Effects of chemical and physical cues in enhancing neuritogenesis and peripheral nerve regeneration
2016-12-02CaseyD.Sigerson,ChristopherJ.Dipollina,MicheleFornaro
PERSPECTIVE
Effects of chemical and physical cues in enhancing neuritogenesis and peripheral nerve regeneration
Peripheral nerve injuries are most often a consequence of axonal damage leading to functional losses that may vary from lack of sensitivity distal to the site of injury to complete loss of motor control. The consequences of such injuries are immense because of the high cost of health care, limited ability to work and emotional trauma that follows the injury.
The spontaneous regrowth that the peripheral nervous system (PNS) undergoes is frequently inadequate to fully restore function. Copious efforts have been invested in studying the mechanism of neuritogenesis and axonal growth as well as the pathology of nerve injuries and the body’s system of repair.
Developments in science have begun to define the role of individual cells in the first step of natural injury response known as Wallerian degeneration (WD). Briefly, WD takes place for 24-48 hours and includes breakdown of the axon and myelin sheath distal to the injury and proliferation of neuroglia (Camara-Lemarroy et al., 2010). As degradation begins, the epineurial nerve-blood barrier is disrupted and constituents of the blood are able to flood the area leading to successive steps in degeneration. As a point of interest, during concurrent degeneration of damaged nerves, the neighboring nerves have actually been shown to undergo hypertrophy (Mizisin and Weerasuriya, 2011). The juxtaposition of destructive and constructive action in close proximity, (assumedly) under the influence of similar biochemical microenvironments, is not well understood. Targeted research focused on this mechanism could prove useful in decoding the proper methods for achieving full regeneration in the future.
Following degeneration, macrophages enter with the influx of blood and allow for phagocytosis of nonfunctional myelin sheaths (Camara-Lemarroy et al., 2010). Interleukin-1 (IL-1) secreted from these macrophages activates Schwann cells (SC) and causes them to proliferate. Furthermore, IL-1 can up-regulate SC-mediated release of nerve growth factor (NGF) which further accelerates growth of the nerve stump (Camara-Lemarroy et al., 2010). As more SCs are attracted to the site, they begin to encapsulate the axon forming cylindrical cavities known as Bands of Büngner. This process will continue until the new neurite reaches its target in the periphery. These Bands of Büngner are natural conduits that provide nourishment, protection and a pathway for regeneration. The proximal stump undergoes a process of retrograde degeneration, debridement, and propagation. The stump initially retracts due to elastic fibers within the epineurial sheath. The myelin sheath undergoes retrograde degeneration for several nodes of Ranvier. The degenerated material is debrided by macrophages, and proliferating Schwann cells regenerate to form a glial conduit for the peripheral stump.
The cell bodies contributing to both sensory and motor fibers peripherally lesioned undergo a series of changes that lead to either increased metabolism and attempted regeneration or apoptosis and cell death (Muratori et al., 2014). The amount of metabolism initiated is inversely proportional to the distance from cell body to proximal stump.
Complete regeneration may fail due to a multitude of issues. It is most commonly insufficient when there is a significant distance between the proximal and distal stump. Scar formation impeding propagation and loss of directionality are also important problems to consider in returning function to the end-organ of interest. Every single aspect of nerve fibers degeneration and regeneration was previously targeted by researcher in the field of regenerative medicine in order to find successful approach to promote neuritogenesis and axonal growth.
Growth, development and repair of the peripheral nerves is highly regulated by soluble factors with a particular role played by a family of molecules called neurotrophins (Boyd and Gordon, 2003). These molecules include NGF, brain derived neurotrophic factor (BDNF), neurotrophin 3 (NT-3) and neurotrophin 4/5 (NT-4/5) (Boyd and Gordon, 2003). Neurotrophins act as a signaling molecule by binding both p75 and tyrosine kinase receptors (trk) (Boyd and Gordon, 2003). The trk receptors seem to have a greater level of specificity than p75. For example, NGF binds to trkA and BDNF to trkB, whereas p75 binds more generally allowing activation of its pathways with all neurotrophins and other signaling molecules. Trk receptor binding causes dimerization and autophosphorylation of internal tyrosine residues, leading to docking of additional messenger proteins, including grb2 and GAB-1 (Boyd and Gordon, 2003). The end result of neurotrophin signaling is promotion of differentiation, survival of the cell and neuritogenesis. All of the possible results are essential when considering the condition of injury and subsequent repair (Boyd and Gordon, 2003). On the other hand, the function of the p75 receptor is not as well understood. Unlike trk, the physiological response of p75 can vary based on the developmental stage of the cell and the ligand that is stimulating the receptor (Boyd and Gordon, 2003). p75 usually binds TNF-alpha as a ligand leading to the release of ceramide, a pre-apoptotic factor. p75 can therefore lead to suppression of the neuritogenesis. Conversely, when NGF activates this receptor there is a wide variety of outcomes that can be initiated, some of which oppose apoptosis. The variety of effector enzymes that are induced can lead to survival of sensory neurons (Boyd and Gordon, 2003). Additionally, p75 may induce neurite growth by down regulating RhoA, a GTPase that has been implicated in suppression of growth cone development and consequently axonal growth (Boyd and Gordon, 2003).
Meanwhile, other studies have been focusing more specifically on the milieu in which axonal growth occurs and particularly but not exclusively, the extracellular matrix (ECM). The ECM provides the physical and chemical environment that allows regeneration to occur. It has been shown to control cell differentiation and cell survival. Moreover, fibrous materials such as laminin and fibronectin seem to be the most influential in promoting cell adhesion and growth, with a combination of both being most beneficial.
Autografts of venous or muscular tissue “conduits” have recently been used to promote regeneration of axons following injury and are considered to be the gold standard for clinical repair. The tunnel like conformation of these tissues provides an excellent guide for the extending neurite. Muscular tissue shares an ECM composition similar to endoneurium and its straight continuous basal lamina is suitable for bridging short gaps of degenerated axon (Dubovy et al., 2013). There is also the added benefit of not experiencing host-recipient immunological reactions, but unfortunately source material for autografting is inherently limited (Dubovy et al., 2013). The alternatives include synthetic materials that can be made to fix growth factors and contain the proper ECM signaling molecules found in vivo and the use of nanoparticles to carry these factors and define specific gradient of release. These factors can even be made in a bioabsorable form that will allow for clearance from the body after a certain period of time, reducing the chances of chronic inflammation.
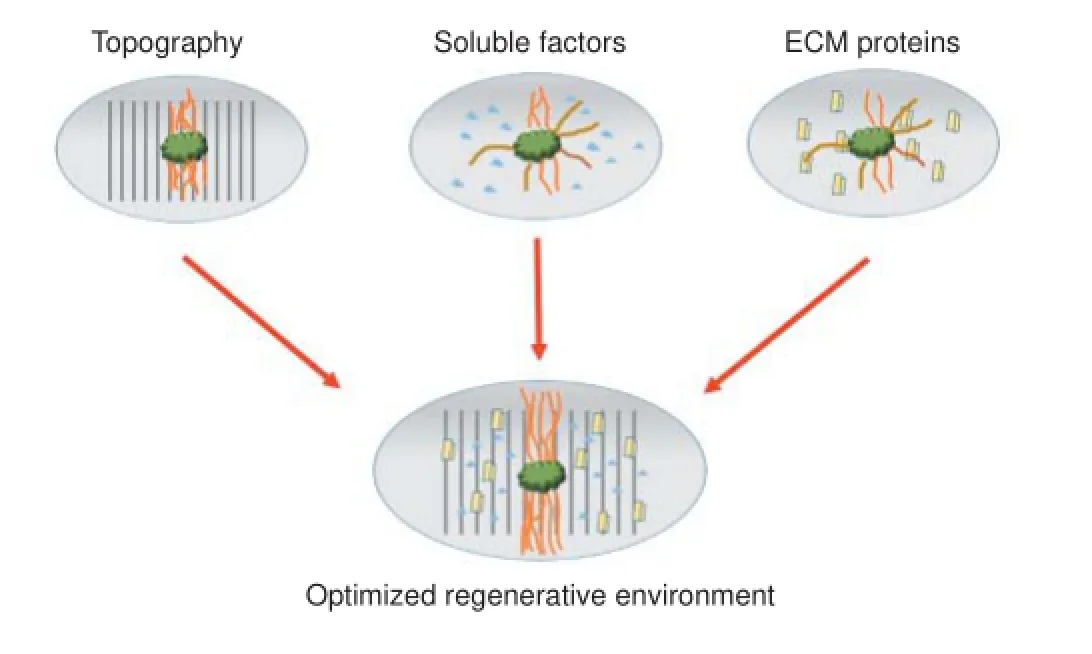
Figure 1 Optimization of multiple factors in peripheral nerve regeneration.
Additionally, new approaches have been investigated to promote axonal growth and nerve repair. It has been known for a long time that the growth cones of axons are influenced by biochemical cues such as soluble molecules, growth factors and components of the extracellular matrix. More recently, physical cues provided by the structure of the surface have also been proven to influence the growth cones. The type of material, texture, surface coating and feature size of topographically patterned sizes seems to play a role in effecting axonal growth and directionality. This sort of phenomenon is known as “contact guidance” or “contact inhibition”. It is a reflexive response in which the growth cone will change direction in response to impediments or cell surface cues it encounters. In the natural environment, different types of collagen, laminin and fibronectin form the structures on which the neurites grow. The arrangement and geometries of these proteins will influence the path of the growth cone and bound cues like netrin, ephrins and semaphorins will also influence the cone (Spedden et al., 2014). The finding that physical cues are equally important has led to an increase in studies in which explants are grown on specially engineered topographic patterns. These patterns include repeated rows of parallel lines, gaps and ridges (Spedden et al., 2014). The ability to manufacture and reproduce nanoscale topography on implantable materials has opened a new method of enhancing neuritogenesis and peripheral nerve regeneration. Many different avenues within this domain are currently being explored. Chemical and physical cues have been adopted to create bioengineered and biomimetic tridimensional scaffolds for nerve repair and axonal growth.
Artificial fibrils have been useful in coordinating the movement of SCs during nerve repair. Silicone and synthetic polymers have been used to form fibrin cables that bridge nerve gaps in rats that are 10-12 mm, these cables facilitate the migration of SCs creating the Bands of Büngner. Such a quality would be favored in vivo as the neuron relies on the SCs heavily for regeneration. The inside of the conduit could be impregnated with growth and chemotactic factors and covered in a topography that would promote axonal regrowth and control direction. Directional fibers have been shown numerous times to demonstrate significant improvement in alignment of both neural and Schwann cells. The cells align parallel with the fibers but do not necessarily run directly with one fiber (Spedden et al., 2014). Conduits containing multiple channels 450 µm in diameter have also been shown to increase the number of regenerating axons delivered over greater distances.
Natural and synthetic nerve regrowth conduits are being used to some extent but this is an area that shows room for significant development in the future. During nerve regeneration, neurites can propagate without definitive direction. This can lead to re-innervation failure and neuroma formation. Nerve conduits aim to channel and direct the growth so that natural regeneration is as effective as possible. While this is a good idea in theory, products that are currently available for clinical use often have poor outcomes and can only be used for short distances (30 mm or less; Pabari et al., 2014) .
An ideal synthetic regrowth conduit could increase the distance over which recovery can occur, block the infiltration of impeding scar tissue, and guide the propagating axons directly to their target.
Currently studied topographical features include grooves, fibers, scaffolds, and repeating projections. The ultimate goal of these is to enhance regeneration to the point that donor nerves are not needed.
Specific groove shapes can further enhance the regenerative effect on recovering neurites. For example, a study demonstrated that 40 degree sloped-wall grooves gave significantly better results than square grooves (Mobasseri et al., 2014). While this does not definitively exclude square grooves from being useful, it is a strong example of another variable to take into account.
Currently used size ranges are 10-20 µm wide by 1-5 µm deep grooves and ridges. This is based on the supported theory that Schwann cells are 5-10 µm wide and need to sit inside the groove (Miller et al., 2001). Preliminary data that suggests grooves as small as 0.7 µm are significantly effective in directing neurite growth without considering Schwann cell alignment (Sigerson et al., 2014).
Conduits that are pre-impregnated with support cells have been shown to be beneficial. While these may be promising in vitro, it would lead to a difficult and complicated transition into clinical and commercial application.
While many individual growth-enhancing techniques have been tested, there are only few studies that combine the most promising methods into one clinically applicable solution. We propose a model using a nerve regrowth conduit made of pliable, implantable, and biodegradable material that not only significantly increases the amount of growth but also directs it. Such a device may use growth factors, surface topography, and channels or fibers in order to achieve such a goal (Figure 1).
Furthermore, developing a model that incorporates several of the relevant factors would be of great interest to the field of neuroregeneration. The creation of microenvironments that simultaneously address topography, bound ECM cues and free neurotrophic factors would allow researchers to test for possible additive or synergistic effects due to the combination of factors. Only myriad combinations of biophysical and biochemical cues will allow neuroscientists to elucidate the optimal environment for neuroregeneration. Discoveries in the area of in vitro regeneration will lead to the most important goal for this field of research: the modification of the neural microenvironment in vivo to promote axonal growth and restore neural function.
Casey D. Sigerson#, Christopher J. Dipollina#, Michele Fornaro*
Department of Anatomy, Midwestern University - Chicago College of Osteopathic Medicine, Downers Grove, IL, USA (Sigerson CD, Fornaro M) Department of Biomedical Sciences, Midwestern University, College of Health Sciences, Downers Grove, IL, USA (Dipollina CJ)
*Correspondence to: Michele Fornaro, Ph.D., mforna@midwestern.edu.
#These authors contributed equally to this work.
Accepted: 2016-01-28
orcid: 0000-0003-1852-9167 (Michele Fornaro)
Boyd JG, Gordon T (2003) Neurotrophic factors and their receptors in axonal regeneration and functional recovery after peripheral nerve injury. Mol Neurobiol 27:277-324.
Cámara-Lemarroy CR, Guzmán-de la Garza FJ, Fernández-Garza NE (2010) Molecular inflammatory mediators in peripheral nerve degeneration and regeneration. Neuroimmunomodulation 17:314-324.
Dubovy P, Jancalek R, Kubek T (2013) Role of inflammation and cytokines in peripheral nerve regeneration. Int Rev Neurobiol 108:173-206.
Miller C, Shanks H, Witt A, Rutkowski G, Mallapragada S (2001) Oriented Schwann cell growth on micropatterned biodegradable polymer substrates. Biomaterials 22:1263-1269.
Mizisin AP, Weerasuriya A (2011) Homeostatic regulation of the endoneurial microenvironment during development, aging and in response to trauma, disease and toxic insult. Acta Neuropathol 121:291-312.
Mobasseri A, Faroni A, Minogue BM, Downes S, Terenghi G, Reid AJ (2014) Polymer scaffolds with preferential parallel grooves enhance nerve regeneration. Tissue Eng Part A 21:1152-1162.
Muratori L, Ronchi G, Raimondo S, Geuna S, Giacobini-Robecchi MG, Fornaro M (2015) Generation of new neurons in dorsal root ganglia in adult rats after peripheral nerve crush injury. Neural Plast 2015: 860546.
Pabari A, Lloyd-Hughes H, Seifalian AM, Mosahebi A (2014) Nerve conduits for peripheral nerve surgery. Plast Reconstr Surg 133:1420-1430.
Sigerson CD, Gasiorowski JZ, Fornaro M (2014) Submicron topographical patterns enhance peripheral nerve regrowth ex vivo. J Am Osteopath Assoc 114:e159-160.
Spedden E, Wiens MR, Demirel MC, Staii C (2014) Effects of surface asymmetry on neuronal growth. PLoS One 9:e106709.
10.4103/1673-5374.177717 http://www.nrronline.org/
How to cite this article: Sigerson CD, Dipollina CJ, Fornaro M (2016) Effects of chemical and physical cues in enhancing neuritogenesis and peripheral nerve regeneration. Neural Regen Res 11(2):220-221.
杂志排行
中国神经再生研究(英文版)的其它文章
- Tissue-type plasminogen activator is a modulator of the synaptic vesicle cycle
- Impaired consciousness caused by injury of the lower ascending reticular activating system: evaluation by diffusion tensor tractography
- Considering calcium-binding proteins in invertebrates: multi-functional proteins that shape neuronal growth
- Cardiovascular dysfunction following spinal cord injury
- Practical application of the neuroregenerative properties of ketamine: real world treatment experience
- Exergames: neuroplastic hypothesis about cognitive improvement and biological effects on physical function of institutionalized older persons
