LMO4基因沉默对Snail诱导的非小细胞肺癌A549细胞上皮-间质转化的影响研究
2016-06-28王雯珺伍思培列璞怡郭敏章何建行
王雯珺,伍思培,列璞怡,郭敏章,何建行
·论著·
LMO4基因沉默对Snail诱导的非小细胞肺癌A549细胞上皮-间质转化的影响研究
王雯珺,伍思培,列璞怡,郭敏章,何建行
510120广东省广州市,呼吸疾病国家重点实验室(王雯珺,列璞怡,郭敏章,何建行);广州医科大学第一附属医院(王雯珺,列璞怡,郭敏章,何建行);广州医科大学(王雯珺,列璞怡,郭敏章,何建行);广东省人民医院肿瘤中心(伍思培);广东省医学科学院(伍思培)
【摘要】目的探讨LMO4基因沉默对Snail诱导的非小细胞肺癌A549细胞上皮-间质转化(EMT)的影响。方法体外培养非小细胞肺癌A549细胞,取对数生长期细胞进行实验,根据转染慢病毒分为sh-NC组(转染sh-NC慢病毒)、sh-LMO4组(转染sh-LMO4慢病毒)、Snail组(转染Snail慢病毒)和Snail+sh-LMO4组(转染Snail慢病毒和sh-LMO4慢病毒)。采用实时定量RT-PCR检测sh-NC组和sh-LMO4组LMO4 mRNA相对表达量,采用Western Bloting法检测sh-NC组和sh-LMO4组LMO4蛋白相对表达量及4组N-cadherin、Vimentin、E-cadherin蛋白相对表达量,采用Transwell实验检测4组细胞侵袭个数,采用细胞划痕实验检测4组细胞迁移距离。结果sh-LMO4组LMO4 mRNA相对表达量和LMO4蛋白相对表达量均低于sh-NC组(P<0.05)。Snail组侵袭细胞个数多于sh-NC组,sh-LMO4组侵袭细胞个数少于sh-NC组,Snail+sh-LMO4组侵袭细胞个数少于Snail组、多于sh-LMO4组(P<0.05)。培养24 h后Snail组细胞迁移距离长于sh-NC组,sh-LMO4组细胞迁移距离短于sh-NC组,而Snail+sh-LMO4组细胞迁移距离短于Snail组、长于sh-LMO4组(P<0.05)。Snail组N-cadherin和Vimentin蛋白相对表达量高于sh-NC组,E-cadherin蛋白相对表达量低于sh-NC组(P<0.05);sh-LMO4组N-cadherin和Vimentin蛋白相对表达量低于sh-NC组,E-cadherin蛋白相对表达量高于sh-NC组(P<0.05);Snail+sh-LMO4组N-cadherin和Vimentin蛋白相对表达量低于Snail组,E-cadherin蛋白相对表达量高于Snail组(P<0.05);Snail+sh-LMO4组N-cadherin和Vimentin蛋白相对表达量高于sh-LMO4组,E-cadherin蛋白相对表达量低于sh-LMO4组(P<0.05)。结论LMO4基因沉默可逆转Snail诱导的非小细胞肺癌A549细胞EMT。
【关键词】癌,非小细胞肺;LMO4;Snail;上皮-间质转化
王雯珺,伍思培,列璞怡,等.LMO4基因沉默对Snail诱导的非小细胞肺癌A549细胞上皮-间质转化的影响研究[J].实用心脑肺血管病杂志,2016,24(5):54-58.[www.syxnf.net]
Wang WJ, Wu SP,Lie PY,et al.Impact of LMO4 gene silencing on snail-induced EMT of non-small cell lung cancer A549 cells[J].Practical Journal of Cardiac Cerebral Pneumal and Vascular Disease,2016,24(5):54-58.
在我国肺癌的发病率在所有肿瘤疾病中居首位,目前其主要治疗方法是以手术治疗为主的综合治疗,而导致患者死亡的主要原因是复发或肿瘤转移[1]。因此,了解肿瘤转移过程中分子作用机制有利于寻找有效的治疗靶点,为提高患者的临床预后提供可能。LMO4是最新发现的核转录协作因子,属于LIM蛋白家族中的一员,其可作为一个桥连接因子与其他组织特定部位的DNA结合蛋白发生相互作用,形成多蛋白复合体,进而调节基因转录。有研究指出,LMO4在多种上皮细胞癌组织中呈高表达,包括前列腺癌、乳腺癌、口腔癌、神经母细胞瘤等[2-5]。据报道LMO4在乳腺癌的细胞周期进程中起重要作用,且有研究者认为LMO4可作为乳腺癌的独立预测因素[3]。Kim等[6]学者认为,LMO4可能通过影响肺癌细胞上皮-间质转化(EMT)而引起肿瘤转移,已明确Snail可促进细胞发生EMT。本研究采用sh-LMO4慢病毒处理被Snail慢病毒转染的非小细胞肺癌A549细胞,旨在探讨LMO4基因沉默对Snail诱导的非小细胞肺癌A549细胞EMT的影响。
1材料与方法
1.1细胞培养人非小细胞肺癌A549细胞株为本实验室自存,将人非小细胞肺癌A549细胞于含有10%胎牛血清(Hyclone,美国)、100 U/ml青霉素、100 μg/ml链霉素(碧云天,杭州)的1640培养基(Gibico,美国)中传代培养,培养条件:温度37 ℃,5%二氧化碳。A549细胞经过消化传代,取对数生长期细胞进行实验。
1.2实验分组及慢病毒转染sh-LMO4慢病毒、sh-NC慢病毒和Snail慢病毒购于吉凯公司,慢病毒转染前24 h,将处于对数生长期的目的细胞消化后接种于6孔板中(细胞数约为5×105)过夜;用含有6 μg/ml Polybrene的新鲜培养基2 ml换液,根据预实验摸索的细胞感染复数(MOI)值,分别加入适量的sh-NC慢病毒(sh-NC组)、sh-LMO4慢病毒(sh-LMO4组)和Snail慢病毒(Snail组),37 ℃孵育。继续培养24 h后换液,转染3 d后采用含嘌呤霉素(浓度为5.00 μg/ml)的培养基继续培养并进行筛选,选取稳定细胞株进行后续实验。然后在转染Snail慢病毒的细胞中转染sh-LMO4慢病毒(Snail+sh-LMO4组),转染3 d后用含G418(浓度5.00 μg/ml)的培养基继续培养并进行筛选,选取稳定细胞株进行后续实验。
名词解释:
转染是将外源性基因导入细胞内的一种专门技术,大致可分为物理介导、化学介导和生物介导3类。物理介导方法包括电穿孔法、显微注射和基因枪;化学介导方法有很多,如经典的磷酸钙共沉淀法、脂质体转染法和多种阳离子物质介导的技术;生物介导方法包括原始的原生质体转染和各种病毒介导的转染技术。
1.3实时定量RT-PCRTrizol法提取sh-NC组和sh-LMO4组细胞总RNA,1%琼脂糖凝胶电泳,定量后使用TAKARA反转录试剂盒(1st Strand cDNA Synthesis Kit)进行反转录,行特定引物PCR扩增,引物合成在吉玛公司进行,具体操作按照说明书执行,采用2-ΔΔct法计算LMO4 mRNA的相对表达量。PCR引物如下:LMO4上游引物:5′-GGA CCG CTT TCT GCT CTA TG-3′;LMO4下游引物:5′-AAG CAC CGC TAT TCC CAA AT-3′;GAPDH上游引物:5′-AAT CCC ATC ACC ATC TTC CA-3′,GAPDH下游引物:5′-CCT GCT TCA CCA CCT TCT TG-3′。
1.4Western Bloting法提取4组细胞总蛋白,进行蛋白定量检测,电泳后转膜,Western封闭液37 ℃封闭2 h,加入相应的一抗(santa cruz,美国),抗体稀释比例分别为ant-LMO4(1∶1 000)、anti-N-cadherin(1∶1 000)、anti-Vimentin(1∶1 000)、anti-E-cadherin(1∶1 000)、anti-GAPDH(1∶500),4 ℃孵育过夜;然后加入稀释比例为1∶500的辣根过氧化酶标记的二抗(santa cruz,USA)。化学发光试剂盒在胶片上显影,晾干后扫描,测定灰度值,并计算扩增出的目的基因与内参基因灰度值的比值,即以LMO4/GAPDH灰度值的比值表示LMO4蛋白相对表达量、N-cadherin/GAPDH灰度值的比值表示N-cadherin蛋白相对表达量、Vimentin /GAPDH灰度值的比值表示Vimentin 蛋白相对表达量、E-cadherin/GAPDH灰度值的比值表示E-cadherin蛋白相对表达量。
1.5Transwell实验将4组细胞制备成单细胞悬液,在Transwell上室(Gibico,美国)中铺好基质胶(BD,美国),每孔加入无血清培养液200 μl(含细胞5×104个/ml),下室加入500 μl含10%胎牛血清的RPMI-1640培养液,常规培养24 h后用棉签擦去基质胶和上室内的细胞进行染色,使用奥林巴斯倒置显微镜拍照(×200),取若干视野计数侵袭细胞个数。
1.6细胞划痕实验取4组细胞,消化后以5×106/L密度接种于12孔细胞培养板,用200 μl枪头在每孔中央划出一道划痕,处理24 h后洗去死细胞,采用奥林巴斯显微镜拍照记录细胞生长情况。采用Image Pro Plus 6.0软件测量每孔多个点划痕间距,取平均值。
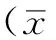
2结果
2.1sh-LMO4慢病毒转染非小细胞肺癌A549细胞后LMO4表达情况sh-LMO4组LMO4 mRNA相对表达量和LMO4蛋白相对表达量均低于sh-NC组,差异有统计学意义(P<0.05,见表1、图1)。
注:A采用实时定量RT-PCR,B采用Western Bloting法
图1sh-NC组和sh-LMO4组LMO4 mRNA和LMO4蛋白表达情况
Figure 1Expression of LMO4 mRNA and LMO4 protein of sh-NC group and sh-LMO4 group

Table 1Comparison of the relative expression quantity of LMO4 mRNA and LMO4 protein between sh-NC group and sh-LMO4 group

组别LMO4mRNA相对表达量LMO4蛋白相对表达量sh-NC组1.10±0.110.97±0.14sh-LMO4组0.28±0.060.38±0.06t值5.513.71P值0.000.02
2.2LMO4基因沉默对Snail诱导的非小细胞肺癌A549细胞侵袭能力的影响Transwell实验结果显示,sh-NC组侵袭细胞个数为(129.66±15.91)个,Snail组为(311.11±15.91)个,sh-LMO4组为(58.00±1.73)个,Snail+sh-LMO4组为(131.66±6.00)个。4组侵袭细胞个数比较,差异有统计学意义(F=43.99,P=0.00),其中Snail组侵袭细胞个数多于sh-NC组,差异有统计学意义(t=6.94,P=0.02);sh-LMO4组侵袭细胞个数少于sh-NC组,差异有统计学意义(t=7.76,P=0.00);Snail+sh-LMO4组侵袭细胞个数少于Snail组、多于sh-LMO4组,差异有统计学意义(t值分别为18.18、9.58,P<0.05,见图2)。

图2 4组细胞侵袭能力
2.3LMO4基因沉默对Snail诱导的非小细胞肺癌A549细胞迁移能力的影响细胞划痕实验结果显示,24 h后sh-NC组细胞迁移距离为(15.17±3.42)μm,Snail组为(63.24±8.71)μm,sh-LMO4组为(5.17±0.92)μm,Snail+sh-LMO4组为(31.23±3.80)μm。4组细胞迁移距离比较,差异有统计学意义(F=28.54,P=0.00),其中Snail组细胞迁移距离长于sh-NC组,差异有统计学意义(t=4.88,P=0.04);sh-LMO4组细胞迁移距离短于sh-NC组,差异有统计学意义(t=4.89,P=0.01);Snail+sh-LMO4组细胞迁移距离短于Snail组、长于sh-LMO4组,差异有统计学意义(t值分别为5.83、7.01,P<0.05,见图3)。
2.4LMO4基因沉默对Snail诱导的非小细胞肺癌A549细胞N-cadherin、Vimentin、E-cadherin表达的影响4组E-cadherin、N-cadherin、Vimentin蛋白相对表达量比较,差异有统计学意义(P<0.05)。Snail组N-cadherin和Vimentin蛋白相对表达量高于sh-NC组,E-cadherin蛋白相对表达量低于sh-NC组,差异有统计学意义(P<0.05);sh-LMO4组N-cadherin和Vimentin蛋白相对表达量低于sh-NC组,E-cadherin蛋白相对表达量高于sh-NC组,差异有统计学意义(P<0.05);Snail+sh-LMO4组N-cadherin和Vimentin蛋白相对表达量低于Snail组,E-cadherin蛋白相对表达量高于Snail组,差异有统计学意义(P<0.05);Snail+sh-LMO4组N-cadherin和Vimentin蛋白相对表达量高于sh-LMO4组,E-cadherin蛋白相对表达量低于sh-LMO4组,差异有统计学意义(P<0.05,见表2、图4)。

图3 4组细胞迁移能力

图4 4组E-cadherin、N-cadherin、Vimentin蛋白表达情况
Figure 4The expression of E-cadherin protein,N-cadherin protein and Vimentin protein of the four groups
表24组N-cadherin、Vimentin、E-cadherin蛋白相对表达量比较

Table 2Comparison of the relative expression quantity of N-cadherin protein,Vimentin protein and E-cadherin protein of the four groups

组别N-cadherin蛋白相对表达量Vimentin蛋白相对表达量E-cadherin蛋白相对表达量sh-NC组0.22±0.100.17±0.530.67±0.04Snail组0.59±0.06ab0.48±0.05ab0.25±0.04absh-LMO4组0.11±0.10ab0.13±0.01ab0.74±0.03abSnail+sh-LMO4组0.39±0.330.35±0.060.43±0.11F值48.959.4925.59P值0.000.000.00
注:与sh-NC组比较,aP<0.05;与Snail+sh-LMO4组比较,bP<0.05
3讨论
肺癌是全球范围内发病率和病死率最高的恶性肿瘤,在我国肺癌的发病率近年来呈逐年上升趋势,目前手术仍是其主要治疗方法。近年来,虽然CT、正电子发射断层扫描(PET)-CT等影像学技术迅速发展,但仍存在漏诊和误诊现象,很多肺癌患者就诊时已是中晚期,已错过最佳治疗时间。临床研究表明,晚期肿瘤患者多存在多脏器的侵袭和转移,如能在基因、蛋白质层面发现早期诊断、预测肿瘤的标志物,或阻断侵袭和转移途径中的关键基因,则对晚期肺癌患者的治疗具有重要意义,因此该方面的研究已引起广大医疗工作者和科研人员的关注。
LIM蛋白是一个非常重要的转录调节因子,其在决定细胞生长与调亡、胚胎发育及肿瘤发生中具有重要作用。LMO蛋白是LIM的亚家族,有LMO1~LMO4 4个家族成员,LMO蛋白主要定位于细胞核,含有可与蛋白质相互作用的LIM锌结合域,但缺乏DNA结合及催化结构域[7]。LMO家族在正常器官发育中起关键作用,且动物实验结果表明,缺乏LMO1和LMO3的小鼠出生后会出现不明原因死亡[8]。临床研究显示,LMO1的表达与T淋巴细胞白血病、急性淋巴细胞白血病密切相关[9];LMO2在胚胎造血和血管生成中是必不可少的,在血管内皮细胞中下调LMO2表达可使 E-cadherin表达下调[10];LMO4作为LMO家族中的一员,含有156个氨基酸的残基,其基本功能是与其他DNA结合蛋白组成大的蛋白复合体,调节转录因子的活性,从而调节一系列基因的表达,在胚胎发育、组织分化及肿瘤形成过程中起重要作用。LMO4可与p53相互作用,但过表达的p53可抑制LMO4[11]。有研究显示,LMO4可调节转化生长因子β(TGF-β)引起的EMT[12]。
在上皮细胞癌中,绝大部分的转移与EMT的发生有关,且同时伴随明显的细胞形态学改变、细胞与细胞间及细胞与基质间的黏附性丢失、迁移和侵袭能力增强等。EMT的发生主要以上皮标志物E-cadherin下降和间质标志物Vimentin上升为生物学特征。研究人员通过在原发性肿瘤中检测EMT相关标志物发现,其表达与肿瘤的转移及患者预后有关。有研究指出在Snail介导的EMT中LMO4起关键作用。Snail属于含有SNAG结构域的锌指(zinc-finger)蛋白家族成员,SNAG位于Snail分子的N末端,具有抑制基因转录的作用[13]。在胚胎发育和肿瘤转移过程中,Snail是诱导EMT的关键因子,是连接多种促肿瘤转移信号通路的中心分子[14]。本实验旨在探讨LMO4基因沉默对Snail诱导的非小细胞肺癌A549细胞EMT的影响。
本研究结果显示,sh-LMO4组LMO4 mRNA相对表达量和LMO4蛋白相对表达量均低于sh-NC组,提示sh-LMO4慢病毒能抑制非小细胞肺癌A549细胞中LMO4的表达;Snail组侵袭细胞个数多于sh-NC组、细胞迁移距离长于sh-NC组,sh-LMO4组侵袭细胞个数少于sh-NC组、细胞迁移距离短于sh-NC组,提示Snail过表达能增强肿瘤细胞的侵袭和迁移能力,进而促进EMT的发生;LMO4基因沉默能减弱肿瘤细胞的侵袭和迁移能力,进而抑制EMT的发生。本研究结果亦显示,Snail+sh-LMO4组侵袭细胞个数少于Snail组、多于sh-LMO4组,细胞迁移距离短于Snail组、长于sh-LMO4组,提示LMO4基因沉默可抑制过表达的Snail对非小细胞肺癌A549细胞侵袭和迁移能力的增强作用。E-cadherin是上皮细胞标志物,N-cadherin、Vimentin是间质细胞标志物,研究显示E-cadherin蛋白表达降低及N-cadherin、Vimentin蛋白表达升高提示EMT的发生。本研究结果显示,Snail组N-cadherin和Vimentin蛋白相对表达量高于sh-NC组,E-cadherin蛋白相对表达量低于sh-NC组;LMO4组N-cadherin和Vimentin蛋白相对表达量低于sh-NC组,E-cadherin蛋白相对表达量高于sh-NC组;Snail+sh-LMO4组N-cadherin和Vimentin蛋白相对表达量低于Snail组,E-cadherin蛋白相对表达量高于Snail组;Snail+sh-LMO4组N-cadherin和Vimentin蛋白相对表达量高于sh-LMO4组,E-cadherin蛋白相对表达量低于sh-LMO4组。提示Snail过表达能促进EMT的发生,LMO4基因沉默能抑制EMT的发生,且LMO4基因沉默可逆转Snail诱导的A549细胞发生EMT。
综上所述,LMO4沉默可逆转Snail诱导的非小细胞肺癌A549细胞发生EMT,为后续研究LMO4对肺癌的侵袭和转移的分子机制提供了实验依据。
作者贡献:王雯珺进行实验设计与实施、资料收集整理、撰写论文、成文并对文章负责;伍思培、列璞怡、郭敏章进行实验实施、评估、资料收集;何建行进行质量控制。
本文无利益冲突。
参考文献
[1]Rajer M,Zwitter M,Rajer B.Pollution in the working place and social status:co-factors in lung cancer carcinogenesis[J].Lung Cancer,2014,85(3):346-350.
[2]Yu J,Ohuchida K,Nakata K,et al.LIM only 4 is overexpressed in late stage pancreas cancer[J].Mol Cancer,2008.doi:10.1186/1476-4598-7-93.
[3]Montanez-Wiscovich ME,Seachrist DD,Landis MD,et al.LMO4 is an essential mediator of ErbB2/HER2/Neu-induced breast cancer cell cycle progression[J].Oncogene,2009,28(41):3608-3618.
[4]Mizunuma H,Miyazawa J,Sanada K,et al.The LIM-only protein,LMO4,and the LIM domain-binding protein, LDB1,expression in squamous cell carcinomas of the oral cavity[J].Br J Cancer,2003,88(10):1543-1548.
[5]Vu D,Marin P,Walzer C,et al.Transcription regulator LMO4 interferes with neuritogenesis in human SH-SY5Y neuroblastoma cells[J].Mol Brain Res,2003,115(2):93-103.
[6]Kim MJ,Lim J,Yang Y,et al.N-myc downstream-regulated gene 2(NDRG2) suppresses the epithelial-mesenchymal transition(EMT) in breast cancer cells via STAT3/Snail signaling[J].Cancer Lett,2014,354(1):33-42.
[7]Racevskis J,Dill A,Sparano JA,et al.Molecular cloning of LMO41,a new human LIM domain gene[J].Biochim Biophys Acta,1999,1445(1):148-153.
[8]Tse E,Smith AJ,Hunt S,et al.Null mutation of the Lmo4 gene or a combined null mutation of the Lmo1/Lmo3 genes causes perinatal lethality,and Lmo4 controls neural tube development in mice[J].Mol Cell Biol, 2004,24(5):2063-2073.
[9]Oram SH,Thoms J,Sive JI,et al.Bivalent promoter marks and a latent enhancer may prime the leukaemia oncogene LMO1 for ectopic expression in T-cell leukaemia[J].Leukemia,2013,27(6):1348-1357.
[10]Peng X,Ye Z,Gao Y.Regulation of E-cadherin promoter activity by LMO2 impact on the progression and metastasis of prostate cancer[J].Zhongguo Ying Yong Sheng Li Xue Za Zhi,2013,29(5):385-389.
[11]Zhou X,Sang M,Liu W,et al.LMO4 inhibits p53-mediated proliferative inhibition of breast cancer cells through interacting p53[J].Life Sci,2012,91(9/10):358-363.
[12]Ferronha T,Rabadán MA,Gil-Guion E,et al.LMO4 is an essential cofactor in the Snail2-mediated epithelial-to-mesenchymal transition of neuroblastoma and neural crest cells[J].J Neurosci,2013,33(7): 2773-2783.
[13]Chang C,Yang X,Pursell B,et al.Id2 complexes with the SNAG domain of Snai1 inhibiting Snai1-mediated repression of integrin β4[J].Mol Cell Biol,2013,33(19):3795-3804.
[14]He L,Lou W,Ji L,et al.Serum response factor accelerates the high glucose-induced Epithelial-to-Mesenchymal Transition(EMT) via snail signaling in human peritoneal mesothelial cells[J].PLoS One,2014,9(10):e108593.
(本文编辑:谢武英)
Impact of LMO4 Gene Silencing on Snail-induced EMT of Non-small Cell Lung Cancer A549 Cells
WANGWen-jun,WUSi-pei,LIEPu-yi,etal.
NationalKeyLaboratoryforRespiratoryDisease,Guangzhou510120,China
【Abstract】ObjectiveTo investigate the impact of LMO4 gene silencing on Snail-induced EMT of non-small cell lung cancer A549 cells.MethodsNon-small cell lung cancer A549 cells were cultured in vitro,and logarithmic growth phase cells were extracted to carry out the experiment.According to the transfection of slow viruses,the cells were divided into A group(transfected with sh-NC slow virus),B group(transfected with sh-LMO4 slow virus),C group(transfected with Snail slow virus)and D group(transfected with Snail and sh-LMO4 slow virus).Quantitative Real-Time RT-PCR was used to detect the relative expression quantity of LMO4 mRNA of A group and B group;Western Bloting method was used to detect the relative expression quantity of LMO4 protein of A group and B group,the relative expression quantity of N-cadherin protein,Vimentin protein and E-cadherin protein of the four groups;Transwell test was used to detect the number of cell invasion of the four groups;cell scratch test was used to detect the cell migration distance of the four groups.ResultsThe relative expression quantity of LMO4 mRNA and LMO4 protein of B group were statistically significantly lower than those of A group(P<0.05).The number of cell invasion of C group was statistically significantly more than that of A group,that of B group was statistically significantly less than that of A group,that of D group was statistically significantly less than that of C group,but was statistically significantly more than that of B group(P<0.05).After 24 hours of culture,the cell migration distance of C group was statistically significantly longer than that of A group,that of B group was statistically significantly shorter than that of A group,that of D group was statistically significantly shorter than that of C group,but was statistically significantly longer than that of B group(P<0.05).The relative expression quantity of N-cadherin protein and Vimentin protein of C group were statistically significantly higher than those of A group,while that of E-cadherin protein of C group was statistically significantly lower than that of A group(P<0.05);the relative expression quantity of N-cadherin protein and Vimentin protein of B group were statistically significantly lower than those of A group,while that of E-cadherin protein of B group was statistically significantly higher than that of A group(P<0.05);the relative expression quantity of N-cadherin protein and Vimentin protein of D group were statistically significantly lower than those of C group,while that of E-cadherin protein of D group was statistically significantly higher than that of A group(P<0.05);the relative expression quantity of N-cadherin protein and Vimentin protein were statistically significantly higher than those of B group,while that of E-cadherin protein of D group was statistically significantly lower than that of B group(P<0.05).ConclusionLMO4 gene silencing can reverse the Snail-induced EMT of non-small cell lung cancer A549 cells.
【Key words】Carcinoma,non-small-cell lung;LMO4;Snail;Epithelial-mesenchymal transition
基金项目:国家自然科学基金(81301939);博士后基金(2015M570697)
通信作者:王雯珺,510120广东省广州市,呼吸疾病国家重点实验室,广州医科大学第一附属医院,广州医科大学;E-mail:wwj01@foxmail.com
【中图分类号】R 730.26
【文献标识码】A
doi:10.3969/j.issn.1008-5971.2016.05.013
(收稿日期:2015-12-16;修回日期:2016-04-28)
