Human neural stem cells promote proliferation of endogenous neural stem cells and enhance angiogenesis in ischemic rat brain
2016-12-02SunRyuSeungHoonLeeSeungKimByungWooYoon
Sun Ryu, Seung-Hoon Lee, Seung U. Kim, Byung-Woo Yoon,*
1 Department of Neurology and Clinical Research Institute, Seoul National University Hospital, Seoul National University, Seoul, Republic of Korea
2 Medical Research Institute, Chung-Ang University School of Medicine, Seoul, Republic of Korea
3 Department of Neurology, UBC Hospital, University of British Columbia, Vancouver, Canada
4 Medical Research Center, Seoul National University, Seoul, Republic of Korea
RESEARCH
Human neural stem cells promote proliferation of endogenous neural stem cells and enhance angiogenesis in ischemic rat brain
Sun Ryu1,4, Seung-Hoon Lee1,4, Seung U. Kim2,3, Byung-Woo Yoon1,4,*
1 Department of Neurology and Clinical Research Institute, Seoul National University Hospital, Seoul National University, Seoul, Republic of Korea
2 Medical Research Institute, Chung-Ang University School of Medicine, Seoul, Republic of Korea
3 Department of Neurology, UBC Hospital, University of British Columbia, Vancouver, Canada
4 Medical Research Center, Seoul National University, Seoul, Republic of Korea
Transplantation of human neural stem cells into the dentate gyrus or ventricle of rodents has been reportedly to enhance neurogenesis. In this study, we examined endogenous stem cell proliferation and angiogenesis in the ischemic rat brain after the transplantation of human neural stem cells. Focal cerebral ischemia in the rat brain was induced by middle cerebral artery occlusion. Human neural stem cells were transplanted into the subventricular zone. The behavioral performance of human neural stem cells-treated ischemic rats was significantly improved and cerebral infarct volumes were reduced compared to those in untreated animals. Numerous transplanted human neural stem cells were alive and preferentially localized to the ipsilateral ischemic hemisphere. Furthermore, 5-bromo-2′-deoxyuridine-labeled endogenous neural stem cells were observed in the subventricular zone and hippocampus, where they differentiated into cells immunoreactive for the neural markers doublecortin, neuronal nuclear antigen NeuN, and astrocyte marker glial fibrillary acidic protein in human neural stem cells-treated rats, but not in the untreated ischemic animals. The number of 5-bromo-2′-deoxyuridine-positive / anti-von Willebrand factor-positive proliferating endothelial cells was higher in the ischemic boundary zone of human neural stem cells-treated rats than in controls. Finally, transplantation of human neural stem cells in the brains of rats with focal cerebral ischemia promoted the proliferation of endogenous neural stem cells and their differentiation into mature neural-like cells, and enhanced angiogenesis. This study provides valuable insights into the effect of human neural stem cell transplantation on focal cerebral ischemia, which can be applied to the development of an effective therapy for stroke.
nerve regeneration; focal cerebral ischemia; middle cerebral artery occlusion; human neural stem cel ls; transplantation; differentiation; infarct size; behavioral analysis; endogenous neurogenesis; angiogenesis; rats; neural regeneration
Accepted: 2015-12-22
Introduction
Stroke is an acute cerebrovascular disorder caused by abnormal blood supply to the brain due to ischemia or hemorrhage, resulting in extensive loss of neurons and their connections in the damaged brain regions (Jin et al., 2005; Go et al., 2014). Current therapy for patients with stroke is restricted to fast vessel recanalization and pharmacological approaches based on thrombolytic drugs (The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group, 1995; Hacke et al., 2008). Despite considerable research efforts, few treatment options for stroke are presently available, and there is an urgent need for approaches that are clinically more effective.
Stem cell-mediated regeneration has emerged as a promising therapeutic strategy to promote regeneration after stroke (Lindvall et al., 2004). Several studies have demonstrated that transplanted neural stem cells (NSCs) restore neurological function and secretion of neurotrophic factors; furthermore, they were able to differentiate into mature neurons and even promote endogenous neurogenesis and angiogenesis in a cerebral ischemia model (Chu et al., 2004; Zhang et al., 2011; Mine et al., 2013). The role that neuroplasticity, neurogenesis, and angiogenesis play in mediating recovery following stroke has been recently reviewed (Ergul et al., 2012; Hermann and Chopp, 2012). However, only a few studies have investigated the relationship between the transplantation of exogenous NSCs and endogenous neurogenesis and angiogenesis after stroke (Jin et al., 2011; Zhang et al., 2011). Previous studies have shown that grafting of human stem/progenitor cells or neural stem cells into the dentate gyrus (DG) or ventricles of rodents promotes neurogenesis of endogenous NSCs (Munoz et al., 2005; Park et al., 2010). In this study, we examined whether human NSCs (hNSCs) directly transplanted into thesubventricular zone (SVZ), a neuroproliferative region, would promote endogenous neurogenesis and angiogenesis in the brain of ischemic rats, as intraventricularly injected NSCs can be transplanted into the ischemic brain without cell loss (Jin et al., 2005).
Materials and Methods
Focal ischemia model
Animal housing, care, and experimental procedures were carried out according to the guidelines of the Institutional Animal Care and Use Committee of Seoul National University Hospital, Korea. An effort was made to minimize the pain and suffering of the animals. Transient focal cerebral ischemia was induced in Sprague-Dawley male rats, weighing 270-300 g, by intraluminal thread occlusion of the left middle cerebral artery (MCAo) for 2 hours followed by reperfusion (Song et al., 2009). During brain ischemia, rectal temperature was maintained at 37 ± 0.5°C using a thermistor-controlled heated blanket.
hNSC preparation and transplantation
Immortalized human neural stem cell (HB1.F3) line (Kim et al., 2008) was grown in Dulbecco’s modified Eagle medium (Sigma, St. Louis, MO, USA) supplemented with 5% fetal bovine serum (FBS), 5% horse serum, 100 U/mL penicillin, 100 mg/mL streptomycin (Sigma), 20 ng/mL human epidermal growth factor (PeproTech, Rocky Hill, NJ, USA), and 10 ng/ mL human basic fibroblast growth factor (PeproTech), and transplanted 24 hours after ischemia. Rat models of cerebral ischemia (n = 7) were reanesthetized and placed in a stereotaxic frame (David Kopf instruments, Tujunga, CA, USA) with a rat head holder, and burr holes were drilled with a dental drill. Human NSCs (1.2 × 105cells/µL in PBS) were injected with a Hamilton syringe into the left lateral ventricle (-0.8 mm anterior to the bregma, 1.3 mm lateral to the midline, and 3.7 mm beneath the dura) in a volume of 5 µL over 5 minutes, and the needle was left for an additional 5 minutes after injection (Jin et al., 2005). The control group (n = 7) was injected with vehicle. Subsequently, bone wounds were sealed with bone wax, and the animals were returned to their cages. The rats were perfused with 4% paraformaldehyde in PBS 14 days after hNSC transplantation.
5-Bromo-2′-deoxyuridine (BrdU) administration
To detect cell mitosis, which is an indirect marker for cell proliferation, BrdU incorporation was used to label DNA-replicating cells. All rats (n = 14) received intraperitoneal injections of 50 mg/kg BrdU (Sigma, St. Louis, MO, USA) in saline daily for 7 days beginning 24 hours after hNSC transplantation, and BrdU labeling in the brain was analyzed by immunohistochemistry.
Behavioral test
One of the most common neurological scales used in animal studies of stroke is the modified Neurologic Severity Score (mNSS), which includes an assessment of motor (muscle status and abnormal movement), sensory (visual, tactile, and proprioceptive), and reflex (pinna, corneal, and startle) functions (Chen et al., 2001). The testing was performed before cerebral ischemia and at days 1, 7, and 14 after cerebral ischemia. Using the mNSS system with minor modifications, we graded the test results from 0 to 20 (normal score, 0; maximal deficit score, 20).
Quantitative RT-PCR
At 14 days after ischemia, the brains were removed, and the ipsilateral hippocampi were quickly frozen in liquid nitrogen and homogenized using a Polytron homogenizer. Total RNA was isolated using the RNeasy Lipid Tissue Kit (Qiagen, Valencia, CA, USA) and treated with DNase. Reverse transcription was performed using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) based on the manufacturer’s protocol. Primer sequences were designed using software program (Applied Biosystems) and synthesized commercially (Bioneer, Korea). Primers for proliferating cell nuclear antigen (Pcna) are as follows: forward, 5′-GAG CAA CTT GGA ATC CCA GAA CAG G-3′; reverse, 5′-CCA AGC TCC CCA CTC GCA GAA AAC T-3′. Gapdh was used as an internal control to normalize the expression levels of the target genes; the primers are as follows: forward, 5′-AAT GCA TCC TGC ACC ACC AA-3′; and reverse, 5′-GTA GCC ATA TTC ATT GTC ATA-3′. Quantitative PCR was performed with SYBR Green Master Mix (Applied Biosystems) using the ABI 7700 system (Applied Biosystems). Amplification was carried out under the following conditions: 20-second denaturation at 94°C followed by 1-minute annealing and extension at 62°C for 40 cycles. PCR products were then electrophoresed on 1% agarose gels and stained with ethidium bromide.
Measurement of infarct volume
Infarct volume was assessed 14 days post-ischemia. After cardiac perfusion-fixation with 4% paraformaldehyde in 0.1 M PBS, the brains were removed and cut into 40-μm-thick coronal sections by using a cryostat microtome. In total, six brain sections from each brain were mounted onto glass slides, and processed for Nissl staining; infarct volumes were measured using an image analysis program (Image-Pro Plus, Media Cybernetics, Silver Spring, MD, USA).
Immunohistochemistry

Figure 1 Transplanted hNSCs exhibited directional migration and differentiation in the ischemic lesion of rats.

Figure 2 Effects of hNSC transplantation on the behavior and infarct volume of ischemic rats.
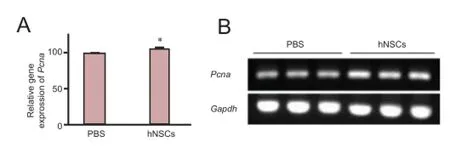
Figure 3 Transplanted hNSCs increased mRNA expression of Pcna in the hippocampus of ischemic rats.

Figure 6 Transplanted hNSCs promoted focal angiogenesis in the ischemic penumbra in ischemic rats.

Figure 4 Transplanted hNSCs promoted proliferation of endogenous NSCs in the SVZ of cerebral ischemic rats.

Figure 5 Transplanted hNSCs promoted proliferation of endogenous NSCs in the DG of the hoppocampus in ischemic rats.
For immunohistochemical staining with a BrdU-specific antibody, the slides were placed into 2 N HCl at 37°C for 10 minutes and then 0.1 M boric acid at room temperature for 3 minutes. After blocking in normal serum, sections were treated with mouse monoclonal anti-BrdU (Biodesign, SanDiego, CA, USA) diluted at 1:200 in phosphate buffered saline (PBS) at 4°C overnight. Following sequential incubation with biotinylated secondary anti-mouse IgG (dilution 1:200; Vector Laboratories, Inc., Burlingame, CA, USA), the sections were treated with an ABC kit (Vector Laboratories). DAB was then used as a sensitive chromogen for light microscopy. For immunofluorescence staining with a BrdU-specific antibody, the sections were first incubated in 1.5 M HCl for 30 minutes at37°C and then in blocking solution. For other immunohistochemical staining, the sections were incubated in blocking solution and then the following primary antibodies were used: mouse and sheep monoclonal anti-BrdU (1:200; Biodesign, San Diego, CA, USA), goat polyclonal anti-doublecortin (Dcx) to label migrating neuroblasts (1:100; Santa Cruz Biotechnology, Santa Cruz, CA, USA), mouse monoclonal anti-human nuclei antibody to label transplanted human neural cells (1:100; Chemicon International, Temecula, CA, USA), mouse monoclonal anti-neuronal nuclei antigen (NeuN) to label mature neurons (1:100; Chemicon International), rabbit polyclonal anti-glial fibrillary acidic protein (GFAP) to label mature astrocytes (1:1,000; Chemicon International), and rabbit monoclonal anti-von Willebrand factor (vWF) to label endothelial cells (1:200; Abcam, Cambridge, UK). The sections were incubated with the primary antibodies at 4°C for 16 hours. After washing, the sections were incubated for 2 hours at room temperature (22 ± 3°C) with the following secondary antibodies diluted at 1:200: Alexa 488-conjugated goat anti-mouse IgG (Molecular Probes, Eugene, OR, USA), Alexa 568-conjugated donkey anti-sheep IgG (Molecular Probes), FITC-conjugated goat anti-rabbit IgG (Jackson ImmunoResearch, West Grove, PA, USA), or rhodamine-conjugated donkey anti-goat IgG (Jackson ImmunoResearch). BrdU-positive cells and cells double-labeled with BrdU and NeuN, Dcx, GFAP, or vWF were counted in the lesioned hemisphere by using a laser scanning confocal microscope (Carl Zeiss, Weimar, Germany). All analyses were accomplished using objective counting methods.
Statistical analysis
The two-sample t-test was used to compare infarct volumes, behavioral data, mRNA expression and cell counts between the groups. Prism 5 for Windows (GraphPad Software Inc., La Jolla, CA, USA) was used for statistical analyses. The data are presented as the mean ± standard deviation (SD); and P < 0.05 was considered statistically significant.
Results
Transplanted hNSCs exhibited directional migration and differentiation in the ischemic lesion
We observed that hNSCs intraventricularly transplanted within the focal cerebral ischemic region migrated toward the ischemic striatum, mostly located in the infarct border zone (Figure 1B). To determine the differentiation potential of transplanted hNSCs in the ischemic brain, we conducted double labeling with antibodies specific to human nuclei (hNu) and GFAP or NeuN. We found that hNu-positive cells were located in the infarct border zone and confirmed that some of the hNu-positive cells were co-localized with the marker for mature neurons, NeuN, whereas a small percentage of hNu-positive cells expressed the astrocyte marker GFAP (Figure 1C, D). However, the transplanted hNSCs were not observed in the contralateral hemisphere.
Transplanted hNSCs enhanced recovery of neurological function and reduced infarct volume
We evaluated how hNSC transplantation influenced reurological function after cerebral ischemia using the mNSS tests in rats. At day 14 after transplantation, hNSCs-transplanted rats showed significantly improved behavioral scores compared with PBS-injected rats (Figure 2A; P < 0.01). Furthermore, when we measured the infarct volume, hNSCs-transplanted animals showed a reduced infarct area compared to PBS-injected rats (Figure 2B, C; P < 0.05).
Transplanted hNSCs promoted endogenous cell proliferation in rats with cerebral ischemia
To explore in detail the effect of transplanted hNSCs on cell proliferation, we analyzed the expression of Pcna in the hippocampus. At day 14 after transplantation, the level of Pcna mRNA in hNSCs-transplanted rats was significantly higher than that in the control PBS-injected rats (Figure 3; P < 0.05), suggesting an increase in cell proliferation. To further evaluate the influence of hNSC grafting on cell proliferation, we assessed the proliferation of newly generated cells in the SVZ and hippocampus. BrdU was administered daily for 7 days after hNSC transplantation and the proportion of BrdU-positive cells was measured at day 14 after transplantation. There were significantly more BrdU-positive cells in the ischemic hemisphere (the SVZ and DG) of hNSCs-transplanted rats than in PBS-injected rats (Figure 4A, B, P < 0.05; Figure 5A, B, P <0.05), whereas hNu were not detected, indicating that hNSCs did not migrate to these brain regions. The results demonstrate that transplanted hNSCs stimulated the proliferation of endogenous neural stem cells within the SVZ and DG of the hippocampus.
Transplanted hNSCs enhanced endogenous NSC differentiation
To analyze the differentiation potential of endogenous NSCs in rats after hNSC transplantation, we performed double labeling with the antibodies against BrdU and against Dcx, GFAP, or NeuN. BrdU-positive cells expressed Dcx, a marker for migrating neuronal cells. The total number of BrdU+/Dcx+(Figure 4C, D, P < 0.05; Figure 5C, D, P < 0.05) and BrdU+/ GFAP+(Figure 4G, H, P < 0.05; Figure 5G, H, P < 0.05) cells was significantly higher in the SVZ and DG of hNSC-transplanted rats than in PBS-injected rats, whereas the proportion of BrdU+/NeuN+cells was slightly higher in hNSCs-transplanted rats than in PBS-treated rats, although this difference was statistically insignificant (Figure 4E, F, P > 0.05; Figure 5E, F, P> 0.05). These results demonstrate that hNSC transplantation promoted the expansion and differentiation of endogenous rat NSCs within the SVZ and DG of the hippocampus.
Transplanted hNSCs promoted focal angiogenesis in rats with cerebral ischemia
To determine the effect of hNSC transplantation on the proliferation of endothelial cells in ischemic rats, we performed double labeling with BrdU and vWF antibodies. In ischemic rats treated with hNSCs, the number of BrdU+/vWF+cells in the ischemic penumbra was higher than that in the PBS-injected rats at day 14 post-MCAo (Figure 6, P < 0.05), indicating that hNSC transplantation enhanced angiogenesis after focal ischemicstroke.
Discussion
In the present study, we demonstrated the beneficial effect of NSC therapy on recovery after ischemic brain damage. Transplantation of hNSCs into the ipsilateral subventricular zone reduced brain infarct volume and enhanced functional recovery at day 14 after ischemic stroke, which was accompanied by increased proliferation of endogenous neural stem cells and angiogenesis.
Functional mechanisms and therapeutic effects of stem cells in stroke have been investigated using various delivery routes, including intravenous, intrastriatal, and intraventricular transplantation (Jin et al., 2005). Although intravenous delivery is the easiest route of stem cell administration, it yields very few donor cells in the ischemic brain region (Jin et al., 2005), prompting us to investigate more effective ways of NSC transplantation. We did not specifically compare the efficiency of NSC delivery by different routes; however, intracerebroventricular implantation of NSCs has been shown to be less invasive and to deliver more cells, resulting in increased cell engraftment compared to intrastriatal administration (Jin et al., 2005). Our data suggest that direct transplantation of hNSCs into the SVZ and DG of the hippocampus, which are neuroproliferative regions, is potentially a more effective delivery method that also promotes endogenous neurogenesis. The mechanism underlying the migration of intraventricularly transplanted human NSCs to the ischemic lesion remains unclear. Several studies have reported that NSCs have specific receptors activated by chemokines released in the injured site that govern cell migration towards the lesion (Kelly et al., 2004). However, the specific mechanisms involved in NSC chemotaxis after transplantation have yet to be investigated.
Our results indicate that transplanted hNSCs can survive, migrate toward damaged areas in the ischemic region, and differentiate into neural cells, resulting in a reduction in infarct lesion size and improved functional neuronal recovery. The exact mechanism underlying the functional improvement observed in the ischemic brain after NSC-based therapy remains unclear. Cell replacement was initially considered as the main mechanism responsible for the beneficial effect of NSC implantation because a number of animal studies have demonstrated that transplanted NSCs differentiate into neuronal and/ or glial phenotypes (Song et al., 2009). However, enhanced functional recovery could be observed even in the absence of neuronal differentiation (Hao et al., 2014), suggesting that cell replacement may not directly contribute to the functional neuronal improvement caused by NSC transplantation. Therefore, our focus in this study was not on the replacement of ischemia-damaged tissue with NSCs, but rather on functional augmentation, including proliferation of endogenous neural stem cells and angiogenesis.
We demonstrated that transplanted hNSCs stimulated the proliferation and differentiation of endogenous neural stem cells within the SVZ and DG of the hippocampus in rats at the acute phase of ischemic stroke (14 days). A previous study has shown that human neural precursor cell grafts increased the number of Dcx-positive cells in the SVZ of both young adult and aged rats with cerebral ischemia (Zhang et al., 2011). It has been demonstrated that NSC transplantation increased the number of BrdU-positive cells in the SVZ at days 7 and 14 after focal cerebral ischemia (Zhang et al., 2011), and that intrastriatal implantation of hNSCs promoted several stages of neurogenesis in the striatum of rats with cerebral ischemia (Mine et al., 2013). Our results were consistent with those of these previous reports.
The enhancement of endogenous neurogenesis contributes to the improvement of neuronal function after ischemia, whereas its ablation has negative effects on functional recovery (Jin et al., 2010; Wang et al., 2012). Our results, together with previous findings, suggest that stimulation of neurogenesis by NSC transplantation is associated with functional improvement after ischemic stroke.
Angiogenesis is coupled to neurogenesis and plays a critical role in neuronal repair (Shen et al., 2004). We observed that the number of BrdU+/vWF+cells in the ischemic region increased following hNSC transplantation, suggesting that hNSCs could promote angiogenesis in the acute phase of ischemic injury. Our results are consistent with those of a previous study that elucidated that NSC transplantation increased the proportion of BrdU+/vWF+cells in cortical peri-infarct regions at days 14 after focal cerebral ischemia; however, the effect was insignificant at later stages (Zhang et al., 2011). Further studies are required to investigate the specific mechanisms underlying hNSCs-induced stimulation of endothelial cells at different times after stroke. The finding that intracerebral transplantation of NSCs can induce angiogenesis is important because angiogenesis is implicated in the formation of neurovascular units and functional recovery after ischemic stroke and thus may significantly contribute to a favorable clinical outcome for stroke patients (Zhang et al., 2011).
In conclusion, we have shown that NSC transplantation promotes the proliferation of endogenous neural stem cells and enhances angiogenesis, suggesting that transplantation of NSCs provides a microenvironment that activates endogenous restorative mechanisms in the ischemic brain. Therefore, the results of the present study suggest that NSC-based therapy may offer an effective treatment for stroke patients. Further studies are required to identify the exact mechanisms underlying the therapeutic effects of transplanted hNSCs and to evaluate the functional outcome provided by NSC-based treatment after ischemic stroke.
The current study was limited in its focus on one phase of rat brain ischemia; however, we have demonstrated for the first time that intraventricular transplantation of hNSCs promotes neurogenesis in the acute phase of stroke in rats. Further work is needed to determine whether NSCs are therapeutic in later phases of cerebral ischemia and to test whether NSC therapy alters the expression of neurotrophins and angiogenic proteins in cerebral ischemia.
Author contributions: SR, SUK, and BWY designed this study. SR performed experiments and statistical analysis. SR and SHL analyzed experimental data. SR and BWY wrote the paper. Allauthors approved the final version of this paper.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M (2001) Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke 32:1005-1119.
Chu K, Kim M, Park KI, Jeong SW, Park HK, Jung KH, Lee ST, Kang L, Lee K, Park DK, Kim SU, Roh JK (2004) Human neural stem cells improve sensorimotor deficits in the adult rat brain with experimental focal ischemia. Brain Res 1016:145-153.
Ergul A, Alhusban A, Fagan SC (2012) Angiogenesis: a harmonized target for recovery after stroke. Stroke 43:2270.
Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, et al. (2014) Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation 129:e28-292.
Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, Larrue V, Lees K, Medeghri Z, Machnig T, Schneider D, Kummer R, Wahlgren N, Toni D (2008) Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 359:1317-1329.
Hao L, Zou Z, Tian H, Zhang Y, Zhou H, Liu L (2014) Stem cell-based therapies for ischemic stroke. BioMed Res Int 2014:468748.
Hermann DM, Chopp M (2012) Promoting brain remodelling and plasticity for stroke recovery: therapeutic promise and potential pitfalls of clinical translation. Lancet Neurol 11:369.
Jin K, Sun Y, Xie L, Mao XO, Childs J, Peel A, Logvinova A, Banwait S, Greenberg DA (2005) Comparison of ischemia-directed migration of neural precursor cells after intrastriatal, intraventricular, or intravenous transplantation in the rat. Neurobiol Dis 18:366-374.
Jin K, Xie L, Mao X, Greenberg MB, Moore A, Peng B (2011) Effect of human neural precursor cell transplantation on endogenous neurogenesis after focal cerebral ischemia in the rat. Brain Res 16:56-62.
Jin K, Wang X, Xie L, Mao XO, Greenberg DA (2010) Transgenic ablation of doublecortin-expressing cells suppresses adult neurogenesis and worsens stroke outcome in mice. Proc Natl Acad Sci U S A 107:7993-7998.
Kelly S, Bliss TM, Shah AK, Sun GH, Ma M, Foo WC, Masel J, Yenari MA, Weissman IL, Uchida N, Palmer T, Steinberg GK (2004) Transplanted human fetal neural stem cells survive, migrate, and differentiate in ischemic rat cerebral cortex. Proc Natl Acad Sci U S A 101:11839-11844.
Kim SU, Nagai A, Nakagawa E, Choi HB, Bang JH, Lee HJ, Lee MA, Lee YB, Park IH (2008) Production and characterization of immortal human neural stem cell line with multipotent differentiation property. Methods Mol Biol 438:103-121.
Lindvall O, Kokaia Z, Martinez-Serrano A (2004) Stem cell therapy for human neurodegenerative disorders-how to make it work. Nat Med 10:S42-50.
Mine Y, Tatarishvili J, Oki K, Monni E, Kokaia Z, Lindvall O (2013) Grafted human neural stem cells enhance several steps of endogenous neurogenesis and improve behavioral recovery after middle cerebral artery occlusion in rats. Neurobiol Dis 52:193-203.
Munoz JR, Stoutenger BR, Robinson AP, Spees JL, Prockop DJ (2005) Human stem/progenitor cells from bone marrow promote neurogenesis of endogenous neural stem cells in the hippocampus of mice. Proc Natl Acad Sci U S A 102:18171-18176.
Park DH, Eve DJ, Sanberg PR, Musso J 3rd, Bachstetter AD, Wolfson A, Schlunk A, Baradez MO, Sinden JD, Gemma C (2010) Increased neuronal proliferation in the dentate gyrus of aged rats following neural stem cell implantation. Stem Cells Dev 19:175-180.
Savitz SI, Rosenbaum DM, Dinsmore JH, Wechsler LR, Caplan LR (2002) Cell transplantation for stroke. Ann Neurol 52:266-275.
Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S (2004) Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science 304:1338-1340.
Song M, Kim YJ, Kim YH, Ryu S, Song I, Kim SU, Yoon BW (2009) MRI tracking of intravenously transplanted human neural stem cells in rat focal ischemia model. Neurosci Res 64:235-239.
The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group (1995) Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 333:1581-1587.
Wang X, Mao X, Xie L, Sun F, Greenberg DA, Jin K (2012) Conditional depletion of neurogenesis inhibits long-term recovery after experimental stroke in mice. PLoS One 7:e38932.
Zhang P, Li J, Liu Y, Chen X, Lu H, Kang Q, Li W, Gao M (2011) Human embryonic neural stem cell transplantation increases subventricular zone cell proliferation and promotes peri-infarct angiogenesis after focal cerebral ischemia. Neuropathology 31:384-391.
Copyedited by Radenovic L, Kim ST, Kim JH, Li CH, Song LP, Zhao M
10.4103/1673-5374.177739 http://www.nrronline.org/
How to cite this article: Ryu S, Lee SH, Kim SU, Yoon BW (2016) Human neural stem cells promote proliferation of endogenous neural stem cells and enhance angiogenesis in ischemic rat brain. Neural Regen Res 11(2):298-304.
Funding: This work was supported by the Korea Health Technology R&D Project, Ministry of Health & Welfare (HI12C0381), Republic of Korea.
*Correspondence to: Byung-Woo Yoon, M.D., Ph.D., bwyoon@snu.ac.kr.
orcid: 0000-0002-8597-807X (Byung-Woo Yoon)
杂志排行
中国神经再生研究(英文版)的其它文章
- Tissue-type plasminogen activator is a modulator of the synaptic vesicle cycle
- Impaired consciousness caused by injury of the lower ascending reticular activating system: evaluation by diffusion tensor tractography
- Considering calcium-binding proteins in invertebrates: multi-functional proteins that shape neuronal growth
- Cardiovascular dysfunction following spinal cord injury
- Practical application of the neuroregenerative properties of ketamine: real world treatment experience
- Exergames: neuroplastic hypothesis about cognitive improvement and biological effects on physical function of institutionalized older persons
