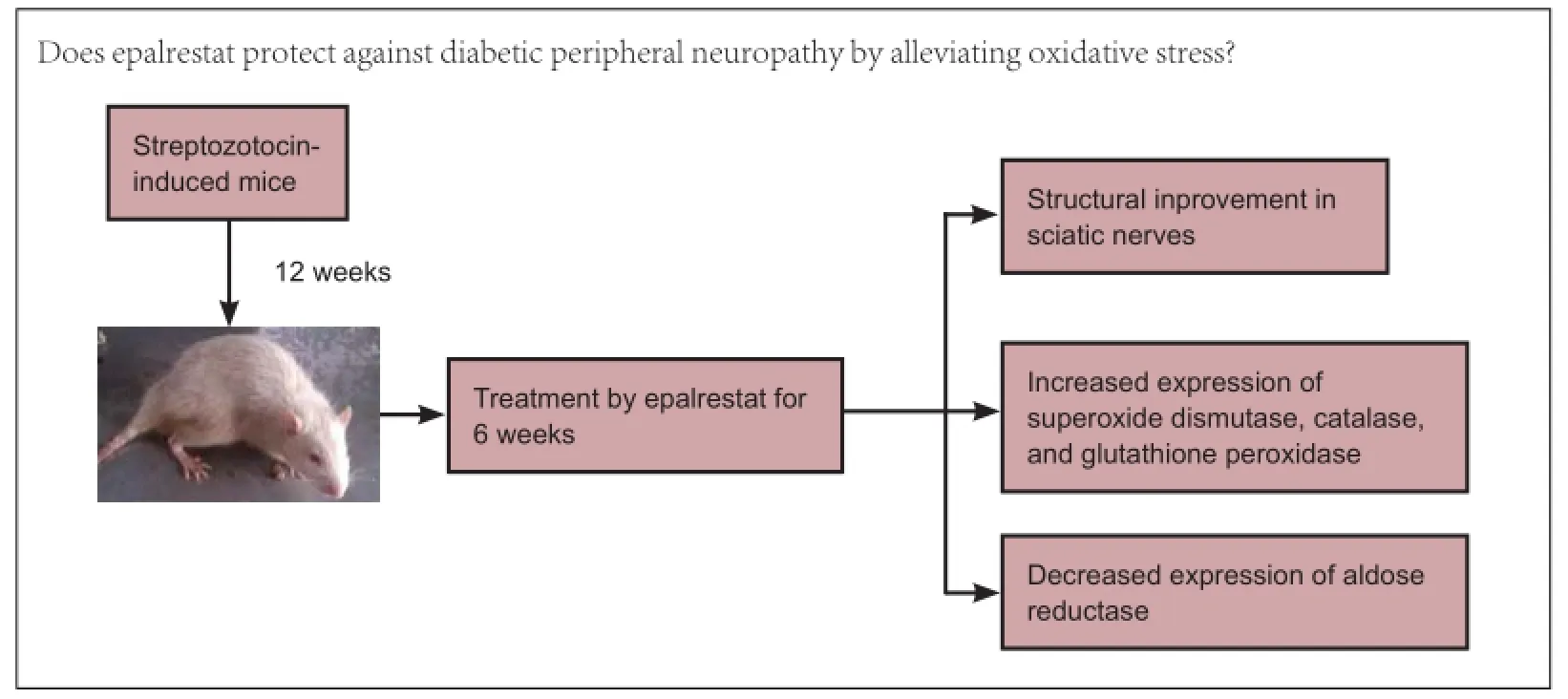
Epalrestat protects against diabetic peripheral neuropathy by alleviating oxidative stress and inhibiting polyol pathway
2016-12-02QingrongLiZhuoWangWeiZhouShouruiFanRunMaLiXueLuYangYashanLiHongliTanQinghuaShaoHongyingYang
Qing-rong Li, Zhuo Wang, Wei Zhou, Shou-rui Fan, Run Ma, Li Xue, Lu Yang, Ya-shan Li, Hong-li Tan, Qing-hua Shao, Hong-ying Yang,
1 Second Affiliated Hospital of Kunming Medical University, Department of Clinical Laboratory, Kunming, Yunnan Province, China
2 Third People’s Hospital of Yunnan Province, Kunming, Yunnan Province, China
RESEARCH
Epalrestat protects against diabetic peripheral neuropathy by alleviating oxidative stress and inhibiting polyol pathway
Qing-rong Li1, Zhuo Wang1, Wei Zhou2, Shou-rui Fan1, Run Ma1, Li Xue1, Lu Yang1, Ya-shan Li2, Hong-li Tan2, Qing-hua Shao2, Hong-ying Yang1,*
1 Second Affiliated Hospital of Kunming Medical University, Department of Clinical Laboratory, Kunming, Yunnan Province, China
2 Third People’s Hospital of Yunnan Province, Kunming, Yunnan Province, China
Graphical Abstract
orcid: 0000-0001-6740-880X (Hong-ying Yang)
Epalrestat is a noncompetitive and reversible aldose reductase inhibitor used for the treatment of diabetic neuropathy. This study assumed that epalrestat had a protective effect on diabetic peripheral nerve injury by suppressing the expression of aldose reductase in peripheral nerves of diabetes mellitus rats. The high-fat and high-carbohydrate model rats were established by intraperitoneal injection of streptozotocin. Peripheral neuropathy occurred in these rats after sustaining high blood glucose for 8 weeks. At 12 weeks after streptozotocin injection, rats were intragastrically administered epalrestat 100 mg/kg daily for 6 weeks. Transmission electron microscope revealed that the injuries to myelinated nerve fibers, non-myelinated nerve fibers and Schwann cells of rat sciatic nerves had reduced compared to rats without epalrestat administuation. Western blot assay and immunohistochemical results demonstrated that after intervention with epalrestat, the activities of antioxidant enzymes such as superoxide dismutase, catalase and glutathione peroxidase gradually increased, but aldose reductase protein expression gradually diminished. Results confirmed that epalrestat could protect against diabetic peripheral neuropathy by relieving oxidative stress and suppressing the polyol pathway.
nerve regeneration; peripheral nerve injury; streptozotocin; reactive oxygen species; diabetic neuropathy; oxidative stress; aldose reductase; antioxidant enzymes; polyol pathway; aldose reductase inhibitor; superoxide dismutase; catalase; glutathione peroxidase; rats; NSFC grant; neural regeneration
Introduction
As one of the most common complications of diabetes mellitus (DM), diabetic peripheral neuropathy (DPN) has long been a topic of research (Obrosova, 2009). Up-regulation of the polyol and hexosamine pathways and increases in the levels of non-enzymatic glycation products and activity of protein kinase C are responsible for the occurrence of diabetic complications (Brownlee, 2005). However, inhibition of those pathways and enzymes has not provided the expected outcomes. Banting laureate, Prof. Brownlee, recently proposed the theory that excess reactive oxygen species in the mitochondria is the major cause of diabetic complications (Brownlee, 2005).
Oxidative stress is manifested as a series of reactions initiated by an imbalance between oxidative substances and antioxidant capacity, which is accompanied by an increase in reactive oxygen species production and impaired antioxidative defenses(Singh et al., 2014). Oxidative stress is widely accepted as a key mediator in the development and progression of diabetic complications (Babizhayev et al., 2014). Clarifying the changes in the concentrations, activities and distribution of antioxidant defenses during diabetic peripheral neuropathy is important to direct therapy (Vincent et al., 2013). Diabetic hyperglycemia can activate the polyol pathway (Tarr et al., 2013), which converts glucose into sorbitol by the rate-limiting enzyme, aldose reductase. Sorbitol can be converted into fructose via fructose reductase (Obrosova, 2009) but the absence of fructose kinase in peripheral nervous tissue results in the accumulation of sorbitol causing intracellular hypertonia and inhibits inositol uptake (Schemmel et al., 2010). Intracellular inositol becomes exhausted, resulting in edema, demyelination and necrosis of peripheral nerves (Polak et al., 2013).
The aim of the current study was to evaluate the effects of epalrestat, an aldose reductase inhibitor (ARI), on oxidative stress and changes in aldose reductase in streptozotocin-induced DM. It was proposed that epalrestat could prevent peripheral nerve injury through inhibition of antioxidant enzymes and aldose reductase expressions in the peripheral nerves of rats. Subsequent analysis would add to an objective evaluation of diabetic peripheral neuropathy.
Materials and Methods
Animals
Seventy-six 8-week-old male Sprague-Dawley rats weighing 170-190 g were purchased from the Laboratory Animal Center of Kunming Medical University of China (license No. SYXK (Dian) K2015-0002). The rats were housed in cages for at least 1 week for choosing healthy rats.
The investigation conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication No. 85-23, revised in 1996). Animal experiments followed the ethical standards approved by the Research Ethics Committee of Kunming Medical University of China.
In the first week, the rats were randomly divided into a control group (n = 30) and a DM group (n = 46). Rats in the DM group were treated with high-fat and high-sugar diet for 4 weeks, and injected with streptozotocin at 4 and 8 weeks. Twelve weeks later, the above two groups were divided into four groups: non-diabetes mellitus (nDM) (ARI-) group (n = 15; nDM rats without epalrestat administration), nDM (ARI+) group (n = 15; nDM rats with epalrestat administration), DPN (ARI-) group (n = 20; DPN rats without epalrestat administration), and DPN (ARI+) group (n = 26; DPN rats with epalrestat administration).
Establishment of DPN models and drug administration
The DM group consisted of 46 rats that were treated with highfat and high-sugar diet for 4 weeks. Food composition was 32% carbohydrate, 28% fat, 17% protein and 23% other to induce insulin resistance. The rats were intraperitoneally injected twice with streptozotocin dissolved in citric acid-sodium citrate buffer (Sigma-Aldrich, St. Louis, MO, USA). The first injection (25 mg/kg body weight) was conducted after 4 weeks and the second injection (40 mg/kg body weight) was conducted after 8 weeks on the diet (Islam and Choi, 2007). Blood glucose was obtained by repeated needle puncture of the tail tip veins to determine hyperglycemia. Blood glucose concentrations were determined using Bayer Glucometer Elite® and compatible blood glucose test strips (Bayer, Pittsburgh, PA, USA). Rats with a blood glucose level > 16 mM were considered DM group and those with blood glucose level < 16 mM were considered non-diabetic group (Chen et al., 2015).
Twelve weeks after the streptozotocin induction, all rats underwent an electrophysiological examination to check that the rats suffered from DPN. Blood samples were obtained by repeated needle puncture of the tail tip veins (Jin et al., 2012) to measure serum insulin, glycated hemoglobin and cholesterol levels to check whether insulin resistance had occurred. Serum insulin was determined with an electrochemical immunoassay. The equation for serum insulin resistance was the fasting blood glucose × fasting serum insulin/22.5. The glycated hemoglobin and cholesterol levels were measured (Olympus 5421 instrument, Olympus Corporation, Tokyo, Japan). The nDM (ARI+) group and DPN (ARI+) group (after they exhibited DNP) were fed with an ARI, epalrestat, by gavage (100 mg/kg/d) intraperitoneally for 6 weeks. After 6 weeks of ARI treatment, all rats were killed, and the sciatic nerves were rapidly removed from the right legs and placed in a freezing tube.
Serum total cholesterol, triglyceride, high-density lipoprotein cholesterol and low-density lipoprotein cholesterol
Blood samples were collected from the femoral artery of rats at week 12 following streptozotocin injection and were transferred to sterilized centrifuge tubes (Pedchenko and Malakhov, 1991). The samples were centrifuged at 4,000 × g for 5 minutes to obtain the serum and stored in a freezer for later analysis of total cholesterol, triglyceride, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol. They were measured by an enzymatic method of the automatic biochemical analyzer (Rainwater et al., 1995).
Neurophysiological examination
At 12 weeks after the second streptozotocin injection, streptozotocin-induced and control rats were anesthetized with 10% chloral hydrate (300 mg/kg body weight) and neuropathy measured with an electric stimulator (SDZ-V, Suzhou Medical Instrument Co., Ltd., Suzhou, China) and muscle power amplifier (AD Instruments, Sydney, Australia) (Zhang et al., 2008). The right sciatic nerve was stimulated proximally at the level of the sciatic notch and distal right side of the foot with an input electrode. A reference electrode was located in the rat’s tail. The rats were treated with an electrical stimulation signal with a pulse width of 0.1 ms and stimulation intensity 1.5 times the threshold. The body temperature was automatically maintained at a mean rectal temperature of 37.5-37.9°C. The computer recorded the time of the action potentials, respectively, from the stimulated nerves to the distal muscle. We repeated electrical stimulation three times, each with a time interval of 1 minute, and took the average value.
Transmission electron microscope observations
The rats were killed at 6 weeks of ARI treatment. The sciatic nerves of DM rats were excised and subdivided into small samples for transmission electron microscopy (JEM-1011, JEOL, Tokyo, Japan). The sciatic nerves of DM rats were rinsed three times with cacodylate buffer, fixed in 1% osmium tetroxide for 2 hours at room temperature, rinsed with cacodylate buffer three times again, and placed in buffer overnight. The samples were then dehydrated in 30%, 50%, 70%, 90% alchohol and 70% acetone, embedded in resin, cut into small sections, and stained with 2% uranyl acetate for 30 minutes, rinsed with distilled water, stained with 2% uranyl acetate for 30 minutes, rinsed with distilled water and lead citrate for an additional 30 minutes and rinsed with distilled water. The above samples were examined with an accelerating voltage of 80 kV.
Protein expression measured by western blot assay
Sciatic nerve proteins from each group were separated on 12% polyacrylamide gels and transferred to a polyvinylidene difluoride membrane. These membranes were incubated for 2 hours at room temperature in 3% bovine serum albumin. The primary polyclonal antibodies were rabbit anti-rat aldose reductase (37 kDa), glutathione peroxidase 1 (GPX, 22 kDa), superoxide dismutase (SOD, 18 kDa), catalase (CAT, 60 kDa) (1:1,000, 1:5,000, 1:1,000, 1:2,000; Abcam, Cambridge, MA,USA). The membranes were incubated with primary antibodies overnight at 4°C and with secondary antibody (goat anti-rabbit IgG, monoclonal antibody; 1:15,000; Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China) over 2 hours at room temperature. After three washes in Tris-buffered saline with Tween, immunoreactive complexes were visualized using an enhanced chemiluminescence system (Bio-Rad, Hercules, CA,
USA). β-Actin served as an internal positive control.
Immunohistochemistry
Samples were fixed in 4% paraformaldehyde, dehydrated in ascending alcohols and embedded in paraffin. Serial sections were cut from a paraffin block. The following primary antibodies were used: rabbit anti-rat aldose reductase (37 kDa), GPX (22 kDa), SOD superoxide dismutase (18 kDa), catalase (60 kDa; 1:1,000, 1:5,000, 1:1,000, 1:2,000; Abcam). The sections were incubated with primary antibody overnight at 4° C, and then incubated with goat anti-rabbit secondary antibody (1:15,000; Zhongshan Golden Bridge Biotechnology) for 2 hours at room temperature. The reactions were visualized using 3,3′-diaminobenzidine reagent (Boster Biological Tech-nology Ltd., Wuhan, Hubei Province, China). Nuclei were counterstained with hematoxylin. The sections were examined under a light microscope (Olympus, Tokyo, Japan).

Table 1 Basic profile of rats in the diabetes mellitus group (week 12 following model establishment) and control group

Figure 1 Effects of epalrestat, an ARI, on the ultrastructure of sciatic nerve cells in DM rats.
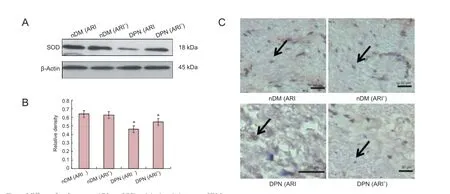
Figure 2 Effects of epalrestat, an ARI, on SOD activity in sciatic nerve of DM rats.
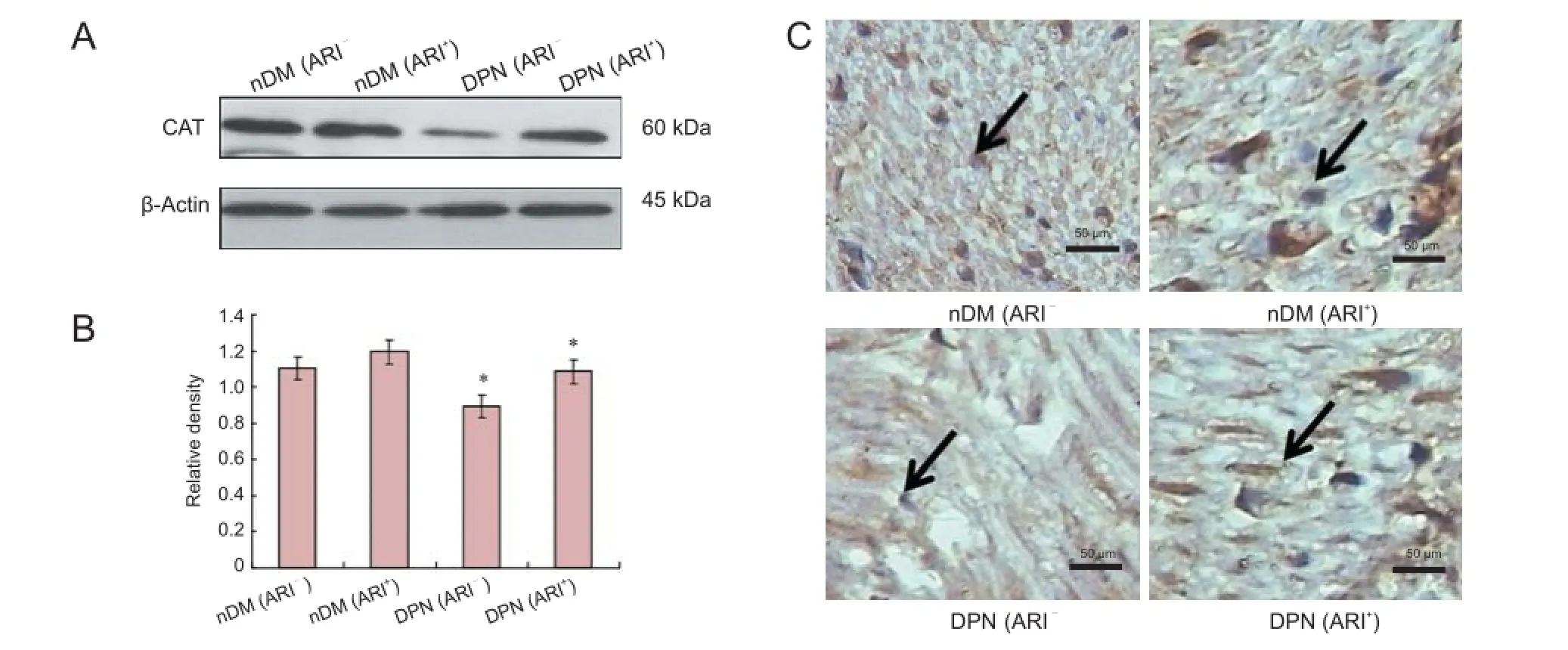
Figure 3 Effects of epalrestat, an ARI, on CAT activity in sciatic nerve of DM rats.
Statistical analysis
Statistical analyses were performed using SPSS 17.0 software (SPSS, Chicago, IL, USA). Data are expressed as the mean ± SEM. One-way analysis of variance and the least significant difference post hoc test were used among groups. P < 0.05 was considered statistically significant.

Figure 4 Effects of epalrestat, an ARI, on GPX activity in sciatic nerve of DM rats.

Figure 5 Effects of epalrestat, an ARI, on AR activity in sciatic nerve of DM rats.
Results
Effects of epalrestat on serological indexes of DPN rats
Blood samples were collected at week 12 following streptozotocin injection in the control group and DM group. Blood glucose, low-density lipoprotein cholesterol, triglyceride, total cholesterol and glycated hemoglobin levels were elevated at week 12 following streptozotocin injection (P < 0.05, P < 0.01), whereas body weights of the DM group were lower than controls (Table 1), but the high-density lipoprotein cholesterol level was not significantly different from the control group.
Effects of epalrestat on sciatic nerve function of DPN ratsSciatic nerve conduction latency was slower in the DM group (1.47 ± 0.10 ms) than in the control group (1.13 ± 0.07 ms) (P < 0.01).
Effects of epalrestat on ultrastructure of cells of sciatic nerve of DPN rats
To study the neural cell changes in each group, the sciatic nerves were analyzed using electron microscopy. In the DPN (ARI-) group, myelin sheath cells became disordered in the medullated fibers of sciatic nerves. Shrinkage, deformation and inhomogeneity were observed in neurites. The structures of Schwann cells blurred and the vacuoles degenerated, but the integrity of cell membranes was not lost. Cells of non-myelinated nerves swelled, with irregular morphology and widened cellular spaces. Schwann cells shrank (Figure 1C). In the DPN (ARI+) group, the pathological structures of neurites and myelin were significantly improved. Deranged myelin sheath cells recovered and Schwann cell swelling was attenuated (Figure 1D).
SOD, CAT, GPX and aldose reductase expression in the streptozotocin-induced DPN rats and ARI-treated DPN rats
To confirm the SOD, CAT and GPX changes of ARI-treated DPN rats, western blot assay and immunohistochemistry were used to detect the protein levels at week 16 following streptozotocin induction. As illustrated in Figures 2-4, SOD, CAT and GPX protein levels were lower in the DPN (ARI-) group than in the nDM (ARI-) group. SOD, CAT and GPX protein levels were higher in the DPN (ARI+) group compared with the DPN (ARI-) group (P < 0.05). No significant differences in SOD, CAT and GPX protein levels were detected between the nDM (ARI-) and nDM (ARI+) groups (P > 0.05) (Figures 2A, 2B, 3A, 3B, 4A, 4B). Immunohistochemical results show that the SOD-, CAT-, and GPX-positive signals were mainly expressed in the cytoplasm and cell membranes, particularly in Schwann cell nuclei, Schwann cytoplasm, and myelin sheath in the DPN (ARI-) group (Figures 2C, 3C, 4C).
In DPN rats, the protein levels of aldose reductase significantly increased in sciatic nerves of DM rats in the DPN (ARI-) group (P < 0.05). There was a decrease in aldose reductase of the DPN (ARI+) group (P < 0.05). There was no significant difference in protein levels of aldose reductase between the nDM (ARI-) and nDM (ARI+) groups (P > 0.05; Figure 5A, B). Consistent with these observations, aldose reductase immunostaining revealed an accumulation of the aldose reductase protein in Schwann cells of sciatic nerve, while aldose reductase expression was low in the myelin sheath and axons in the DPN (ARI-) group (Figure 5C).
Discussion
In the present study, no significant difference in SOD activity was detected between the nDM (ARI-) and nDM (ARI+) groups. With the progression of DM, SOD protein expression levels reduced gradually, and oxidative stress damage aggravated, which led to DPN. After intragastric administration of epalrestat, SOD protein expression levels increased. Currently, it is believed that the decreased SOD concentration in various body tissues reduced the active oxygen radical scavenging ability. This in turn may function as a direct damaging factor in parenchymal cells and the mesenchyme, thereby leading to structural and functional damages in related tissues (Cui et al., 2008). Results from this study demonstrated that no apparent different CAT expression was found between the nDM (ARI-) and nDM (ARI+) groups. As the course of diabetes progressed to the DPN stage, CAT protein expression decreased and oxidative stress aggravated, contributing to DPN. Moreover, the protein expression levels of CAT increased with intragastric administration of epalrestat. There are studies, however, reporting that changes in CAT activity were quite distinct from the expected results (Babizhayev et al., 2014), with some reduced, some increased, or some unchanged significantly, which may result from different rat species, ages or disease durations (Yang et al., 2013). That finding suggested alternative reimbursement mechanisms for the enhancement of anti-oxidative stress. The findings in the present study revealed no evident difference in GPX expression between the nDM (ARI-) and nDM (ARI+) groups. As the course of diabetes progressed to the DPN stage, GPX protein expression levels reduced gradually and oxidative stress aggravated, resulting in DPN. Intragastric administration of epalrestat increased the protein expression level of GPX.
With the progression of DM, aldose reductase protein expression was gradually increased and oxidative stress aggravated, and resulting in DPN. However, the increase in aldose reductase protein expression was not quite significant. Subsequent intragastric epalrestat administration reduced the level of aldose reductase protein expression. Clinically the efficacy of ARIs is confirmed in attenuating peripheral neuropathy (Schemmel et al., 2010). However, the exact mechanisms and targets of ARIs have not been elucidated despite several reported studies (Obrosova et al., 2007). The exact role of ARIs is still an active topic in diabetes research (Colciago et al., 2002).
The present study focuses on biomarkers, including SOD, catalase, GPX and aldose reductase, which are closely related to DPN. By recording changes in the biomarkers after ARI therapy, the influence of ARIs on the biomarkers can be determined (Gabbay, 2004). Changes in the biomarkers for the occurrence of diabetic complications can provide evidence for specifying whether a certain biomarker is predictive of diabetic peripheral neuropathy (Huang et al., 1999). Damage caused by oxidative stress in type II DM is now universally recognized (Liu et al., 2013). Accordingly, antioxidant therapy is applied in clinics all over the world (Russo, 2010). However, very few studies have explored the best time for intervention with an antioxidant therapy (Kalekar et al., 2013). No reliable marker has yet been recognized as an indicator of the recovery of the balance between oxidants and antioxidants. It has been proposed that by analyzing the changes in the DPN biomarkers, the key target of ARI could be specified (Hosseini and Abdollahi, 2013). The effective biomarkers identified could be used as indicators in laboratory examinations for evaluating drug efficacy and nerve damage.
The results of the present study support other reports that antioxidase expression is decreased when DPN occurs (Kawai et al., 2010). As the disorder progresses, further decrease in antioxidase exacerbates reactive oxygen species damage (Yagi-hashi et al., 2001). Western blot assay and immunohistochemistry both indicated that SOD, CAT and GPX changed in the same direction, but aldose reductase changed in the opposite way. Aldose reductase was activated in DPN, but decreased after ARI administration. Aldose reductase, oxidative stress and subsequent metabolism, pathological and pathophysiological reactions jointly contributed to the occurrence and progression of DPN. This established the relationship of DM metabolic disorder as follows: aldose reductase activation - oxidative stress - vascular and nervous pathological changes - DPN. In summary, the present study provides experimental basis for how ARIs act to reduce DPN, reported elsewhere (Kawai et al., 2010). As a selective polyol pathway blocker, ARI can exclusively inhibit aldose reductase and reduce sorbitol accumulation in erythrocytes of DM patients and sorbitol and fructose accumulation in nervous tissues (Sullivan and Feldman, 2005). ARI can up-regulate the nitric oxide production of endothelial cells by inhibiting protein kinase pathways and down-regulate the expression of hyperglycemia-mediated neutrophil-endothelial adhesions and endothelial adhesions. Consequently, ARI can attenuate DPN.
Author contributions: WZ provided technical assistance. ZW designed the study. QRL, HLT, HYY and SRF performed experiments. RM, LX and LY analyzed data. YSL and QHS wrote the paper. All authors approved the final version of the paper.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Babizhayev MA, Strokov IA, Nosikov VV, Savel’yeva EL, Sitnikov VF, Yegor EY, Lankin VZ (2014) The role of oxidative stress in diabetic neuropathy: generation of free radical species in the glycation reaction and gene polymorphisms encoding antioxidant enzymes to genetic susceptibility to diabetic neuropathy in population of type i diabetic patients. Cell Biochem Biophys 71:1425-1443.
Brownlee M (2005) The pathobiology of diabetic complications: a unifying mechanism. Diabetes 54:1615-1625.
Chen K, Xu J, He H, Zhao L, Xiong J, Mo Z (2015) Protective effect of silibinin on islet beta cells in C57BL/6J mice fed a highfat diet. Zhong Nan Da Xue Xue Bao Yi Xue Ban 40:165-170.
Colciago A, Negri-Cesi P, Celotti F (2002) Pathogenesis of diabetic neuropathy--do hyperglycemia and aldose reductase inhibitors affect neuroactive steroid formation in the rat sciatic nerves? Exp Clin Endocrinol Diabetes 110:22-26.
Cui XP, Li BY, Gao HQ, Wei N, Wang WL, Lu M (2008) Effects of grape seed proanthocyanidin extracts on peripheral nerves in streptozocin-induced diabetic rats. J Nutr Sci Vitaminol (Tokyo) 54:321-328.
Gabbay KH (2004) Aldose reductase inhibition in the treatment of diabetic neuropathy: where are we in 2004? Curr Diab Rep 4:405-408.
Hosseini A, Abdollahi M (2013) Diabetic neuropathy and oxidative stress: therapeutic perspectives. Oxid Med Cell Longev 2013:168039.
Huang WC, Juang SW, Liu IM, Chi TC, Cheng JT (1999) Changes of superoxide dismutase gene expression and activity in the brain of streptozotocin-induced diabetic rats. Neurosci Lett 275:25-28.
Islam MS, Choi H (2007) Nongenetic model of type 2 diabetes: a comparative study. Pharmacology 79:243-249.
Jin HY, Lee KA, Song SK, Liu WJ, Choi JH, Song CH, Baek HS, Park TS (2012) Sulodexide prevents peripheral nerve damage in streptozotocin induced diabetic rats. Eur J Pharmacol 674:217-226.
Kalekar SA, Munshi RP, Thatte UM (2013) Do plants mediate their anti-diabetic effects through anti-oxidant and anti-apoptotic actions? an in vitro assay of 3 Indian medicinal plants. BMC Complement Altern Med 13:257.
Kawai T, Takei I, Tokui M, Funae O, Miyamoto K, Tabata M, Hirata T, Saruta T, Shimada A, Itoh H (2010) Effects of epalrestat, an aldose reductase inhibitor, on diabetic peripheral neuropathy in patients with type 2 diabetes, in relation to suppression of N(varepsilon)-carboxymethyl lysine. J Diabetes Complications 24:424-432.
Liu JF, Liu YH, Chen CM, Chang WH, Chen CY (2013) The effect of almonds on inflammation and oxidative stress in Chinese patients with type 2 diabetes mellitus: a randomized crossover controlled feeding trial. Eur J Nutr 52:927-935.
Obrosova IG (2009) Diabetes and the peripheral nerve. Biochim Biophys Acta 1792:931-940.
Obrosova IG, Ilnytska O, Lyzogubov VV, Pavlov IA, Mashtalir N, Nadler JL, Drel VR (2007) High-fat diet induced neuropathy of pre-diabetes and obesity: effects of “healthy” diet and aldose reductase inhibition. Diabetes 56:2598-2608.
Pedchenko VV, Malakhov VN (1991) Enzymatic methods for quantitative determination of total cholesterol in blood serum. Vopr Med Khim 37:85-91.
Polak G, Wertel I, Kwasniewski W, Derewianka-Polak M, Kotarski J (2013) The role of iron metabolism and oxidative stress in the pathogenesis of endometriosis. Ginekol Pol 84:62-64.
Rainwater DL, Ludwig MJ, Haffner SM, VandeBerg JL (1995) Lipid and lipoprotein factors associated with variation in Lp(a) density. Arterioscler Thromb Vasc Biol 15:313-319.
Russo AJ (2010) Increased serum Cu/Zn SOD in individuals with clinical depression normalizes after zinc and anti-oxidant therapy. Nutr Metab Insights 3:37-42.
Schemmel KE, Padiyara RS, D’Souza JJ (2010) Aldose reductase inhibitors in the treatment of diabetic peripheral neuropathy: a review. J Diabetes Complications 24:354-360.
Singh R, Kishore L, Kaur N (2014) Diabetic peripheral neuropathy: current perspective and future directions. Pharmacol Res 80:21-35.
Sullivan KA, Feldman EL (2005) New developments in diabetic neuropathy. Curr Opin Neurol 18:586-590.
Tarr JM, Kaul K, Chopra M, Kohner EM, Chibber R (2013) Pathophysiology of diabetic retinopathy. ISRN Ophthalmol 2013:343560.
Vincent AM, Calabek B, Roberts L, Feldman EL (2013) Biology of diabetic neuropathy. Handb Clin Neurol 115:591-606.
Yagihashi S, Yamagishi SI, Wada Ri R, Baba M, Hohman TC, Yabe-Nishimura C, Kokai Y (2001) Neuropathy in diabetic mice overexpressing human aldose reductase and effects of aldose reductase inhibitor. Brain 124:2448-2458.
Yang H, Fan S, Song D, Wang Z, Ma S, Li S, Li X, Xu M, Xu M, Wang X (2013) Long-term streptozotocin-induced diabetes in rats leads to severe damage of brain blood vessels and neurons via enhanced oxidative stress. Mol Med Rep 7:431-440.
Zhang XY, Liu JH, Cui Y, Tang P (2008) Electrophysiological examination of peripheral nerve injury and its significance in forensic medicine. Fa Yi Xue Za Zhi 24:280-283.
Copyedited by Dawes EA, Maxwell R, Wang J, Qiu Y, Li CH, Song LP, Zhao M
10.4103/1673-5374.177745 http://www.nrronline.org/
How to cite this article: Li QR, Wang Z, Zhou W, Fan SR, Ma R, Xue L, Yang L, Li YS, Tan HL, Shao QH, Yang HY (2016) Epalrestat protects against diabetic peripheral neuropathy by alleviating oxidative stress and inhibiting polyol pathway. Neural Regen Res 11(2):345-351.
Funding: The present study was supported by a grant from the National Natural Science Foundation of China, No. 81060141.
Accepted: 2015-12-22
*Correspondence to: Hong-ying Yang, M.D., yyh8657@163.com.
杂志排行
中国神经再生研究(英文版)的其它文章
- Tissue-type plasminogen activator is a modulator of the synaptic vesicle cycle
- Impaired consciousness caused by injury of the lower ascending reticular activating system: evaluation by diffusion tensor tractography
- Considering calcium-binding proteins in invertebrates: multi-functional proteins that shape neuronal growth
- Cardiovascular dysfunction following spinal cord injury
- Practical application of the neuroregenerative properties of ketamine: real world treatment experience
- Exergames: neuroplastic hypothesis about cognitive improvement and biological effects on physical function of institutionalized older persons
