Effects of Lianhua Qingwen on Pulmonary Oxidative Lesions Induced by Fine Particulates (PM2.5) in Rats
2016-10-13FenPingZhenshengLiFengruiZhangDexinLiandShuzhiHan
Fen Ping*, Zhen-sheng Li, Feng-rui Zhang, De-xin Li, and Shu-zhi Han
Effects of Lianhua Qingwen on Pulmonary Oxidative Lesions Induced by Fine Particulates (PM2.5) in Rats
Fen Ping1*, Zhen-sheng Li2, Feng-rui Zhang1, De-xin Li2, and Shu-zhi Han1
1Department of Respiratory Medicine, Hebei General Hospital, Shijiazhuang, 050000 China2Department of Respiratory Medicine, Hebei Chest Hospital, Shijiazhuang, 050000 China
fine particulates (PM2.5); pulmonary lesion; oxidative stress; Lianhua Qingwen; rats
Objective To investigate the antagonistic effects of different doses of Lianhua Qingwen on pulmonary injury induced by fine particulates PM2.5 in rats.
Methods Fine particulates suspended in the environment were collected. Forty-eight healthy adult wistar rats were randomly divided into 6 groups with 8 rats in each group. Four groups of rats were exposed to PM2.5 by intratracheally dripping suspensions of fine particulates PM2.5 (7.5 mg/kg)as dust-exposed model rats. Among them 24 rats in three groups received Lianhua Qingwen treatment (crude drug) at a dose of 2 g/kg, 4 g/kg, 8 g/kg per day for 3 daysbefore dust exposure and were defined as low-dose, middle-dose and high-dose Lianhua Qingwen treatment groups respectively. The other dust-exposed model rats without treatment were assigned as PM2.5 control group. The un-exposed rats were set as saline control group (1.5 ml/kg saline) and blank control group. All rats were killed after 24 hours of the exposure. Lung tissue, serum and bronchoalveolar lavage fluid (BALF) were collected. The levels of malonaldehyde (MDA), lactate dehydrogenase (LDH), and glutathione peroxidase (GSH-PX) in blood serum and BALF, and superoxide dismutase (SOD) in blood surum were measured using fluorescent quantitation PCR; Expression of NF-E2-related factor 2(NRF-2), heme oxygenase 1 (HO-1) and quinone oxidoreductase 1 (NQO1) in lung tissues were measured using Western blot. Pathological changes of lung tissues in each group were also examined.
Results Pathology revealed thickened alveolar septum, congestion of capillary, interstitial edema and infiltration of lymphocyte and neutrophil surrounding bronchiole in the PM2.5 control group, which were significantly relieved in the Lianhua Qingwen treatment groups. Compared to the blank and saline control groups, the PM2.5 control group had significantly higher levels of LDH and MDA (p<0.01) and lower level of GSH-PS (p<0.01) in BALF, significantly higher levels of LDH and MDA (p<0.05) and lower level of GSH-PS (p<0.05) in rat serum. The levels of MDA in blood serum and BALF were significantly lower in each treatment group than that in PM2.5 control group (all<0.05). In both middle-dose and high-dose treatment group the measurements of LDH in serum and BALF as well as GSH-PX in serum were significant difference from those of PM2.5 control group (all<0.05). Expressions of NRF-2, HO-1 and NQO1 in lung tissues were significantly different among middle-dose and high-dose treatment group compared with those in PM2.5 control group (all<0.05).
Conclusion Fine particulates PM2.5 in environment may induce pulmonary oxidative lesions in rats. Middle-dose and high-dose Lianhua Qingwen has antagonist effece on the injuries induced by fine particulates.
Chin Med Sci J 2016; 31(4):233-238
S fine particulates knowledge grows, the impact of environmental fine particulates (PM2.5) on public health is attracting more attention. The overseas and domestic studies on pulmonary lesion induced by fine particulates show that fine particulates getting into pulmonary interstitium can induce chronic inflammation and oxidative stress injury in pulmonary tissues,1and dramatically increase morbidities and mortalities of chronic obstructive pulmonary diseases (COPD), asthma and other respiratory diseases.2Further studies were needed for better treatment of oxidative stress injury induced by PM2.5. Lianhua Qingwen Capsule, a formula of traditional Chinese medicine including Forsy- thia, Honeysuckle, Ephedra, Male Fern Rhizome, Herba Houttuyniae, Pogostemon Cablin, Rheum Officinale, Rhodiola Rosea, Menthol and Licorice Root, has been demonstrated to have antivirus activity and regulate pulmonary immunity, and has been used in inflammation diseases such as acute pulmonary infection. Thisanimal study investigated oxidative lesion of pulmonary tissues induced by PM2.5 using rat contamination models, in order to explore the relationship between the dosage of Lianhua Qingwen and its effect on pulmonary injuries induced by PM2.5.
MATERIALS AND METHODS
Rats, exposure and intervention
This animal study was approved by the Animal Care and Utilization Committee of Hebei General Hospital. Forty- eight healthy adult Wistar rats from Experimental Center, Hebei Medical University (male, weight 200–250g, and age 8–10 weeks) were matched and maintained on a chow diet in a 12-h light/12-h dark environment at 25°C. The rats were randomly divided into 6 groups, with 8 rats in each group. Two groups were used for blank control and saline control; the other four groups were exposed with PM2.5, and three of them were treated with Lianhua Qingwen.
PM2.5, provided by Shijiazhuang Environmental Moni- toring Center, was made into suspension of 5 mg/ml for the experiment. When sampling, fine particles were released from filter which had been heated at 100°Cfor 24 hours, and put into deionized water and sonicated 3×15 minutes with a soni-cator. The fine particles suspension was treated through mul- tilayer gauze to filter glass fiber, and then dried by vacuum- freeze machine and stored at -20°C until experiment.
Four groups of rats were exposed to PM2.5 by intratra- cheally dripping suspensions of PM2.5 at 7.5 mg/kg as dust exposed model rats. Among them 24 rats in three groups were assigned into the low-dose group, middle-dose group and high-dose group, and were intragastrically treated with 2 g/kg·d, 4 g/kg·d and 8 g/kg·d crude Lianhua Qingwen (Shi- jiazhuang Yiling Pharmaceutical Co., Ltd, China) suspension respectively for 3 days before dust exposure. There was one group of 8 dust exposed model rats that did not recieve the treatment and was defined as PM2.5 control group. In saline control group, the rats were only given 1.5 ml/kg normal salinetrachea once instead of PM2.5 suspension. In blank control group, the rats were given no treatment and no PM2.5 exposure.
Sample collection
All rats were killed 24 hours after PM2.5 exposure by cutting off femoral artery. 10% chloral hydratewere injectedintraperitoneally for anesthesia. Supernatant of blood was reserved after centrifugation. Left lung lobes underwent bronchoalveolar lavage, and bronchoalveolar lavage fluid (BALF) was taken for centrifugation.
Histopathology
Left lungs of rats in each group were sliced and HE staining was performed after dehydration with dehydrated alcohol and paraffin embedding. Pathological changes of samples were observed under light microscope (BX51; Olympus, Tokyo, Japan).
PCR
Lactic dehydrogenase (LDH), malondialdehyde (MDA), superoxide dismutase (SOD) and glutathione peroxidase (GSH-PX) in serum and BALF were measured using a PCR kit (Jiancheng Biologics Co., Ltd. Nanjing) by Fluorescent Quantitation PCR System (ABI prime 7300; American ABI Company, USA).
Western blot
Fresh lung tissues were sampled and packed in 1.5 ml EP tubes and immediately stored in liquid nitrogen. NF-E2- related factor 2 (NRF-2)is a central regulator of cellular antioxidant response. Heme oxygenase enzyme-1 (HO-1) can decompose heme to produce bilirubin, which has a high antioxidative effect. NQO1 has a protective mechanism against injury to oxidant response. Levels of NRF-2, HO-1 and NQO1 were detected using Western-blot Vertical Cells (DYCZ-24D type, Beijing Liuyi Instrument Plant, Beijing) and Gel Imaging System (BioDoc-It, American UVP Company,USA). Antibodies, including anti-NRF-2(1:1000), anti-HO-1 (1:250), and anti-NQO1 (1:1000), were purchased from Abcam (Cambridge, UK).
Statistical analysis
Statistical analysis was performed using SPSS software version 19.0 (Chicago, IL). All the measurement data were expressed as mean and standard deviation; one-way analysis of variance was used for measurements comparison among groups. When variance was found not homogeneous, the Dunnett T3 test was use for multiple comparisons.Andtest was used for comparison between two groups.0.05 was considered statistical significance.
RESULTS
Pathological changes of pulmonary tissues
There was no abnormal histological change under light microscope in blank control group (Fig. 1A) and saline control group (Fig. 1B). For PM2.5 control group, alveolar septum became thickening; congestion of blood capillary and interstitial edema were found; a large number of lymphocytes infiltration and neutrophils infiltration were found surrounding bronchiole (Fig. 1C). The microscopic manifestations of pulmonary tissues in low-, middle- and high-dose Lianhua Qingwentreatment groups were shown in Fig. 1D, 1E and 1F, which relieved in a dose-dependent manner.
LDH, MDA, GSH-PX and SOD in serum and BALF
The measurements of LDH, MDA, GSH-PX in rats BALF are shown in Table 1, where the LDH and MDA in PM2.5 group were significant higher than those in controls (<0.01), but were significantly reduced in each of treatment group, with the lowest level detected in the high dose Lianhua Qingwen group (<0.05). GSH-PX in BALF of PM2.5 group was significantly lower than that in the controls (<0.01), but was elevated in each of the treatment group, with the highest level detected in high dose group (<0.05), despite no significant difference among three treatment groups (>0.05). The measurements of LDH, MDA, GSH-PX and SOD in rats serum were shown in Table 2, where the LDH, MDA in PM2.5 group were significant higher than that in controls (<0.05), but were significantly reduced in middle- and high-dose treatment group, with the lowest level detected in the high-dose group (<0.05). SOD and GSH-PX in serum of PM2.5 group were significantly lower than that in the controls (<0.05), but only GSH-PX was detected to be significantly elevated in Middle- and high- dose group (<0.05).

Figure 1. Pathological changes of rats pulmonary tissue in variable doses of Lianhua Qingwenunder light microscope. HE ×100 Compared to the blank control group (A) and saline control group (B), PM2.5 control group (C) showed thickened alveolar septum, congestion of capillary, interstitial edema, lymphocytes infiltration and neutrophils infiltration surrounding bronchiole. The above changes were relieved in that of low-dose (D), middle-dose (E) and high-dose treatment group (F) compared with PM2.5 control group(C) in a dose-dependent manner.
Protein expression in pulmonary tissues
Western blot showed that protein expressions of HO-1, NRF-2 and NQO-1 in pulmonary tissues increased remarkably in middle- and high-dose Lianhua Qingwen groups (Fig. 2, Table 3).HO-1, NRF-2 and NQO-1 protein expression incr- eased after treatment of middle- and high-dose Lianhua Qingwen, and statistical difference was found (<0.05).These results suggested that Lianhuaqingwen could protect pulmo- nary tissues against oxidative injury induced by PM2.5.
Table 1. Comparisons of LDH, MDA and GSH-PX in rat bronchoalveolar lavage fluid among the study groups and the controls (n=8, ±s)
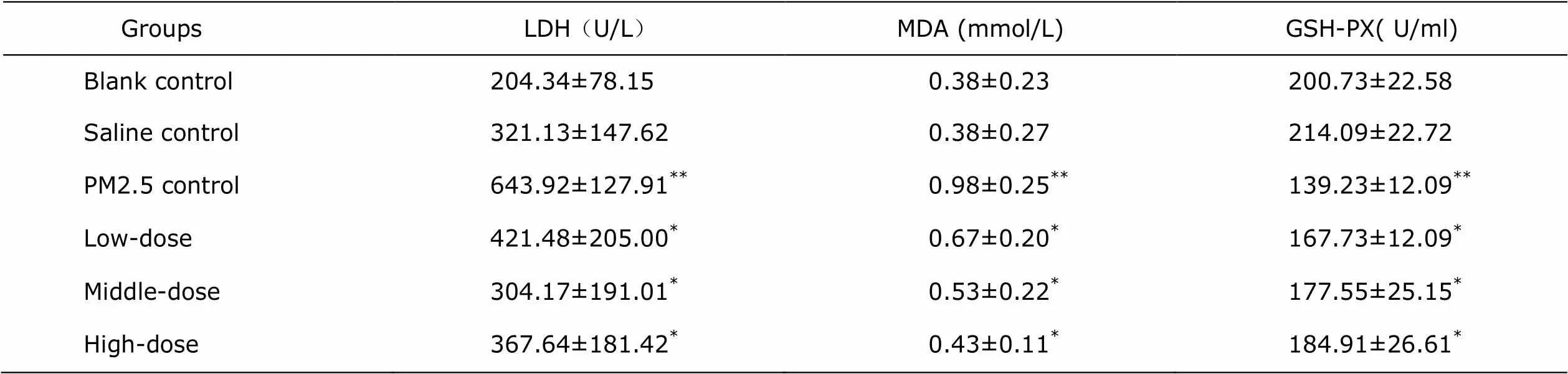
Table 1. Comparisons of LDH, MDA and GSH-PX in rat bronchoalveolar lavage fluid among the study groups and the controls (n=8, ±s)
GroupsLDH(U/L)MDA (mmol/L)GSH-PX( U/ml) Blank control 204.34±78.150.38±0.23200.73±22.58 Saline control321.13±147.620.38±0.27214.09±22.72 PM2.5 control643.92±127.91**0.98±0.25**139.23±12.09** Low-dose 421.48±205.00*0.67±0.20*167.73±12.09* Middle-dose 304.17±191.01*0.53±0.22*177.55±25.15* High-dose 367.64±181.42*0.43±0.11*184.91±26.61*
*0.05 as comparing with PM2.5 control group;**0.05 as comparing with blank control group or saline control group. LDH: lactate dehydrogenase; MDA: malonaldehyde; GSH-PX: glutathione peroxidase.
Table 2. Comparisons of LDH, MDA, GSH-PX and SOD in rat serum among thestudy groups and the controls (n=8, ±s)
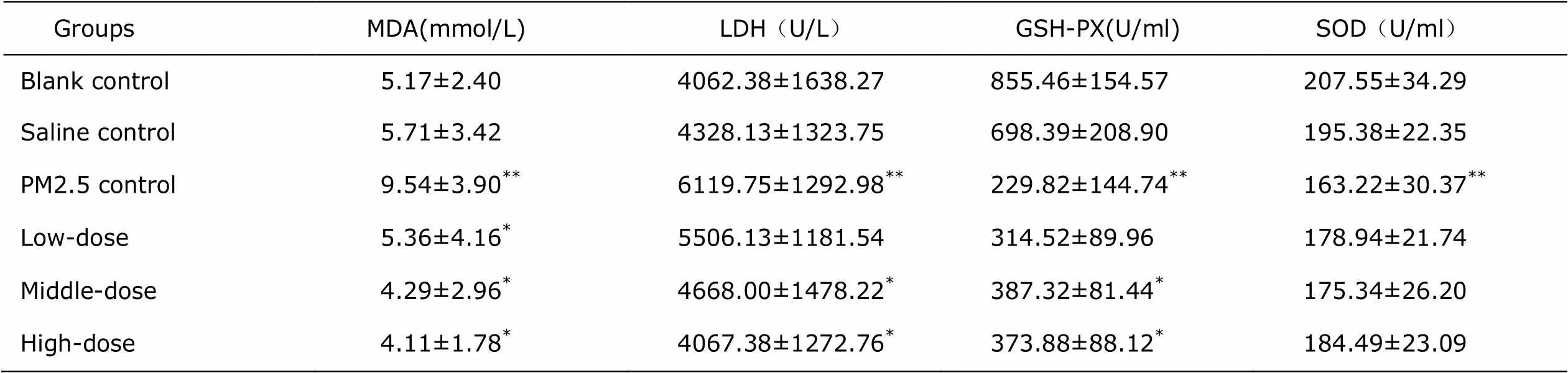
Table 2. Comparisons of LDH, MDA, GSH-PX and SOD in rat serum among thestudy groups and the controls (n=8, ±s)
GroupsMDA(mmol/L)LDH(U/L)GSH-PX(U/ml)SOD(U/ml) Blank control5.17±2.404062.38±1638.27855.46±154.57207.55±34.29 Saline control5.71±3.424328.13±1323.75698.39±208.90195.38±22.35 PM2.5 control9.54±3.90**6119.75±1292.98**229.82±144.74**163.22±30.37** Low-dose 5.36±4.16*5506.13±1181.54314.52±89.96178.94±21.74 Middle-dose 4.29±2.96*4668.00±1478.22*387.32±81.44*175.34±26.20 High-dose 4.11±1.78*4067.38±1272.76*373.88±88.12*184.49±23.09
*<0.05 as comparing with PM2.5 control group.**0.05 as comparing with blank control group or saline control group. SOD: superoxide dismutase.
Table 3. Protein expressions of HO-1, NQO1 and NRF-2 in rat pulmonary tissues of the study groups and the PM2.5 control group (n=8, ±s)
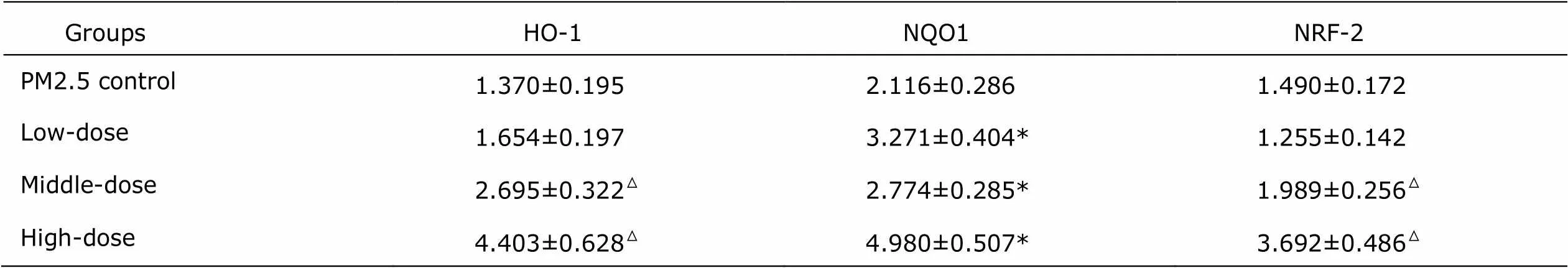
Table 3. Protein expressions of HO-1, NQO1 and NRF-2 in rat pulmonary tissues of the study groups and the PM2.5 control group (n=8, ±s)
GroupsHO-1NQO1NRF-2 PM2.5 control 1.370±0.1952.116±0.2861.490±0.172 Low-dose 1.654±0.1973.271±0.404*1.255±0.142 Middle-dose 2.695±0.322△2.774±0.285*1.989±0.256△ High-dose 4.403±0.628△4.980±0.507*3.692±0.486△
*<0.05 as comparing with PM2.5 control group. △<0.05 as comparing with PM2.5 control group by Dunnett T3 test.HO-1: heme oxygenase enzyme; NQO1: quinone oxidoreductase; NRF-2: NF-E2-related factor 2.
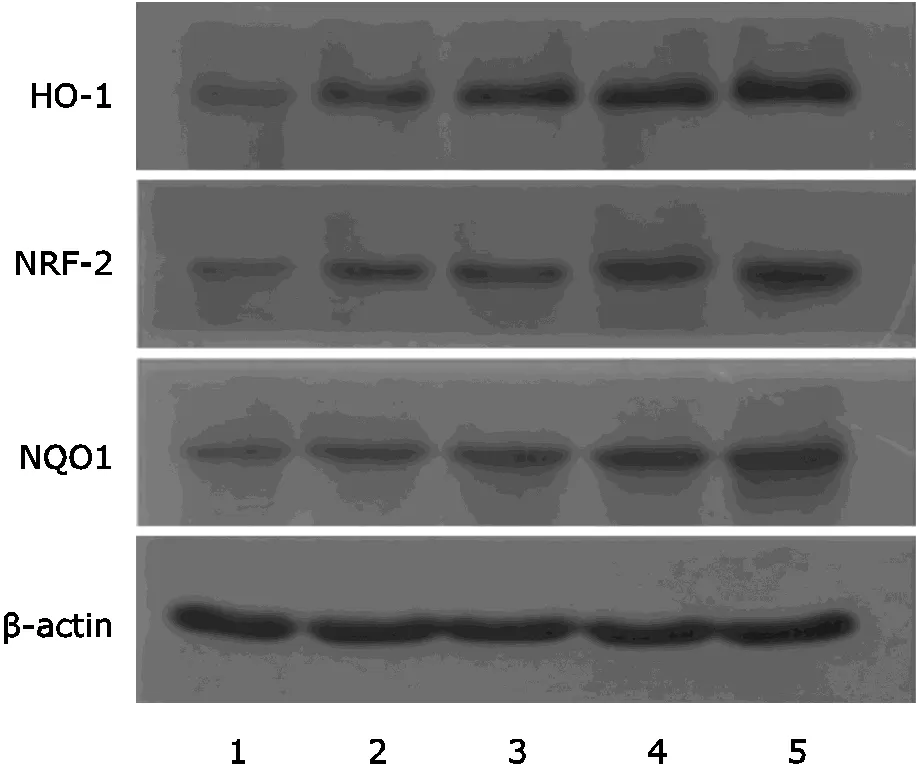
Figure 2. Protein expressions of HO-1, NRF-2 and NQO1 in pulmonary tissues by Western blot. HO-1, NRF-2 and NQO1 protein expression were signifiantly higher in the groups of middle- and high- dose Lianhua Qingwen than controls (<0.05). 1: Control group; 2: PM2.5 control group; 3: Low-dose group; 4: Middle-dose group; 5: High-dose group.
DISCUSSION
In recent years, studies have shown that PM2.5 can induce chronic inflammation and oxidative stress injury for pulmonary tissues, reduce lung function, affect lung development at some extent, and was associated with remarkable increase of morbidities and mortalities from COPD, asthma and other respiratory diseases.1Elevated concentration of PM2.5 in environment could increase mortality of people who was suffering from heart and lung diseases, including lung cancer, and might also increase all-cause mortality.2
In this studywe found that intratracheal instillation of PM2.5 suspension for 24 hours induced oxidative injury of pulmonary tissues in rats. LDH is a kind of metabolic enzyme with abundant content in cells, and can be released into extracellular space after damage of cell membrane. Changes of LDH can indirectly reflect extent of cytotoxic damage. MDA can be used to evaluate cellular oxidative injury and oxidative level.3GSH-P and SOD are important enzymes with anti-oxidation effects in human body. We found that exposure to PM2.5 suspension for 24 hours significantly increased metabolic enzyme LDH in BALF, indicating PM2.5 induced oxidative injuries of pulmonary parenchyma and biological membrane. This results was consistent withstudies by Xiao4and Cao5Besides, we found that, inPM2.5 control group, oxidative stress product MDA increased in serum and BALF, and GSH-P in BALF and serum and SOD in serum decreased, which indicated the elevated peroxidation level and that GSH-P and SOD were consumed asantioxidant system to protect against oxidative injury induced by PM2.5. These results were also consistent with the previous studies.6-8
With consideration of components and formula of Lianhua Qingwen Capsule from the point of view ofTraditional Chinese Medicine (TCM), it has been used in thetreatment of inflammation diseases such as acute pulmonary infection, for its antivirus activity and regulating pulmonary immunity. In this study, different doses of Lianhua Qingwen were used to treat PM2.5 exposed rats. Compared to the PM2.5 control group, oxidative stress marker,LDH and MDA in both BALF and serum weresignificantly reduced in middle- and high-dose pre-treated groups, GSH-P activity was significantly increased in middle- and high-dose pre-treated groups as well, which indicated that middle- and high-dose Lianhua Qingwen could protect against or treat pulmonary oxidative injury. No statistical difference of GSH-P in rat serum was found between low-dose Lianhua Qingwen pre-treated group and the control groups (>0.05).These results suggested that middle- or high-dose Lianhua Qingwen could increase GSH-P activity in BALF, and it might help to treat pulmonary oxidative injury induced by PM2.5 in a dose- dependent manner.
NRF-2 is a central regulator of cellular antioxidant response, and regulates expression of antioxidant protein and phase Ⅱ detoxification enzyme with interaction ofantioxidant responsive element (ARE). After NRF-2 binding with ARE promoter site, downstream target genes were activated that there were expression of catalase (CAT), SOD, glutathione S-transferase (GST), quinone oxidore- ductase (NQ1), heavy chain γ-glutamate synthetase (γ- GCSH), light chain γ-glutamate synthetase (γ-GCSH) and HO-1.9-10HO-1 can decompose heme to produce bilirubin, which has a high antioxidative effect.11NQO1 can catalyze quinones, an important part of PM2.5, to directly revert to hydroquinone, most of which can be stably combined and excreted in cells. Consequently, production of unstable semiquinone materials was avoided.12In this study we found that the NQO1 protein expression increased in all three Lianhua Qingwen pre-treated groups, and HO-1, NRF-2 protein expressionsignificantly increased in the middle- and high-dose treated group. These results suggested that Lianhua qingwen could protect pulmonary tissues against oxidative injury induced by PM2.5.
Qu13found that PM2.5 had acute toxic effects on rat pulmonary tissues. On pulmonary pathological slide, inflammatory changes including hyperplasia of neutrophil, monocyte and lymphocyte were observed. In our study, no abnormal histological change of rat lungs was observed in blank control group and saline control group. For PM2.5 control group, histological changes such as thickened alveolar septum, congestion of blood capillary and interstitial edema,distinctive lymphocytes infiltration and neutrophils infiltration were found surrounding bronchiole. This sug- gested that fine particulates had a toxic damage to lung tissues. These pathological changes were relieved in the Lianhua Qingwen pretreated groups. This demonstrated the protective effect of Lianhua Qingwen on pulmonary injury induced by PM2.5.
In conclusion, our study demonstrated the stress injury of pulmonary tissues induced by PM2.5 in rats, and providedevidence that Lianhua Qingwen in middle- and high-dose could alleviate this injury. Given the damage of PM2.5, our observations suggested it would be necessary to perform clinical trail in the future to study the effects of Lianhua Qingwen on patients with respiratory diseases that are related to PM2.5, as well as the underlying mechanism.
1. Kulkarni N, Pierse N, Rushton L, et al. Carbon in airway macro- phages and lung function in children. N Engl J Med 2006; 355:21-30.
2. PoP CA, Burnett RT, Thun MJ, et al. Lung cancer, cardiopulmornary mortality, and longterm esposures to fine particulate airpollution. JAMA 2002; 287: 1132-41.
3. Dellinger B, Pryor W A, Cueto R, et al. Role of free radicals in the toxicity of air borne fine particulate matter. Chem Res Toxicol 2001; 14:1371-7.
4. Xiao CL, Xi SH, Wang RQ, et al. Study on deep respiratory injury induced by air pollutants in rats. Chin J of Public Health 2003; 19:555-7.
5. Cao Q, Qian X, Zhang S, et al. Cytotoxicity of water-soluble components and water-insoluble components in environ- mental fine particulates. Acta Scientiae Circumstantiae 2008; 28:1167-72.
6. Sørensen M, Daneshvar B, Hansen M, et al. Personal PM2.5 exposure and markers of oxidative stress in blood. Environ Health Perspect 2003; 111:161-5.
7. Kim K, Park EY, lee K H. Differential oxidative stress response in young children and the elderly following exposure to PM2.5. Society for Hygiene 2009; 14:60-6.
8. Romien I, Garcia-Esteban R, Sunyer J, et al. The effect of supplementation with omega-3 polyunsaturated fatty acid on markers of oxidative stress in elderly exposed to PM2.5. Envior Health Perspect 2008; 116:1237-42.
9. Kwak MK, Wakabayashi N, Kensler TW. Chemoprevention through the Keap1-Nrf2 signaling pathway by phase 2 enzyme inducers. Mutation Res 2004; 555: 133-48.
10. Min KJ, Kim JH, Jou I, et al. Adenosine induces hemeoxy- genase-lexpression in microglia through the activation of phosphatidylinositol 3-kinase and nuclear factor E2- related factor 2. Glia 2008; 56:1028-37.
11. Zhang YL, Zhao JY. Cellular protective effect of heme oxygenase-1. Journal of Environ and Occupa Med 2008; 25:197-202.
12. Xia XJ, Jin ZC. Research advances on NQO1 enzyme and its inducible expression in oxygen environment. Progress in Physiological Science 2002; 33:225-9.
13. Qu HM, Niu JP, et al. Study on pulmonary toxicity induced by PM2.5 in atmosphere of Lanzhou city. Chin J of Public Health2006; 22:598-9.
for publication June 14, 2016.
Tel: (86) 311 85988293; E-mail: pingfen2003@126.com
杂志排行
Chinese Medical Sciences Journal的其它文章
- Expression of miRNA-140 in Chondrocytes and Synovial Fluid of Knee Joints in Patients with Osteoarthritis△
- The Effect of Sleep Deprivation on Coronary Heart Disease△
- Uterine Artery Embolization for Management of Primary Postpartum Hemorrhage Associated with Placenta Accreta
- Meta-analysis of aspirin-heparin therapy for un-explained recurrent miscarriage
- Pseudohyperkalemia with Myelofibrosis after Splenectomy
- A Case Report of Acute Arterial Embolization of Right Lower Extremity As the Initial Presentation of Nephrotic Syndrome with Minimal Changes△
