Chinese preparation Xuesaitong promotes the mobilization of bone marrow mesenchymal stem cells in rats with cerebral infarction
2016-12-02JinshengZhangBaoxiaZhangMeimeiDuXiaoyaWangWeiLi
Jin-sheng Zhang, Bao-xia Zhang, Mei-mei Du Xiao-ya Wang Wei Li
1 First Affiliated Hospital of Henan University of Traditional Chinese Medicine, Zhengzhou, Henan Province, China
2 Third Affiliated Hospital of Henan University of Traditional Chinese Medicine, Zhengzhou, Henan Province, China
RESEARCH
Chinese preparation Xuesaitong promotes the mobilization of bone marrow mesenchymal stem cells in rats with cerebral infarction
Jin-sheng Zhang1, Bao-xia Zhang2,*, Mei-mei Du2, Xiao-ya Wang2, Wei Li1
1 First Affiliated Hospital of Henan University of Traditional Chinese Medicine, Zhengzhou, Henan Province, China
2 Third Affiliated Hospital of Henan University of Traditional Chinese Medicine, Zhengzhou, Henan Province, China
Graphical Abstract
orcid: 0000-0002-8720-9055 (Jin-sheng Zhang)
After cerebral ischemia, bone marrow mesenchymal stem cells are mobilized and travel from the bone marrow through peripheral circulation to the focal point of ischemia to initiate tissue regeneration. However, the number of bone marrow mesenchymal stem cells mobilized into peripheral circulation is not enough to exert therapeutic effects, and the method by which blood circulation is promoted to remove blood stasis influences stem cell homing. The main ingredient of Xuesaitong capsules is Panax notoginseng saponins, and Xuesaitong is one of the main drugs used for promoting blood circulation and removing blood stasis. We established rat models of cerebral infarction by occlusion of the middle cerebral artery and then intragastrically administered Xuesaitong capsules (20, 40 and 60 mg/kg per day) for 28 successive days. Enzyme-linked immunosorbent assay showed that in rats with cerebral infarction, middle- and high-dose Xuesaitong significantly increased the level of stem cell factors and the number of CD117-positive cells in plasma and bone marrow and significantly decreased the number of CD54- and CD106-positive cells in plasma and bone marrow. The effect of low-dose Xuesaitong on these factors was not obvious. These findings demonstrate that middle- and high-dose Xuesaitong and hence Panax notoginseng saponins promote and increase the level and mobilization of bone marrow mesenchymal stem cells in peripheral blood.
nerve regeneration; Panax notoginseng saponin; Xuesaitong; bone marrow mesenchymal stem cell; cerebral infarction; mobilization; peripheral circulation; homing; nerual regeneration
Introduction
Bone marrow mesenchymal stem cells (BMSCs) are one of the main sources of endogenous stem cells. They are a type of multi-potent cell with non-immunogenic capacity and are ideal seed cells for gene and cell therapy. Moreover, BMSCs have strong mobilizing effects and increase the potential value of cells for clinical applications (Maijenburg et al., 2011; Zhang et al., 2012). There is evidence that when cerebral ischemia occurs, BMSCs are mobilized from the bone marrow to the focal point through peripheral circulation and initiate tissue regeneration(Maijenburg et al., 2011). The agents of mobilization multiply and drive stem cells from the bone marrow pool to the circulating pool and then enable stem cells to enter the blood circulation through the basilar membrane and vascular endothelium (Zhang, 2011). However, there are three unsolved problems: an insufficient number of homing BMSCs differentiate into specific neurons for functional repair; the low survival rate of homing BMSCs in the infarct area; and most homing BMSCs die within 7 days because of a lack of blood supply and insufficient nutrition in the infarct area. Therefore, a valid method is needed to stimulate neovascularization in the infarct area and increase the survival rate of BMSCs (Qiu and Jin, 2008; Roemeling-van Rhijn et al., 2012). The key to realizing stem cell therapy in clinical applications is to encourage more BMSCs into the bloodstream. Some traditional Chinese medicines have proved useful in the mobilization of stem cells (Wang et al., 2009; Yuan et al., 2012). Activating blood and resolving blood stasis is effective for stem cell homing from multiple aspects; it can improve the microenvironment of the infarct area, increase the survival rate of stem cells in the infarct area and promote the differentiation of stem cells into nerve cells (Zhang et al., 2014a). Panax notoginseng saponins (PNS), one of the main chemical constituents of Xuesaitong capsules (Zhang et al., 2014b), can decrease intracellular adhesion molecule-1 expression and increase vascular endothelial growth factor and vascular endothelial growth factor receptor expression in the peripheral blood of patients with acute cerebral infarction (Guo and Li, 2010; Huang et al., 2014) and improve neurological function deficits after cerebral ischemia. We investigated the effects of PNS on the mobilization of BMSCs after cerebral infarction.
Materials and Methods
Experimental animals
Two hundred healthy specific pathogen-free grade adult male Sprague-Dawley rats weighing 280-300 g and aged 3 months were purchased from Beijing Vital River Company, China (license No. SCXK (Jing) 2006-0009) and used in this study. All rats were raised for 1 week at the Experimental Animal Center, Henan University of Traditional Chinese Medicine, China before use. They were randomly divided into five groups with 40 rats per group: normal, middle cerebral artery occlusion (MCAO), and low-, middle-, and high-dose Xuesaitong groups. All animal experiments were approved by the Animal Ethics Committee. All efforts were made to minimize the suffering and number of rats used in this study.
Establishment of MCAO rat models
MCAO was induced by a method described by Longa et al. (1989). Rats were anesthetized with chloral hydrate (35 mg/ 100 g, i.p.). A median incision was cut on the neck to expose the left common carotid (CCA), external carotid artery (ECA) and internal carotid artery (ICA). A nylon thread was carefully inserted from the CCA into the ICA and advanced towards the origin of the middle cerebral artery until a slight resistance was felt. The thread was inserted towards the bifurcation of the ICA and ECA at a depth of 18 ± 0.5 mm. After 2 hours of MCAO, the intraluminal filament was withdrawn until a slight resistance was felt. This indicated that the tip of the nylon thread was back to the ICA and that reperfusion was accomplished. After recovering from anesthesia, the rats were continuously given penicillin 40,000 units (i.m.) for 3 days during which sufficient food and water were provided. Rats that met all of the following criteria were included for further experiments: survived the first 24 hours after surgery; weight decreased after 24 hours; left Horner’s syndrome; and right neurologic impairment.
Drug administration
Xuesaitong capsules (main ingredient: PNS, 100 mg/capsule; batch No. Z20040016) were purchased from Kunming Pharmaceutical Corporation (KPC, Kunming, Yunnan Province, China) and dissolved into normal saline at concentrations of 20, 40 and 60 mg/mL.
One day after MCAO induction, rats in the normal and MCAO groups were administered normal saline (1 mL/ 100 g, i.g.) every day. Rats in the low-, middle- and high-dose Xuesaitong groups were administered Xuesaitong (20, 40, 60 mg/ mL, i.g., respectively) until all rats were sacrificed.
Harvesting peripheral blood and bone marrow
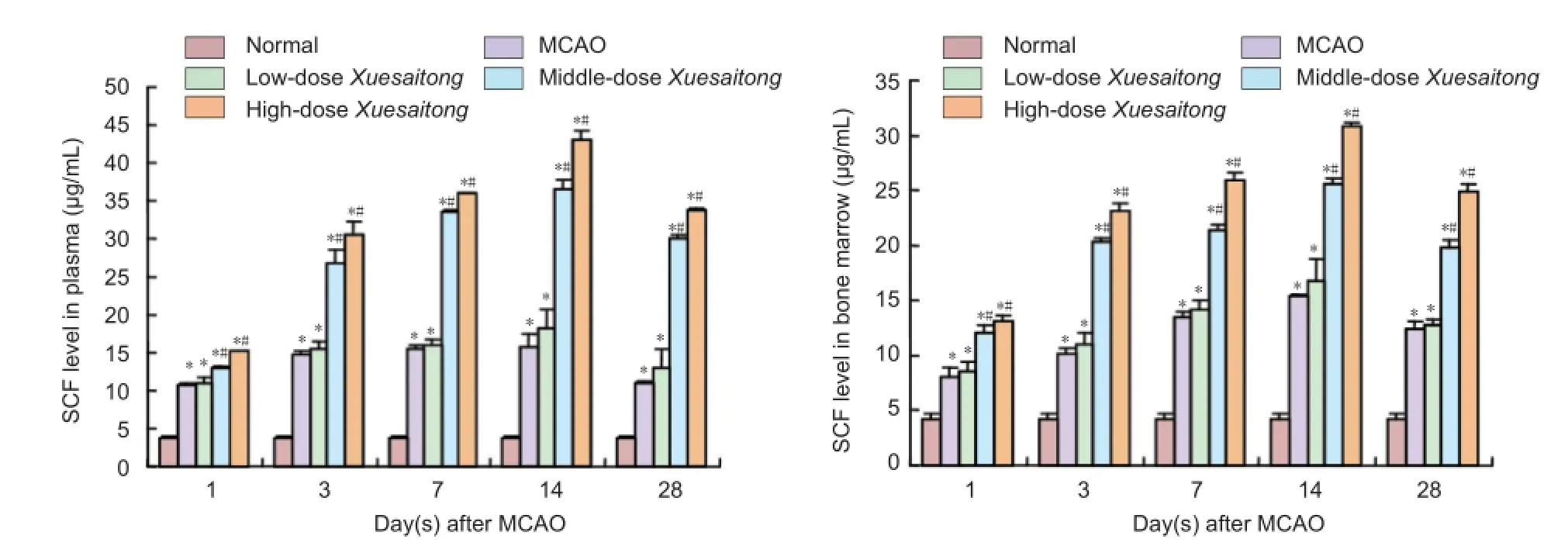
Figure 1 Effect of Xuesaitong (Panax notoginseng saponins [PNS]) on stem cell factor (SCF) level in plasma and bone marrow of rats with cerebral infarction.
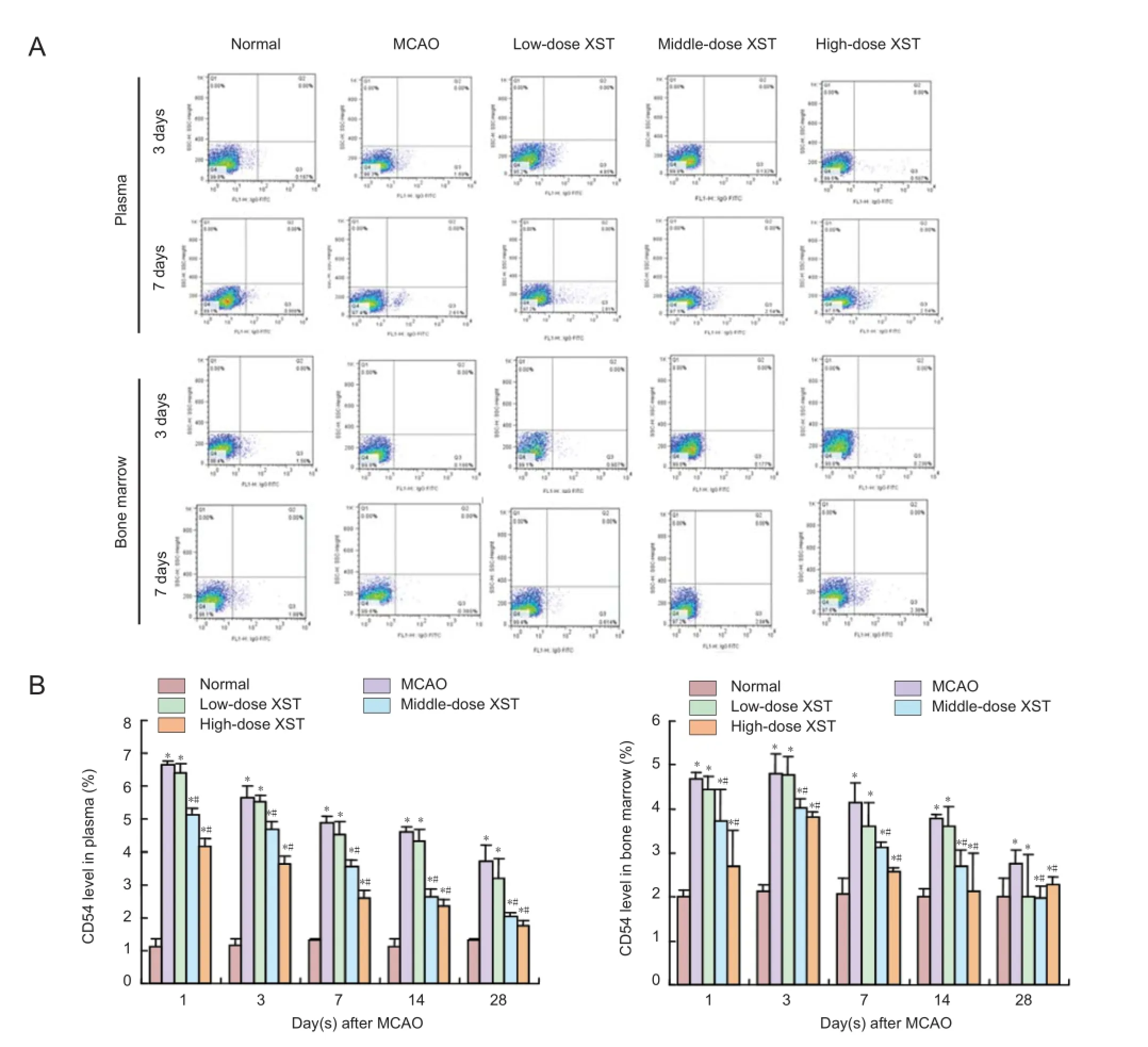
Figure 2 Effect of Xuesaitong (Panax notoginseng saponins [PNS]) on the number of CD54-positive cells in plasma and bone marrow of rats with cerebral infarction.
At 1, 3, 7, 14 and 28 days after MCAO induction, eight rats per group were selected for harvesting peripheral blood and plasma. The blood and plasma were collected from the abdominal aorta. Plasma was used for enzyme-linked immunosorbent assay and blood for flow cytometry. Rats were sacrificed and the thigh bone was exposed. The bone marrow of the thigh bone was flushed out with buffer (3.8 mL of Dulbecco’s modified Eagle’s medium and 0.2 mL of heparin sodium). The samples were then filtered through 40-µm mesh filters and centrifuged at 1,500 r/min for 10 minutes. The supernatant was then collected and kept at -80°C. Sediments were diluted with 250 μL of PBS and recollected for testing.
ELISA assay
The levels of stem cell factors (SCF) in plasma and bone marrow were measured using ELISA kits. The measurements were performed step-by-step as outlined in the protocol booklet of the ELISA kit (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) and according to the manufacturer’s specification.
Flow cytometry assay
Approximately 50 μL of plasma or bone marrow liquid was placed into the flow cytometry chamber (BD Pharmingen, San Diego, CA, USA). CD106-PE, CD54-FITC, CD117-FITC (Beijing Solarbio Science & Technology Co., Ltd., China) and control reagent were added afterwards. 1 × 105cells were counted in each sample and the percentages of CD54, CD106,and CD117-positive cells were calculated using CellQuest Pro software (Olympus, Tokyo, Japan).

Figure 3 Effect of Xuesaitong (Panax notoginseng saponins [PNS]) on the number of CD106-positive cells in plasma and bone marrow of rats with cerebral infarction.
Statistical analyses
All collected data were analyzed using statistical software SPSS Version 17.0 (SPSS, Chicago, IL, USA). Analysis of variance (ANOVA) was used to find the significance of study parameters between groups. All results were expressed as the mean ± SD. P < 0.05 was considered statistically significant.
Results
Effect of PNS on the SCF levels in plasma and bone marrow in rats with cerebral infarction
SCF levels in plasma and bone marrow were significantly higher in MCAO, low-, middle-, and high-dose Xuesaitong groups compared with the normal group (P < 0.05). Plasma and bone marrow SCF levels in the MCAO group began to increase 1 day after MCAO, reached a peak at 14 days after MCAO, and gradually decreased thereafter. Plasma and bone marrow SCF levels in the middle- and high-dose Xuesaitonggroups increased significantly compared with the MCAO group. There was no significant difference in plasma and bone marrow SCF level between low-dose Xuesaitong and MCAO groups (P > 0.05; Figure 1).

Figure 4 Effect of Xuesaitong (Panax notoginseng saponins [PNS]) on the number of CD117-positive cells in plasma and bone marrow of rats with cerebral infarction.
Effect of PNS on the number of CD54- and CD106-positive cells in plasma and bone marrow of rats with cerebral infarction
Flow cytometry results showed that the number of CD54- and CD106-positive cells in plasma and bone marrow of rats in the MCAO, low-, middle-, and high-dose Xuesaitong groups was significantly higher than in the normal group (P < 0.05).
The number of CD54- and CD106-positive cells in plasma and bone marrow of rats in the MCAO and low-, middle-, and high-dose Xuesaitong groups increased to a peak level 1 day after MCAO and then gradually reduced thereafter. At different time points, the number of CD54- and CD106-positive cells in plasma and bone marrow in the middle- and highdose Xuesaitong groups was significantly lower than that in the MCAO group, and the reduction in the low-dose Xuesaitong group was the most significant (P < 0.05). There was no significant difference in the number of CD54- and CD106-positive cells in plasma and bone marrow between low-dose Xuesaitong and MCAO groups at each time point (P > 0.05; Fig-ures 2, 3).
Effect of PNS on the number of CD117-positive cells in plasma and bone marrow of rats with cerebral infarction
Flow cytometry results showed that the number of CD117-positive cells in plasma and bone marrow of rats in the MCAO, low-, middle-, and high-dose Xuesaitong groups was significantly higher than in the normal group (P < 0.05). Plasma and bone marrow SCF levels in the MCAO group began to increase 1 day after MCAO and reached a peak at 14 days. The number of CD117-positive cells gradually decreased thereafter. The number of plasma and bone marrow CD117-positive cells in the middle- and high-dose Xuesaitong groups increased more significantly than in the MCAO group. There was no significant difference in the number of CD117-positive cells in plasma and bone marrow between low-dose Xuesaitong and MCAO groups (P > 0.05; Figure 4).
Discussion
Recently, multiple factors such as chemotactic factors, growth factors and adhesion molecules have been discovered to be involved in the mobilization of BMSCs. However, tissue damage and external effects such as drugs can interfere with the process of mobilization. SCFs are considered to be one of the most powerful agents of mobilization and can drive more stem cells into plasma and greatly contribute to stem cell migration, accumulation and multiplication (Li et al., 2011; Baker et al., 2013).
Adhesion molecules such as CD106 and CD54 are common surface receptors of BMSCs that mediate the adhesion of hematopoietic cells and play an important role in information transmission between the two. Wang and Cheng (2009) discovered an obvious increase in serum CD106 and CD54 in patients with acute cerebral ischemia. The difference in serum CD106 and CD54 levels in the recovery phase remained significant compared with the control group. In addition, increased levels of CD106 and CD54 were positively correlated with the size of the infarct area. Yuan et al. (2012) found that the components of Yangxintongmai, an effective recipe for treatment of blood stasis syndrome of cardiovascular disease, could mobilize BMSCs into plasma and guide them to the myocardium infarct.
Our study showed that PNS stimulated the production of SCF from bone marrow and promoted the mobilization of BMSCs into plasma by reducing the adhesion effects of adhesion molecules on BMSCs. This phenomenon supports the traditional Chinese medicine theory of promoting blood circulation to remove blood stasis and thereby initiating tissue regeneration (Zhang, 2012a, b).
Author contributions: MMD and XYW provided and integrated the data. JSZ conceived and designed this study, analyzed the data, and was responsible for fundraising. BXZ wrote the paper. JSZ and WL authorized the paper and performed statistical analyses. WL provided assistance in technique application. All authors approved the final version of this paper. Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Baker DJ, Dawlaty MM, Wijshake T, Jeganathan KB, Malureanu L, van Ree JH, Crespo-Diaz R, Reyes S, Seaburg L, Shapiro V, Behfar A, Terzic A, van de Sluis B, van Deursen JM (2013) Increased expression of BubR1 protects against aneuploidy and cancer and extends healthy lifespan. Nat Cell Biol 15:96-102.
Guo ZC, Li L (2010) Effects of Breviscapine and Panax Notoginseng Saponins in ICAM-1 expression in rats with acute cerebral infarction. Zhongxiyi Jiehe Xinnao Xueguan Bing Zazhi 8:1213-1215.
Huang NF, Tang GC, Yu ZH, Yan ZS, Tang SW, Chen Q, Li WZ, Lao XL, Huang Y, Fang CL, Song ZQ (2014) Effect of Panax Notoginseng Saponins in the concentration of plasma VEGF and VEGFR of the patients with acute cerebral infarction. Shenjing Sunshang yu Gongneng Chongjian:388-390.
Li S, Zhang K, Wang Z (2011) Influential factors in mobilization of endothelial progenitor cells. Shengming de Huaxue 31:779-784.
Longa EZ, Weinstein PR, Carlson S, Cummins R (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20:84-91.
Maijenburg MW, van der Schoot CE, Voermans C (2011) Mesenchymal stromal cell migration: possibilities to improve cellular therapy. Stem Cells Dev 21:19-29.
Qiu L, Jin XQ (2008) Biological characteristics and clinical application of bone marrow mesenchymal stem cells. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu 12:2347-2350.
Roemeling-van Rhijn M, Weimar W, Hoogduijn MJ (2012) Mesenchymal stem cells: application for solid-organ transplantation. Curr Opin Organ Transplant 17:55-62.
Wang C, Zhang YJ, Feng Y, Zhuang PW, Wang J, Liu H, Wang LL (2009) Effect of astragaloside IV on neurogenesis in adult hippocampus of rats after transient forebrain ischemia. Zhongcaoyao 40:754-758.
Wang XX, Cheng GP (2009) Expression changes and clinical significance of TNF-α,VCAM-1 and MCP-1 in the serum of patients with acute cerebral infarction. Xin Nao Xueguan Bing Fangzhi 9:359-360.
Yuan ZK, Huang XP, Li YH, Zheng JH, Wang LP, Jian WX, Sun GX, Wang P, Huang B, Yu SR (2012) Experimental study of Yangxin Tongmai effective components recipe promoting bone marrow mesenchymal stem cells homing to infarcted myocardium in rats. Zhonghua Zhongyiyao Zazhi 27:2321-2325.
Zhang JS (2011) Effect of study the theory of bone marrow stem cells recycle to the application of clinic and foundation. Zhongguo Shiyan Fangji Xue Zazhi 17:263-264,266.
Zhang JS (2012a) Huoxuehuayu and atem cell cycle. Zhongyi Zazhi 53:451-454.
Zhang JS (2012b) Connotation of dispelling stasis and producing the new in theory of activating blood and resolving stasis therapy. Beijing Zhongyiyao Daxue Xuebao 35:230-232.
Zhang JS, Zhang BX, Du MM, Wang XY, Fan HH (2014a) Effects of Xuesaitong capsules on the repair and regeneration of autologous neural stem cells in infarcted brain tissue of rats. Zhongyi Zazhi 55:1494-1497.
Zhang JS, Zhang BX, Zhu HF, Du MM, Zhang YY (2014b) Panax notoginseng saponins promote proliferation and differentiation of CD105+-BMSC homing to cerebral infarction marginal zone. Beijing Zhongyiyao Daxue Xuebao 37:777-780.
Zhang JY, Liu G, Ma D, Fu WL, Yu JK (2012) Mobilization, culture, isolation and identification of mesenchymal stem cells from peripheral blood of Sprague-Dawley rats. Zhongguo Yundong Yixue Zazhi 31:811-816.
Copyedited by Paul P, Raye W, Yu J, Li CH, Song LP, Zhao M
10.4103/1673-5374.177738 http://www.nrronline.org/
How to cite this article: Zhang JS, Zhang BX, Du MM, Wang XY, Li W (2016) Chinese preparation Xuesaitong promotes the mobilization of bone marrow mesenchymal stem cells in rats with cerebral infarction. Neural Regen Res 11(2):292-297.
Funding: This study was financially supported by a grant from Henan Medical Science and Technology Innovative Talents Project in 2010, No. 1041000510010.
Accepted: 2015-08-24
*Correspondence to: Bao-xia Zhang, 1064979341@qq.com.
杂志排行
中国神经再生研究(英文版)的其它文章
- Tissue-type plasminogen activator is a modulator of the synaptic vesicle cycle
- Impaired consciousness caused by injury of the lower ascending reticular activating system: evaluation by diffusion tensor tractography
- Considering calcium-binding proteins in invertebrates: multi-functional proteins that shape neuronal growth
- Cardiovascular dysfunction following spinal cord injury
- Practical application of the neuroregenerative properties of ketamine: real world treatment experience
- Exergames: neuroplastic hypothesis about cognitive improvement and biological effects on physical function of institutionalized older persons
