85] Liu L,Xiao W,Li L,et al.Effect of exogenously applied molybdenum on its absorption and nitrate metabolism in strawberry seedlings [J].Plant Physiology and Biochemistry,2017,115:200-211.
(责任编辑:顾浩然,冯珍珍)
Progressinfunctionandregulationofnitratetransportersinplants
Song Tianli, Zhou Jianjian, Xu Chenxi, Cai Xiaofeng, Dai Shaojun, Wang Quanhua, Wang Xiaoli*
(Development Center of Plant Germplasm Resources,College of Life and Environmental Sciences,Shanghai Normal University,Shanghai 200234,China)
Nitrate transporters can not only uptake and transport nitrate in plants,but also play key roles in many other physiological processes.This article reviewed the multiple functions of nitrate transporter in nitrate accumulation,lateral root development,hormone transport,and stress tolerance,etc.The expression regulation of nitrate transporters was also discussed.
nitrate transporters; nitrate accumulation; root system development; hormone transport; stress response
Q 945.12; S 60
A
1000-5137(2017)05-0740-11
2017-08-31
国家自然科学基金青年基金(31601744);上海市自然科学基金(15ZR1431300)、上海植物种质资源工程技术研究中心项目(17DZ2252700).
宋田丽(1993-),女,硕士研究生,主要从事植物分子育种方面的研究.E-mail:1903972321@qq.com
导师简介: 王全华(1963-),女,博士,研究员,主要从事植物分子育种方面的研究.E-mail:wqh6352083@126.com
*
王小丽(1980-),女,博士,讲师,主要从事植物营养生理与分子育种方面的研究.E-mail:wxl2006by@163.com
