宁夏枸杞园土壤线虫和微生物群落多样性研究
2016-10-27张俊华
张俊华 张 翼 李 明
(1.宁夏大学环境工程研究院, 银川 750021; 2.宁夏大学教育学院, 银川 750021)
宁夏枸杞园土壤线虫和微生物群落多样性研究
张俊华1张翼2李明1
(1.宁夏大学环境工程研究院, 银川 750021; 2.宁夏大学教育学院, 银川 750021)
为了揭示宁夏枸杞园土壤质量变化趋势,以宁夏枸杞之乡——中宁县为研究区,选取不同树龄宁夏枸杞园土壤,分析不同季节、土层和树龄条件下土壤线虫和微生物群落特征变化规律。结果表明,随树龄的增加,枸杞园0~20 cm土壤线虫总数先增加后减少,6 a树龄时达最大值。不同树龄表层土壤均为食细菌线虫所占比例最大(夏季和秋季平均分别为57.23%和61.19%),植物寄生线虫次之;亚表层植物寄生线虫比例显著提高。夏季表层土壤总磷脂脂肪酸(PLFA)和细菌PLFA浓度随树龄呈减小-增大-减小的趋势,9 a树龄各菌群PLFA浓度普遍最大。随树龄的增加,夏季0~20 cm土壤线虫多样性和丰富度指数先增大后减小,但土壤微生物多样性指数、均匀度指数都逐渐减小,线虫和微生物群落优势度指数都逐渐增大;20~40 cm土壤线虫和微生物数量、多样性指数和优势度指数变化趋势一致。土壤EC与微生物总PLFA浓度、细菌PLFA浓度达显著负相关;土壤有机质、全氮、速效磷含量与土壤各线虫数量和微生物浓度相关性普遍达显著或极显著水平。土壤线虫总数、食细菌线虫数量与细菌、真菌和放线菌PLFA浓度均显著或极显著相关。总之,季节、土层和树龄对土壤线虫和微生物群落均有不同程度的影响,但树龄对其影响相对最小;季节、土层和树龄对土壤微生物群落的影响比对土壤线虫群落更显著。在相同季节和土层条件下,土壤微环境质量随着树龄的增加呈现出先改善后退化的趋势。
宁夏枸杞; 土壤线虫群落; 磷脂脂肪酸; 生态指数; 土壤理化性状
引言
土壤生物作为土壤生态功能的关键驱动者,其群落结构的变化在农业生态系统服务功能中占据重要的地位[1]。其中,线虫作为土壤食物网的重要组成部分,是农田土壤中多样性最为丰富的土壤动物,能够灵敏地反映环境变化时土壤生态系统的综合状况[2],在评价农田土壤生态系统变化方面具有很大的优势[3-4];而土壤微生物在生态系统营养物质循环过程,特别是碳、氮循环过程中扮演着重要的角色[5-6]。
单一植物长期种植会引起土壤微生物多样性减少,细菌数量减少,真菌数量增加,土壤类型从细菌型向真菌型转变,土壤线虫结构也会向不利于土壤质量的方向发展[7]。随树龄的增加,土壤细菌、微生物碳、氮生物量均先增大后减小,但真菌数量呈一直增大的趋势[8]。海南省澄迈县香蕉园土壤中植物寄生线虫的数量随树龄的增加而增加,食细菌线虫、食真菌线虫和捕食-杂食性线虫的数量则表现出相反趋势,香蕉园土壤的生态环境遭到破坏[9]。在树龄较小的果树根围线虫群落的多样性指数和均匀度指数均高于树龄较大的果树,而优势度指数相反;且植物线虫更易于侵袭幼龄果树[10]。随着线虫群落的改变,细菌和真菌都有显著变化[11],且随植物生长年限的增长, 土壤线虫总数及属数、密度、多样性指数和土壤微生物数量呈相似变化趋势[12-13]。食细菌线虫数量与细菌生物量呈极显著正相关关系[14],并且能够显著提高土壤氮和有效磷[15]。土壤总碳能够显著影响土壤微生物生物量,土壤pH值能显著影响土壤线虫总数。此外,施用氮肥能够显著降低土壤微生物碳代谢功能群多样性指数、真菌/细菌(F/G)和食真菌线虫数量[16-17],同时提高食细菌线虫数量[18];施用磷肥则能显著提高食细菌和食真菌线虫的数量[19]。胡锋等[20]指出食微动物虽然在某些条件下可抑制微生物种群,但总体上起着激活、增殖“培养”微生物的作用。
宁夏枸杞(LyciumbarbarumL.)为茄科枸杞属多年生落叶灌木,具有极高的药用价值和营养价值。因独特的地理气候条件,宁夏成为枸杞的原产地和最佳生态区。近年来由于过量施肥、频繁采摘和喷洒农药等,局部地区出现枸杞产量减少、品质下降的问题,为了揭示该现象的根本原因,本文选择对土壤环境变化敏感的土壤线虫和微生物群落进行研究,分析不同季节、土层和树龄宁夏枸杞园土壤微生物和线虫群落多样性、相互作用及其与土壤理化性状的关系,旨在探讨宁夏枸杞园土壤质量动态变化规律,为改善枸杞园土壤质量、推进宁夏枸杞产业的可持续发展提供理论依据。
1 材料与方法
1.1研究区概况
宁夏回族自治区中卫市中宁县(105°15′~106°05′E,36°49′~37°47′N)是全国枸杞生产基地县、中国枸杞之乡,也是宁夏枸杞的主产区。该地区年平均气温9.5℃,年降水量202.1 mm;日平均气温大于等于0℃积温3 200~3 300℃。本研究在中宁县宁安镇南桥村分别选择树龄为当年(小于1 a)、3、6、9、12 a枸杞地各3块(各块面积大于660 m2),共15块地。该地区土壤以灌淤土为主, 质地为砂壤土。
研究区枸杞地每年灌水3~4次,翻地1~2次,除草4~5次,采摘8~10次;施用化肥约2次(多为春施和秋施),每年喷洒农药8~11次。
1.2样品采集
分别于夏季(盛果期,7月14日)和秋季(落叶期,10月25日)采集土壤样品。在树冠投影范围外,先除去表层枯枝落叶后,用土钻取表层(0~20 cm)和亚表层(20~40 cm)土壤。样品采集时每块地选择9个点分别分层采样,然后将同一地块相同层次的土壤充分混匀,将新鲜土样分成2份,一份低温冷藏带回实验室,在-20℃下冷冻保存,用于土壤线虫的分离和鉴定以及土壤微生物磷脂脂肪酸的提取、测定,另一份在室内风干用于测定土壤pH值、电导率(EC)、有机质及养分含量。
1.3样品分析
1.3.1土壤样品理化性状测定
土壤pH值采用酸度计法测定;EC采用电导率法测定;有机质采用外加热法测定;全氮采用凯氏定氮法测定;碱解氮采用碱解扩散法测定;速效磷采用Oslen法测定;速效钾采用乙酸铵提取-火焰光度计法测定[21]。
1.3.2土壤线虫分离和鉴定
每个样品称取土样100 g,利用改良的浅盘法[22]对土壤线虫进行分离提取。线虫总数通过解剖镜直接计数,然后在光学显微镜下参照BONGERS的分类图进行科属鉴定[23-24]。
1.3.3土壤PLFA测定
采用修正的Bligh-Dyer方法提取脂类[25]。利用HP6890气相色谱-HP5973质谱联用仪。内标为19: 0用于定量。峰面积通过计算机自动积分,各脂肪酸的识别与定量分别参照 BAME (Bacterial acid methyl esters) Mix 和Supelcoe 37 Component FAME Mix。
1.3.4生态指数计算
土壤线虫和微生物生态指数公式[26-27]为:
多样性指数(Diversity index)
H′=-∑(pilnpi)
均匀度(Evenness)
J′=H′/lnS
丰富度(Species richness)
SR=(S-1)/lnN
优势度指数(Dominance index)
式中pi——第i个分类单元中个体所占的比例
S——所鉴定分类单元的个数
N——鉴定的线虫或微生物磷脂脂肪酸(PLFA)生物标记的个体数量
1.4数据处理
利用SAS中Duncan法检验不同树龄、季节和土层枸杞园土壤线虫及微生物数量差异显著性,用MANOVA法研究季节、土层和树龄对土壤线虫和微生物指标的交互影响,采用Pearson相关进行土壤理化性状与土壤线虫和微生物数量间的相关性分析。
2 结果与分析
2.1宁夏枸杞园土壤线虫群落特征
本研究夏季和秋季共获得土壤线虫2 145条,个体密度平均107条/(100 g干土),分属于2纲6目11科38属,基本情况见表1。
夏季共分离得到土壤线虫36属904条,个体密度平均90条/(100 g干土),其中螺旋属Helicotylenchus、拟丽突属Acrobeloides和小杆属Rhabditidae为优势属,个体数量占所有已知个体总数的44.82%;13个常见属共占所有已知属个体总数的48.91%;23个稀有属仅占所有已知属个体总数的6.34%。秋季共分离得到土壤线虫30属1 241条,个体密度平均124条/(100 g干土),其中拟丽突属、小杆属和螺旋属为优势属,占所有已知属个体总数的43.40%;17个常见属占所有已知属个体属总数的51.49%;15个稀有属占所有已知属个体总数5.11%。
随着树龄的增加,夏季0~20 cm土壤线虫总数先增加后减少,6 a树龄达到最大值(比当年树龄增加47.85%),树龄为12 a时线虫总数低于当年树龄33.91%(图1)。秋季不同树龄表层土壤线虫变化趋势与夏季相同,但当年树龄线虫总数最小。随着树龄的增加,20~40 cm夏季土壤线虫总数无明显变化规律,秋季则先减少后增加。秋季表层土壤线虫总数比夏季平均增加24.49%,亚表层增加33.36%;不同树龄秋季表层线虫总数平均值最大。
不同树龄表层土壤均为食细菌线虫所占比例最大(夏季和秋季平均分别为57.23%和61.19%),植物寄生线虫次之(夏季和秋季平均分别为25.67%和21.64%),食真菌线虫和捕食-杂食性线虫比例平均小于10%(图2)。亚表层植物寄生线虫比例显著提高(夏季和秋季分别为41.68%和40.04%),尤其在秋季,树龄为小于1、6、9 a时植物寄生线虫比例高于食细菌线虫。随树龄的增加,食真菌线虫所占比例先增大后减小,而捕食-杂食性线虫变化普遍与之相反。
随着树龄的增加,夏季和秋季0~20 cm土壤线虫多样性和丰富度指数先增大后减小;优势度指数则呈一直增大趋势。线虫各生态指数表层与亚表层无明显差异。秋季线虫丰富度指数高于夏季,而其他3种生态指数普遍略低于夏季;秋季各生态指数间差异相对较小,如表2所示。
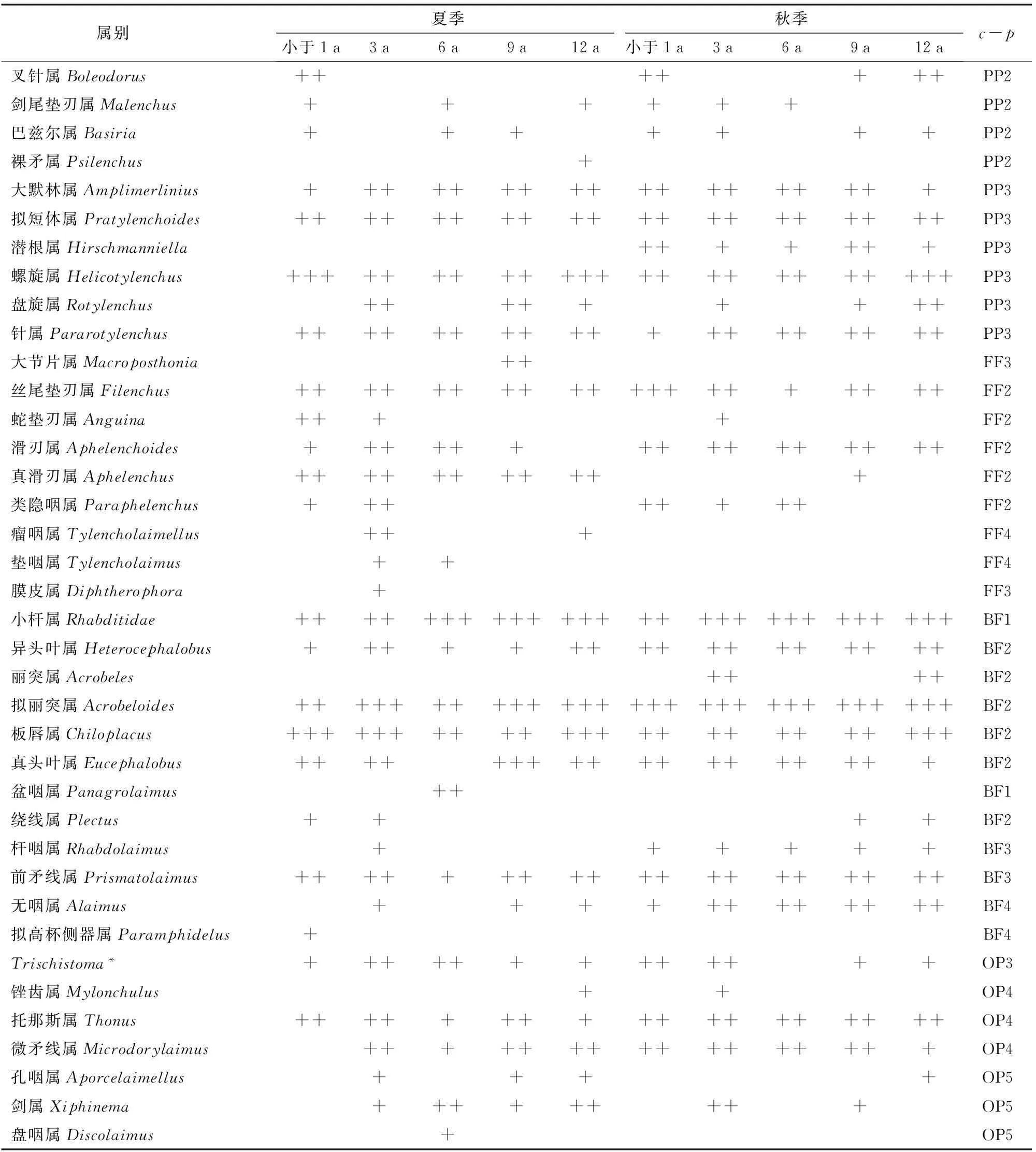
表1 宁夏枸杞园0~20 cm土壤线虫属优势度和功能类群Tab.1 Dominance and functional group of soil nematodes in 0~20 cm soil of Lycium barbarum L.orchard
注:*表示未查到其中文名。BF、PP、FF和OP分别表示食细菌线虫、植物寄生线虫、食真菌线虫和捕食-杂食性线虫。+,为稀有属比例小于1%;++,为常见属比例1%~10%;+++,为优势属比例大于10%[28]。
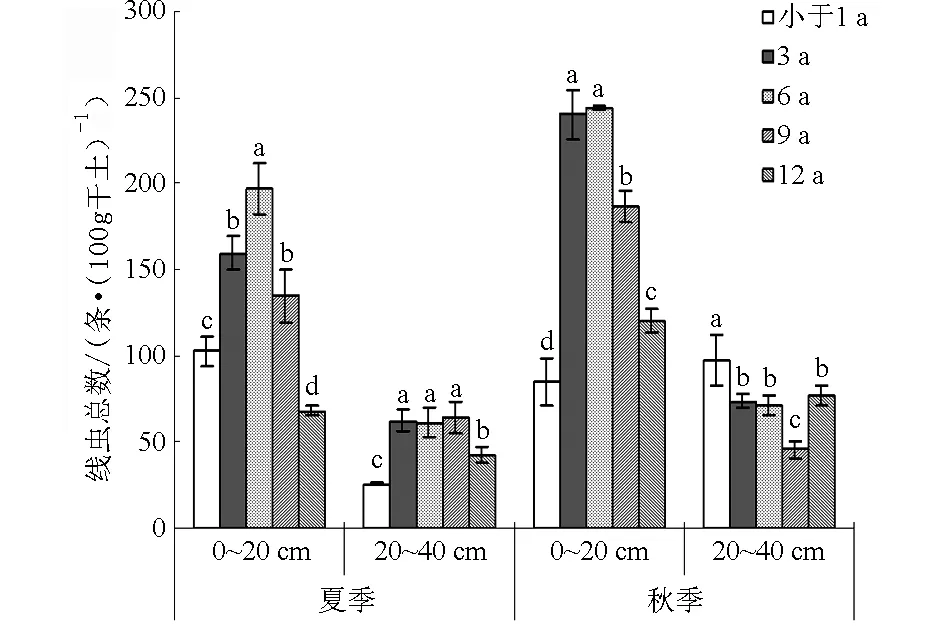
图1 宁夏枸杞园0~20 cm土壤线虫总数Fig.1 Abundance of nematodes in 0~20 cm soil of Lycium barbarum L. orchard
从多因素方差分析结果来看(表3),季节对线虫总数、优势度和丰富度指数有显著和极显著影响;除植物寄生线虫数量、优势度指数和均匀度指数外,土层对其他土壤线虫指标均有极显著或显著影响;树龄可以显著影响食细菌线虫数量、捕食-杂食线虫数量和线虫优势度指数。在季节、土层和树龄多因素交互中,只有土层和树龄2因子交互对食细菌线虫树龄有显著影响,其他2因子或3因子交互对土壤线虫各指标均无显著影响。
2.2宁夏枸杞园土壤微生物群落特征
2.2.1土壤各菌群PLFA质量摩尔浓度
供试土壤中细菌PLFA浓度占微生物总PLFA质量摩尔浓度(以下简称浓度)的70%~82%,真菌占8%~15%,放线菌占6%~11%(图3,图中不同字母表示相同季节和土层不同树龄土壤总PLFA浓度间的差异性)。随着枸杞树龄的增加,夏季表层
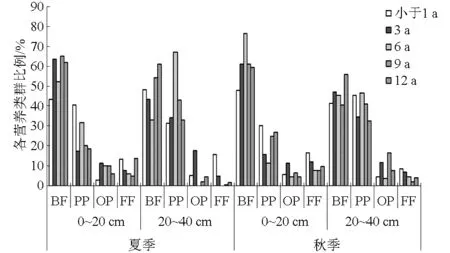
图2 不同季节和树龄枸杞园土壤0~20 cm不同营养类群线虫比例Fig.2 Proportions of different trophic groups of nematodes in different seasons and planting ages in surface soil of Lycium barbarum L. orchard
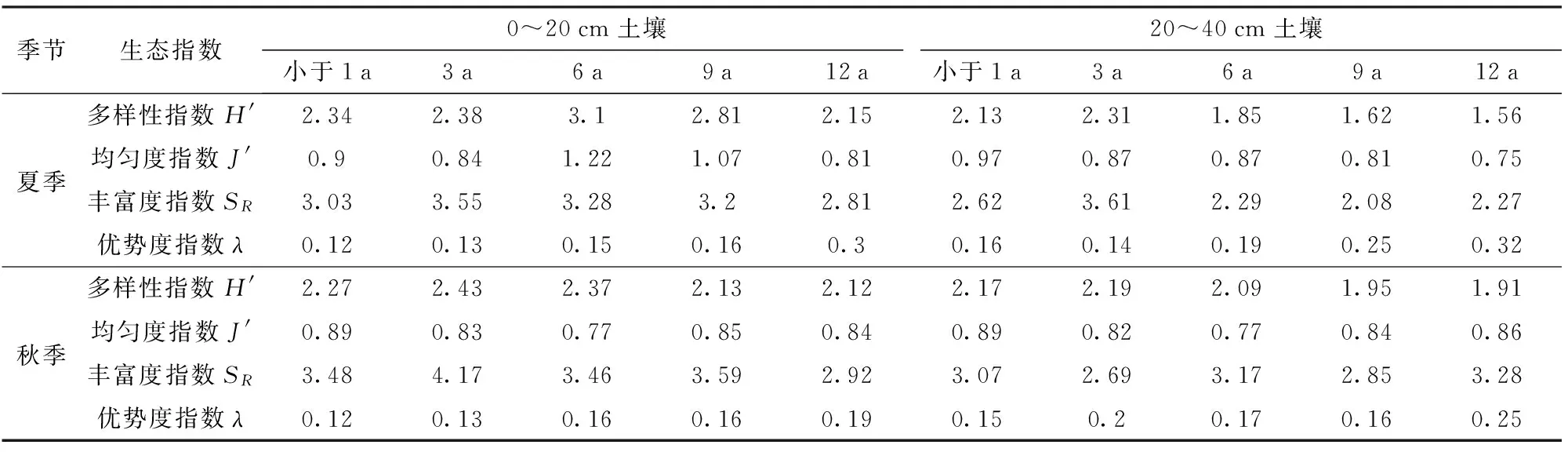
表2 不同树龄土壤线虫群落多样性指数Tab.2 Characteristics of diversity of soil nematode communities at different stand ages
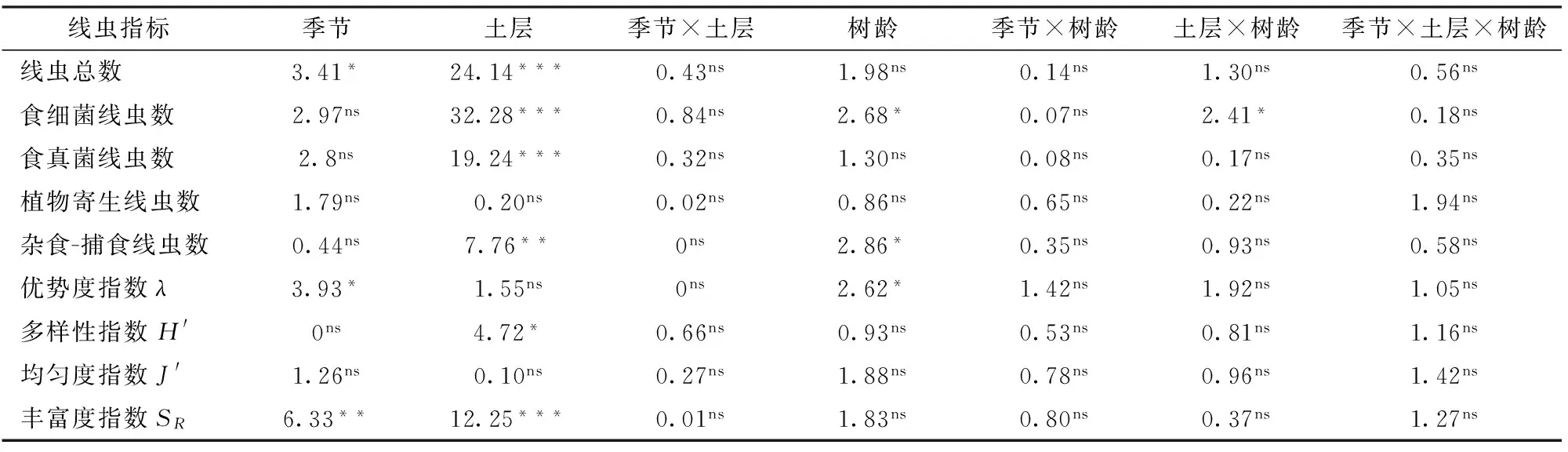
表3 季节、土层、树龄及其交互作用影响枸杞园土壤线虫因子的多因素方差分析结果Tab.3 Results from three-way ANOVA testing effects of season, layer, planting age, and their interactions on soil nematode index in orchard of Lycium barbarum L.
注:*、 **和***分别表示在0.05、0.01和0.001水平上的显著性,ns表示不显著,下同。
土壤细菌PLFA浓度先减小后增大,到9 a树龄时达到最大值,树龄为12 a时又有所减小;而亚表层土壤细菌PLFA浓度先增大后减小;秋季表层土壤细菌PLFA浓度变化与夏季亚表层相似,但各树龄间无显著差异;秋季亚表层土壤细菌PLFA浓度先减小后增大,6 a树龄时最小, 当年树龄相对最大。随树龄的增加,土壤真菌和放线菌PLFA浓度变化趋势与细菌相似。夏季表层总PLFA浓度大于亚表层及秋季0~40 cm土层,其中9 a树龄最高,依次分别高于其他树龄32.97%、50.45%、13.72%和10.67%。秋季亚表层总PLFA浓度最小,平均值只有39.70 nmol/g,且除6 a树龄外,其他4个树龄间均无显著差异。
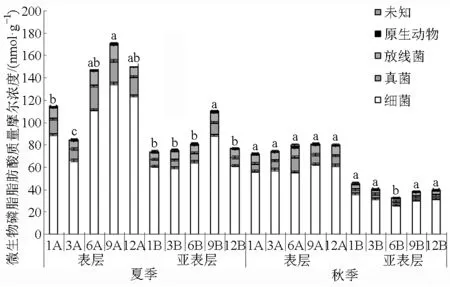
图3 不同种植年限土壤微生物磷脂脂肪酸质量摩尔浓度Fig.3 Soil microbial PLFAs contents at different stand ages of Lycium barbarum L.
2.2.2土壤微生物群落生态指数
随着树龄的增加,夏季表层土壤微生物多样性、均匀度和丰富度指数都逐渐减小(表4),而优势度指数变化与之相反,所以12 a树龄的土壤微生物群落多样性最差。随树龄的增加,夏季和秋季亚表层多样性指数呈增大-减小的趋势;秋季表层多样性指数有减小-增大的现象,9 a和12 a树龄各指数基本相等。
相对于线虫各指标来讲,季节、土层和树龄对土壤微生物的影响更显著(表5)。除F/G和均匀度指数外,季节对微生物PLFA浓度和各生态指数的影响都达到极显著或显著水平。土层对土壤微生物PLFA浓度和生态指数也普遍达到显著和极显著水平,但对土壤G-/G+及微生物多样性指数无显著影响。树龄对放线菌PLFA浓度、优势度、均匀度和丰富度均无显著影响,但对其他微生物指标都产生显著或极显著影响。季节、土层和树龄每2个因子交互对微生物总PLFA浓度、细菌、真菌PLFA浓度和多样性指数都有显著或极显著影响。整体而言,3个因子对土壤微生物的影响程度由大到小依次为:季节、土层、树龄。季节、土层和树龄3因子交互对微生物多样性能够产生极显著影响。
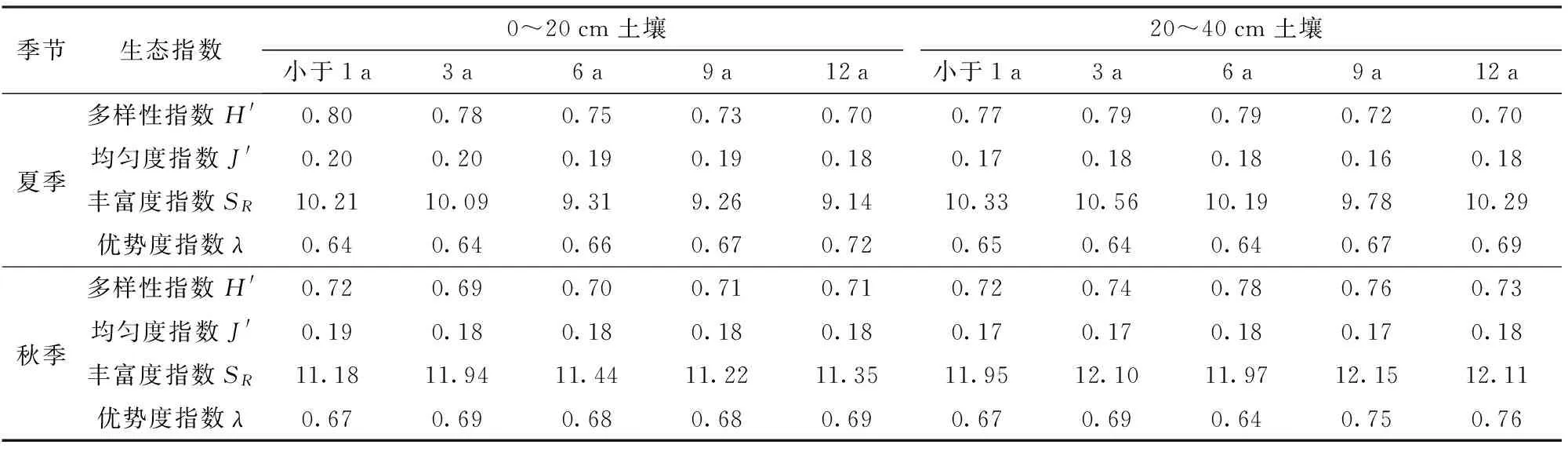
表4 不同树龄土壤微生物群落多样性指数Tab.4 Characteristics of diversity of soil microbe communities at different stand ages
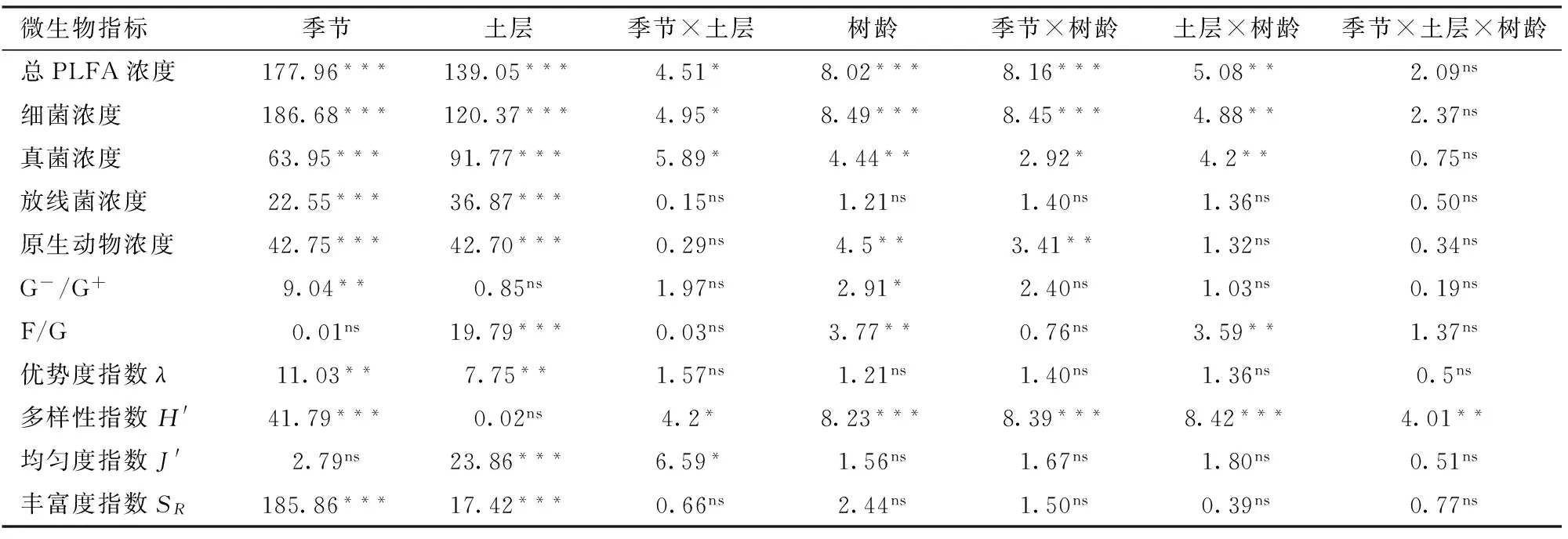
表5 季节、土层、树龄及其交互作用影响枸杞园土壤微生物因子的多因素方差分析结果Tab.5 Results from three-way ANOVA testing effects of season, layer, planting age, and their interactions on soil microbe index in orchard of Lycium barbarum L.
2.3土壤理化性质与土壤线虫和微生物间的相关性
供试区域土壤有机质含量与全氮、碱解氮、速效磷含量之间呈极显著或显著正相关,碱解氮含量与全氮、速效磷含量呈极显著正相关关系,速效钾含量与pH值、EC呈显著性负相关(表4)。土壤pH值与EC和线虫数量、微生物数量普遍呈负相关关系,其中EC与微生物总PLFA浓度和细菌PLFA浓度达显著相关。除植物寄生线虫外,土壤有机质、全氮含量与各线虫数量和微生物浓度均呈显著或极显著正相关关系。除植物寄生线虫和食真菌线虫外,土壤速效磷含量与土壤各线虫数量和微生物浓度相关性均达显著或极显著。土壤速效钾含量与微生物总PLFA浓度、细菌PLFA浓度呈显著正相关关系。线虫总数与细菌、真菌和放线菌PLFA浓度呈显著和极显著相关,食细菌线虫数量与总PLFA、细菌、真菌和放线菌均显著或极显著相关,捕食-杂食性线虫数量与放线菌PLFA浓度显著相关。
土壤EC与微生物多样性和优势度分别呈极显著负相关和正相关(表7)。土壤有机质含量与线虫丰富度指数、微生物均匀度呈显著性正相关;全氮、碱解氮、速效磷含量与微生物丰富度呈极显著和显著负相关;速效钾含量与微生物多样性、优势度指数呈显著负相关和正相关关系。土壤线虫丰富度与微生物均匀度间呈显著性正相关。
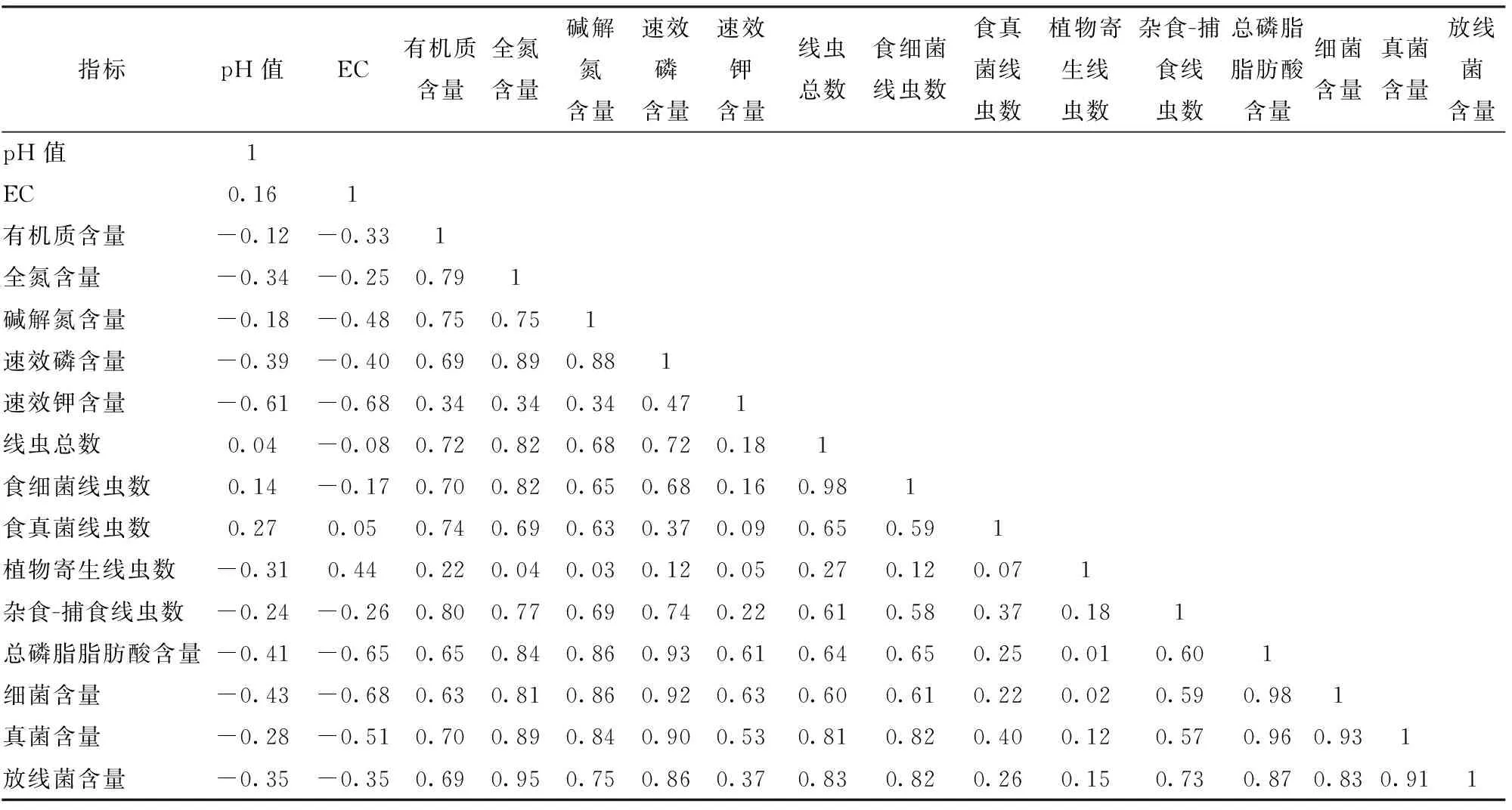
表6 土壤理化性状与土壤线虫和微生物数量的相关性分析Tab.6 Correlation between soil PLFAs and soil physicochemical properties
注:P0.05=0.602,P0.01=0.735,下同。
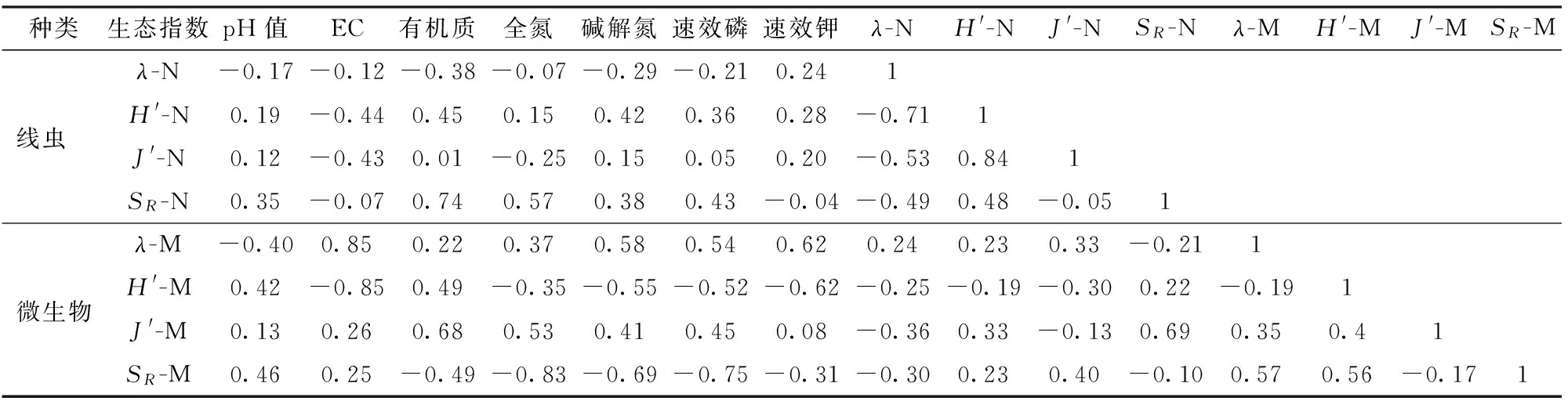
表7 土壤理化性状与土壤线虫和微生物生态指数的相关性分析Tab.7 Correlation between soil PLFAs and soil physicochemical properties
注:表中-N和-M分别代表土壤线虫和土壤微生物。
3 讨论
研究区地处卫宁平原引黄灌区,土壤有不同程度的次生盐渍化,土壤线虫和微生物群落在不同季节、土层和树龄都有其独特的变化规律。本研究得到不同树龄土壤线虫类群属数2纲6目11科38属,个体密度平均107条/(100 g干土),低于其他果园的报道结果[29-30]。
夏季枸杞园表层土壤食细菌线虫所占比例最大,植物寄生线虫次之,亚表层植物寄生线虫数量显著增加,秋季树龄为小于1、6、9 a的土壤植物寄生线虫比例居各营养类群最高值。植物寄生线虫是引起连作障碍的原因之一[31],可诱发或加重某些病害的发生,造成经济损失[32],所以需要抑制这几个树龄秋季土壤植物寄生线虫的繁殖。使用除草剂和化肥会抑制植物寄生线虫的生长[33]。但食细菌线虫也可以显著提高木本植物对土壤氮和磷的吸收[34],促进植物根系生长[35],提高植物对污染/退化土壤的忍耐力[36],所以土壤食细菌线虫还应该保留合理的数量。
随树龄的增加,表层土壤线虫总数与微生物PLFA浓度变化并不一致:土壤线虫总数先增加后减少,6 a树龄土壤线虫总数最多;但土壤微生物总PLFA浓度先减小后增大再减小,9 a树龄土壤总PLFA浓度最大。这是由于线虫摄食的细菌通过肠道后大部分仍保持活性[37],而且这些细菌可能在肠道内获得某些激素和限制性营养物质,因而当排出后生长加快;此外,线虫的分泌和排泄物为细菌生长提供了更易于利用的基质及无机营养,这对非根际土壤环境中细菌的增殖尤为重要,因此食细菌线虫能够促进微生物种群的增长[20]。陈小云等[38]指出食细菌线虫显著增加了土壤细菌、真菌和放线菌的数量,且对真菌和放线菌数量的促进作用比对细菌更为明显,与本研究结论完全一致,这种现象可能是由于速生型细菌和慢生型真菌存在较激烈的资源竞争[39]。食细菌线虫对细菌的捕食(减弱细菌对资源的竞争)给真菌(或放线菌)提供了生长的竞争优势[40]。相对于线虫各指标来讲,季节、土层和树龄对该地区枸杞园土壤微生物的影响更显著,且这3个因素的交互对土壤微生物多样性有显著影响,这也反映了微生物群落组成对外部环境影响的敏感性。
土壤EC和微生物PLFA总浓度、细菌PLFA浓度、微生物多样性呈显著负相关关系,说明土壤盐分会抑制土壤微生物的多样性,但会促进适合高盐分环境微生物的繁殖使其成为优势种群。土壤有机质与各线虫数量和微生物浓度均呈显著或极显著正相关关系,可以提高土壤线虫丰富度和微生物群落的均匀度[41-42]。土壤速效氮含量与线虫数量呈极显著正相关,这是由于土壤氮能够直接通过新陈代谢、铵态氮释放或间接通过侵染和(或)食用细菌、真菌来增加土壤线虫数量[43]。除食真菌线虫和植物寄生线虫外,土壤速效磷与各线虫数量和微生物PLFA浓度均存在显著或极显著正相关关系,但本地区枸杞园长期大量施用复合肥,土壤氮磷钾含量均处于较高水平,故与低磷水平下土壤菌根真菌数量与土壤磷含量呈显著负相关关系的结论相反[44]。枸杞园通过施肥来显著影响土壤微环境,土壤速效氮和速效磷与线虫总数和食细菌线虫呈显著和极显著整相关,与LIU等[45]研究结论完全一致。本研究发现枸杞园土壤微生物学性质和养分含量与土壤线虫群落组成表现出密切的关系,这表明树龄的增加改变了土壤资源有效性及微生物群落,进而对土壤线虫群落或土壤碎屑食物网的结构和功能产生影响[46]。本试验结果表明,不同树龄枸杞园土壤线虫生态指数和微生物生态指数变化趋势并不一致。线虫丰富度指数与微生物均匀度指数呈显著正相关,这一现象也与线虫和微生物群落生长、取食密切相关[20]。
4 结束语
通过对土壤线虫、微生物数量及其生态指数的分析,可知研究区宁夏枸杞园0~20 cm土壤线虫和微生物数量多于20~40 cm,但二者生态指数变化规律不同;20~40 cm土壤线虫和微生物在数量、多样性指数和优势度指数变化趋势一致。土壤有机质、全氮、速效磷含量与土壤各线虫数量和微生物浓度相关性普遍达显著或极显著水平,土壤线虫总数、食细菌线虫数量与细菌、真菌和放线菌PLFA浓度均显著或极显著相关。季节和土层对土壤线虫和微生物群落多样性的影响普遍大于树龄;季节、土层和树龄对微生物群落的影响较对线虫群落更显著。在相同季节、相同土层的前提下,宁夏枸杞园土壤微环境质量随树龄的增加呈先改善后退化的趋势。
1NAIR A, NGOUAJIO M. Soil microbial biomass, functional microbial diversity, and nematode community structure as affected by cover crops and compost in an organic vegetable production system[J]. Applied Soil Ecology, 2012, 58: 45-55.
2陈婧, 陈法军, 刘满强, 等. 温度和CO2浓度升高下转Bt水稻种植对土壤活性碳氮和线虫群落的短期影响[J]. 生态学报, 2014, 34(6): 1481-1489.
CHEN Jing, CHEN Fajun, LIU Manqiang, et al. Short-term effects of CO2concentration elevation, warming and transgenic Bt rice cropping on soil labile organic carbon and nitrogen, and nematode communities[J]. Acta Ecologica Sinica, 2014, 34(6): 1481-1489. (in Chinese)
3LU Z B, DONG D F, YANG B, et al. Effects of crop species richness on the community of soil nematodes in an experimental agro-ecosystem[J]. European Journal of Soil Biology, 2016, 73: 26-33.
4WAGNER D, EISENHAUER N, CESARZ S. Plant species richness does not attenuate responses of soil microbial and nematode communities to a flood event[J]. Soil Biology and Biochemistry, 2015, 89: 135-149.
6HORTAL S, BASTASTIDA F, MORENO J L, et al. Benefactor and allelopathic shrub species have different effects on the soil microbial community along an environmental severity gradient[J]. Soil Biology and Biochemistry, 2015, 88: 48-57.
7CHAPARRO J M, BADRI D V,VIVANCO J M. Rhizosphere microbiome assemblage is affected by plant development[J]. The ISME Journal, 2014, 8(4): 790-803.
8FU Q X, GU J, LI Y D, et al. Analyses of microbial biomass and community diversity in kiwifruit orchard soils of different planting ages[J]. Acta Ecologica Sinica, 2015, 35(3): 22-28.
9XIAO H F, TIAN Y H, ZHOU H P, et al. Intensive rubber cultivation degrades soil nematode communities in Xishuangbanna, southwest China[J]. Soil Biology and Biochemistry, 2014, 76: 161-169.
10孙晓铭, 段玉玺, 赵磊, 等. 辽宁果树根围土壤线虫的多样性研究[J]. 果树学报, 2010, 27(3): 410-415.
SUN Xiaoming, DUAN Yuxi, ZHAO Lei, et al. Diversity of soil nematodes in orchards in Liaoning province[J]. Journal of Fruit Science, 2010, 27(3): 410-415. (in Chinese)
11MOCALI S, LANDI S, CERTO G, et al. Resilience of soil microbial and nematode communities afterbiofumigant treatment with defatted seed meals[J]. Industrial Crops and Products, 2015, 75: 79-90.
12刘雨迪, 陈小云, 刘满强, 等. 不同稻作年限下土壤微生物学性质和线虫群落特征的变化[J]. 生物多样性, 2013, 21(3): 334-342.
LIU Yudi, CHEN Xiaoyun, LIU Manqiang, et al. Changes in soil microbial properties and nematode assemblage over time during rice cultivation[J]. Biodiversity Science, 2013, 21(3): 334-342. (in Chinese)
13张丹桔, 张健, 杨万勤, 等. 一个年龄序列巨桉人工林植物和土壤生物多样性[J]. 生态学报, 2013, 33(13): 3947-3962.
ZHANG Danju, ZHANG Jian, YANG Wanqin, et al. Plant’s and soil organism’s diversity across a range ofEucalyptusgrandisplantation ages[J]. Acta Ecologica Sinica, 2013, 33(13): 3947-3962. (in Chinese)
14JIANG Y J, SUN B, JIN C, et al. Soil aggregate stratification of nematodes and microbial communities affects the metabolic quotient in an acid soil[J]. Soil Biology and Biochemistry, 2013, 60: 1-9.
15BONKOWSKI M, VILLENAVE C, GRIFFITHS B. Rhizosphere fauna: the functional and structural diversity of interactions of soil fauna with plant roots[J]. Plant and Soil, 2009, 321: 213-233.
16SARATHCHANDRA S U, GHANI A, YEATES G W, et al. Effect of nitrogen and phosphate fertilizers on microbial and nematode diversity in pasture soils[J]. Soil Biology and Biochemistry, 2001, 33(7): 953-964.
17BRADLEY K, DRIJBER R A, KNOPS J. Increased N availability in grassland soils modifies their microbial communities and decreases the abundance of arbuscular mycorrhizal fungi[J]. Soil Biology and Biochemistry, 2006, 38(7):1583-1595.
18FORGE T A, BITTMAN S, KOWALENKO C G. Responses of grassland soil nematodes and protozoa to multi-year and single-year applications of dairy manure slurry and fertilizer[J]. Soil Biology and Biochemistry, 2005, 37(10):1751-1762.
19齐莎, 赵小蓉, 郑海霞, 等. 内蒙古典型草原连续5年施用氮磷肥土壤生物多样性的变化[J]. 生态学报, 2010, 30(20): 5518-5526.
QI Sha, ZHAO Xiaorong, ZHENG Haixia, et al. Changes of soil biodiversity in Inner Mongolia steppe after 5 years of N and P fertilizer applications[J]. Acta Ecologica Sinica, 2010, 30(20): 5518-5526. (in Chinese)
20胡锋, 李辉信, 谢涟琪, 等. 土壤食细菌线虫与细菌的相互作用及其对N、P矿化生物固定的影响及机理[J]. 生态学报, 1999, 19(6): 914-920.
HU Feng, LI Huixin, XIE Lianqi, et al. Interactions of bacterivorous nematode and bacteria and their effects on mineralization-immobiolizat ion of nitrogen and phosphorus[J]. Acta Ecologica Sinica, 1999, 19(6): 914-920. (in Chinese)
21鲁如坤. 土壤农业化学分析方法[M]. 北京: 中国农业科技出版社, 2000: 1-638.
22LIANG W J, LOU Y L, LI Q, et al. Nematode faunal response to long-term application of nitrogen fertilizer and organic manure in northeast China[J]. Soil Biology and Biochemistry, 2009, 41(5): 883-890.
23BONGERS T. Nematodes of Netherland[M]. Utrecht: Pirola Schoorl, 1988.
24尹文英. 中国土壤动物检索图鉴[M]. 北京: 科学出版社, 1998.
25BOSSIO D A, SCOW K M. Impacts of carbon and flooding on soil microbial communities: phospholipid fatty acid profiles and substrate utilization patterns[J]. Microbial Ecology, 1998, 35(3): 265-278.
26王峰源, 张建丽, 刘峰, 等. 河流对河岸带落水区土壤线虫生态指数的影响[J]. 水生态学杂志, 2013, 34(1): 7-13.
WANG Fengyuan,ZHANG Jianli,LIU Feng, et al. Effect of river on the ecological index of the soil nematode in the riparian falling zone[J]. Journal of Hydroecology, 2013, 34(1): 7-13. (in Chinese)
27ZHEN Z, LIU H, WANG N, et al. Effects of manure compost application on soil microbial community diversity and soil microenvironments in a temperate cropland in China[J]. PLOS ONE, 2014, 9(10): e108555.
28王诚楠, 张伟东, 王雪峰, 等. 沿海区土壤线虫对海水入侵土壤盐渍化的响应[J]. 土壤学报, 2015, 52(5): 1135-1143.
WANG Chengnan, ZHANG Weidong, WANG Xuefeng, et al. Respone of soil nematodes to soil salinizaion induced by seawater intrusion in coastal areas[J]. Acta Pedologica Sinica, 2015, 52(5): 1135-1143. (in Chinese)
29杨树泉, 沈向, 毛志泉, 等. 环渤海湾苹果产区老果园与连作果园土壤线虫群落特征[J]. 生态学报, 2010, 30(16): 4445-4451.
YANG Shuquan, SHEN Xiang, MAO Zhiquan, et al. Characterization of nematode communities in the soil of long-standing versus replanted apple orchards surrounding Bohai Gulf[J]. Acta Ecologica Sinica, 2010, 30(16): 4445-4451. (in Chinese)
30REARDON C L, STRAUSS S L, MAZZOLA M. Changes in available nitrogen and nematode abundance in response toBrassicaseed meal amendment of orchard soil[J]. Soil Biology and Biochemistry, 2013, 57: 22-29.
31李琪, 梁文举, 姜勇. 农田土壤线虫多样性研究现状及展望[J]. 生物多样性, 2007, 15(2): 134-141.
LI Qi, LIANG Wenju, JIANG Yong. Present situation and prospect of soil nematode diversity in farmland ecosystems[J]. Biodiversity Science, 2007, 15(2): 134-141. (in Chinese)
32CHAUVIN C, DOREL M, VILLENAVE C, et al. Biochemical characteristics of cover crop litter affect the soil food web, organic matter decomposition, and regulation of plant-parasitic nematodes in a banana field soil[J]. Applied Soil Ecology, 2015, 96: 131-140.
33PALOMARES-RIUS J E, CASTILLO P, MONTES-BORREGO M, et al. Nematode community populations in the rhizosphere of cultivated olive differs according to the plant genotype[J]. Soil Biology and Biochemistry, 2012, 45: 168-171.
34IRSHAD U,VILLENAVE C,BRAUMAN A, et al. Grazing by nematodes on rhizosphere bacteria enhances nitrate and phosphorus availability toPinuspinasterseedlings[J]. Soil Biology and Biochemistry, 2011, 43(10): 2121-2126.
35JIANG Y, WU Y, XU W S, et al. IAA-producing bacteria and bacterial-feeding nematodes promoteArabidopsisthaliana, root growth in natural soil[J]. European Journal of Soil Biology, 2012, 52(9): 20-26.
36HUA J F, JIANG Q, BAI J F, et al. Interactions between arbuscular mycorrhizal fungi and fungivorous nematodes on the growth and arsenic uptake of tobacco in arsenic-contaminated soils[J]. Applied Soil Ecology, 2014, 84: 176-184.
37SMERDA S M, JENSEN H J, ANDERSON A W. Escape of Salmonel laef rom chlorination during ingestion by pristionchus lheritieri (Nematoda: Diplogasterinae) [J]. Journal of Nematology, 1971, 3(3): 201-204.
38陈小云,李辉信,胡锋, 等. 食细菌线虫对土壤微生物量和微生物群落结构的影响[J]. 生态学报, 2004, 24(12): 2825-2831.
CHEN Xiaoyun, LI Huixin, HU Feng, et al. Effect of bacterivorous nematode on soil microbial biomass and microbiocoenosis[J]. Acta Ecologica Sinica, 2004, 24(12): 2825-2831. (in Chinese)
39TURNER S M, NEWMAN E I, CAMPBELL R. Microbial population of ryegrass root surface: influence of nitrogen and phosphorus supply[J]. Soil Biology and Biochemistry, 1985, 17(5): 711-715.
40GRIFFITHS B S, BARDGETT R D. Int eractions between micro-feeding invertebrates and soil microorganisms[M]∥Van Elsas. Modern Soil Microbiology. New York: Marcel Dekker, 1997: 165-182.
41HODSON A K, FERRIS H, HOLLANDER A D, et al. Nematode food webs associated with native perennial plant species and soil nutrient pools inCaliforniariparianoak woodlands[J]. Geoderma, 2014, 228-229(5): 182-191.
42LI W, ZHENG Z C, LI T X, et al. Effect of tea plantation age on the distribution of soil organic carbon fractions within water-stable aggregates in the hilly region of Western Sichuan, China[J]. CATENA, 2015, 133: 198-205.
43REARDON C L, STRAUSS S L, MAZZOLA M. Changes in available nitrogen and nematode abundance in response toBrassica, seed meal amendment of orchard soil[J]. Soil Biology and Biochemistry, 2013, 57: 22-29.
44ATUL-NAYYAR, HAMEL C, FORGE T, et al. Arbuscular mycorrhizal fungi and nematodes are involved in negative feedback on a dual culture of alfalfa and Russian wildrye[J]. Applied Soil Ecology, 2008, 40(1): 30-36.
45LIU T, WHALEN J K, RAN W, et al. Bottom-up control of fertilization on soil nematode communities differs between crop management regimes[J]. Soil Biology and Biochemistry, 2016, 95: 198-201.
46OKADA H, HARADA H. Effects of tillage and fertilizer on nematode communities in a Japanese soybean field[J]. Applied Soil Ecology, 2007, 35(3): 582-598.
Soil Nematode and Microbial Community Diversity inLyciumbarbarumL. Orchard
Zhang Junhua1Zhang Yi2Li Ming1
(1.InstituteofEnvironmentalEngineering,NingxiaUniversity,Yinchuan750021,China2.CollegeofEducation,NingxiaUniversity,Yinchuan750021,China)
Soil nematode and microbes are essential and very sensitive to any upsets in terrestrial ecosystems. In order to reveal the tendency of soil quality of wolfberry (LyciumbarbarumL.) orchard, make the origin ofL.barbarumas the objective region, different stand ages of soil were selected in wolfberry orchard. The objective of the study was achieved by nematode and phospholipid fatty acid (PLFA) biomarker analysis of soil samples fromL.barbarumorchards in the objective region. The change rule of soil nematode and microbial community diversity with the change of season, soil layer and stand age was analyzed. The results showed that the abundance of nematode was increased and then decreased in 0~20 cm of soil, with the highest nematode at the stand age of 6. The proportion of bacterivores was the highest (57.23% and 61.19% in summer and autumn, respectively), and plant parasites nematode was next, fungivorous nematode and predators-omnivore had the lowest abundance. Plant parasites nematode was relatively higher at 20~40 cm than that at 0~20 cm. The average concentrations of total and bacterial PLFAs in the surface soil were initially decreased and then increased, and the highest microbial PLFA concentrations were obtained in 9thyear. The tendency change of total and bacterial PLFAs were similar to nematode abundance at 20~40 cm in summer. With longer stand age, Shannon diversity index (H′) and richness index (SR) of nematode were increased and then decreased, however,H′ andSRof microbial were decreased, and dominant index was increased. The change tendency of abundance,H′ andSRof soil nematode were similar to soil microbial. There was significant negative correlation between soil pH value, EC, microbial and bacteria PLFA. The organic matter, total nitrogen and available P were significantly positively correlated to the abundance of nematodes and concentration of microbial PLFA, respectively. Total abundance of nematode, bacterivores and bacteria, fungi and actinomyces PLFA were significantly positively correlated. On the whole, the season, layer and stand age had different effects on the nematode and microbial community, and the stand age had the least effect; the season, soil layer and stand age had more significant effect on microbe than nematode. Furthermore, the microenvironment of soil was improved and then declined gradually as the stand age increased in the same season as well as the soil layer.
LyciumbarbarumL.; soil nematode community; phospholipid fatty acid; ecological index; soil physicochemical characteristics
10.6041/j.issn.1000-1298.2016.09.024
2016-03-22
2016-04-11
国家自然科学基金项目(41261080)和宁夏自治区环保专项
张俊华(1977—),女,副研究员,主要从事土壤质量提升研究,E-mail: zhangjunhua728@163.com
S154.3; S154.5
A
1000-1298(2016)09-0161-10
