胃癌根治术联合脾脏切除治疗胃癌的Meta分析
2016-06-07刘启领
刘启领
上海市公共卫生临床中心普外一科,上海 201508
胃癌根治术联合脾脏切除治疗胃癌的Meta分析
刘启领
上海市公共卫生临床中心普外一科,上海 201508
目的 评估胃癌根治术(radical gastrectomy, RG)联合脾脏切除术(splenectomy, SE)治疗进展期胃癌的长期效果。方法 以PubMed、Cochrane、Web of knowledge、Ovid SpringerLink、中国知网、万方、维普为数据源,检索相关文献,采用RevMan对切脾与保脾的RG随机对照试验(RCT)进行Meta分析,结局变量为患者5年生存率、手术操作相关并发症、术后30 d死亡率。结果 符合纳入标准的RCT研究共5篇,包括1 344例病例,切脾组588例,保脾组756例,切脾组与保脾组5年生存率比较,差异无统计学意义(OR=0.80,95%CI: 0.60~1.06,P>0.05),灵敏性分析切脾组患者5年生存率低于保脾组(OR=0.72,95%CI: 0.53~0.97,P<0.05);切脾组操作相关并发症发生率明显高于保脾组(OR=2.51,95%CI: 1.90~3.33,P<0.05),两组术后30 d死亡率比较差异无统计学意义(OR=1.57,95%CI: 0.59~4.23,P>0.05)。结论 SE并不能有效改善患者预后,反而增加了术后并发症的发生,暂不推荐SE作为进展期胃癌的常规术式。
进展期胃癌;胃癌根治术;脾切除;随机对照试验;Mate分析
胃癌根治术(radical gastrectomy, RG)是进展期近端胃癌的标准术式[1]。临床上由于近端胃癌、胃食管交界癌以及淋巴转移性癌更易出现于脾门,因此,常联合脾切除术(splenectomy, SE)清扫脾动脉旁及脾门淋巴结,以保证手术根治的彻底性[2];然而,SE对预后的影响是有争议的。先前的报道表明,胃切除术联合脾切除术(radical gastrectomy combined splenectomy, RGSE)较单纯的RG能更为有效地延长患者生存期[3]。但有学者认为,SE非但不会提高胃癌患者存活率[4],反而脾切除后其免疫功能的丧失将导致不良后果[5]。最近的临床试验显示,RGSE可能导致更高的术后发病率和死亡率[6-7]。本Meta分析旨在评价SE对胃癌患者远期生存的影响,并比较切脾和保脾患者术后手术相关并发症发生率和死亡率的差异。
1 资料与方法
1.1 资料来源 研究以PubMed、Cochrane临床试验中心、Web of knowledge、Ovid医学期刊数据库、SpringerLink为外文检索数据库;中国知网、万方、维普为中文文献检索数据库;外文检索关键词为“Stomach Neoplasms” (Mesh)、 “Carcinoma” (Mesh)、“splenectomy” (MeSH)、“spleen dissection” (textword)、“spleen resection” (textword)、“splenic preservation” (textword)、“Comparative Study” (Publication Type)、“follow-up studies” (Mesh)、“Clinical Trial” (Publication Type)、“Evaluation Studies” (Publication Type)、“Multicenter Study” (Publication Type)、“Random allocation” (Subheading)、“Randomized”。中文检索关键词为胃癌、胃肿瘤、胃新生物、脾切除术。此外,研究通过引用列表筛选相关文章,合格的未发表的论文中也被纳入在内,筛选文献过程中未设定发表日期与语言限制,检索截止日期2014年6月30日。
1.2 排除与纳入标准 文献纳入标准:(1)随机对照试验(RCTs);(2)评估RGSE和RG手术的有效性和安全性;(3)术前通过内镜或活检确诊。排除标准:(1)其他类型的胃肿瘤,如淋巴瘤或多个胃肿瘤;(2)其他器官肿瘤;(3)文章重复发表。
1.3 数据提取 严格按照预先制定的纳入标准,由2人独立查阅文献题目、摘要和关键词选择纳入的研究,如文章满足纳入标准则通读全文提取相关信息,包括作者、出版年份、样本量、研究对象年龄、性别、肿瘤部位、TNM分期、手术入路、切除范围、术后并发症、5年生存率、复发率。所有数据提取工作由2人独立完成,出现不一致时由第三人或协商确定。
1.4 文献治疗评估 采用如下7项评价指标对纳入文章进行质量评估:(1)是否真正做到随机;(2)是否有分配隐藏;(3)RGSE与RG手术患者基线是否保持均衡;(4)是否采用盲法干预和评估结局;(5)纳入排除标准是否合适;(6)是否有失访;(7)是否为意向性分析。7项中如果满足6项则被评为高质量;5项或4项为中质量;3项或更少为低质量[8]。
1.5 结局事件定义 结局事件为5年总体生存率、术后死亡率以及与手术操作相关的并发症发生率。其中术后死亡率为住院期间死亡率或手术后30 d死亡率,手术操作相关的并发症包括胰漏、胰腺炎、胸腔积液、腹腔脓肿、伤口感染、肠梗阻和吻合口漏、肺部感染等多种术后不良反应的总和。
1.6 统计学方法 分类数据采用相对危险度(relative risk,RR)表示,采用Review manager 5.3对数据进行分析。根据齐性检验结果选择计算模型,若研究效应量呈同质。则采用固定效应模型加权合并:若为异质,则采用随机效应模型加权合并,最终获得OR值、95%CI及P值,P<0.05为差异有统计学意义
2 结果
2.1 纳入研究基本特征 共检索到相关文献564篇,通过题目和摘要阅读, 493篇文献被排除,其余文章通读全文后,共纳入RCT研究5篇,包括1 344例病例,切脾组588例,保脾组756例。最终纳入文献和提取数据见表1。
2.2 纳入文献质量评估 纳入文献均为随机对照研究,但是否分配隐藏和是否盲法均不清楚,文献质量中等,具体见表2。
表1 纳入RCT试验资料来源和数据提取
Tab 1 RCT test data source and data extraction

纳入研究年份总例数切脾组保脾组5年生存切脾组保脾组并发症切脾组保脾组术后死亡切脾组保脾组Csendes等[9]200290973241613943Toge等[10]198541382921————Yu等[11]2006104103575016921Oh等[12]20099926745173293121Sano等[13]2010254251——774212
表2 纳入文献质量评估
Tab 2 Quality assessment of included literature

资料来源年份随机分配隐藏基线特征评估标准盲法失访意向性分析研究质量Csendes等[9]2002是不清楚是是不清楚是否中Toge等[10]1985是不清楚否否不清楚不清楚不清楚差Yu等[11]2006是不清楚是是不清楚是不清楚中Oh等[12]2009是不清楚是是不清楚是否中Sano等[13]2010是不清楚是是不清楚是否中
2.3 切脾组与保脾组生存率比较 切脾组和保脾组5年生存率比较差异无统计学意义(OR=0.80,95%CI:0.60~1.06,P=0.12),为保证研究结果的可靠性,通过灵敏性分析,排除低质量文献后,Meta分析结果显示切脾组患者5年生存率低于保脾组(OR=0.72,95%CI:0.53~0.97,P=0.03,见图1)。
2.4 切脾术与保脾术安全性比较 5篇RCT文献中,仅有4篇报道了术后并发症、死亡发生情况,对文献提取数据进行分析,结果显示,切脾组并发症发生率明显高于保脾组,差异有统计学意义(OR=2.51,95%CI:1.90~3.33,P<0.05,见图2)。两组术后死亡率比较,差异无统计学意义(OR=1.57,95%CI:0.59~4.23,P=0.37,见图2、图3)。

图2 切脾组与保脾组术后并发症发生率Meta分析
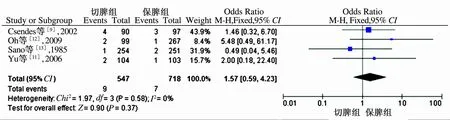
图3 切脾组与保脾组术后死亡率Meta分析
3 讨论
流行病学调查显示,近端胃癌的发病率有所增加。RG是近端胃癌的标准术式,但在手术中胃的切除范围、淋巴结清扫范围以及是否联合脾脏切除等细节问题尚缺乏统一认识。淋巴管造影结果表明淋巴流从左侧胃上部区域进入脾门淋巴结,沿着脾动脉主干前往腹腔周围的节点[14],因此,近端胃癌发生脾门淋巴结或脾动脉转移的概率为8%~10%[16]。在近端胃癌手术治疗时,通常需要联合SE以彻底清除脾门淋巴结和脾动脉淋巴结。但脾切除术为高危手术,且脾脏为机体免疫系统的一部分,行SE后患者获益多少已经引起了关注[16]。国内外学者进行了多项前瞻性随机对照试验和回顾性分析探讨SE的临床价值[17],但主要结果尚存争议。
在进行数据收集时,共查找到4篇RCT研究和17篇回顾性研究,在回顾性分析研究中,接受RGSE患者的病变通常出现在上腹部,肿瘤大,伴有3~4级浸润性病变,浆膜侵犯深度更大,淋巴结转移率高。Meta分析结果显示,切脾组5年生存率低于保脾组,与手术操作相关的并发症发生率明显高于保脾组。基于本研究结果,Meta分析显示脾切除术并不能有效改善患者预后,反而增加了术后并发症的发生。这与既往基于回顾性研究的Meta分析结果相似[18]。
脾脏是人体的“血库”,具有造血、储血功能,脾脏切除会导致机体储备的血液大量丢失,如术中操作不当导致脾动脉损伤则会使术中大出血的风险增加。脾脏是机体细胞免疫和体液免疫的中心,脾脏切除后,机体免疫功能失衡,使正常的免疫因子促吞噬和清除体内外抗原无法发挥作用,易造成感染综合征的发生;同时,脾脏切除后,外周血T淋巴细胞亚群发生改变,辅助性T淋巴细胞数量减少,抑制性T淋巴细胞数量相对增加,易导致肿瘤免疫抑制,造成肿瘤复发。最近欧洲胃切除术的临床试验表明,脾切除术是术后并发症发生和死亡的一个重要风险因素[19-20]。SE后常见的并发症是胰腺炎、胸腔积液、腹腔脓肿、伤口感染、胰漏、肠梗阻和吻合口漏。脾切除术易诱发胃残余缺血,可能导致吻合口漏和死亡率高发。
本研究为保证研究结果的信度和效度,仅纳入RCT研究,由于符合条件的高质量的相关研究过少,可能对最终的结果带来偏倚。因此,需要实施设计良好的随机对照试验来探索脾切除术的有效性。基于本研究,暂不推荐切脾术作为进展期胃癌的常规术式。
[1]Ajani JA, Bentrem DJ, Besh S, et al. Gastric cancer, version 2.2013: featured updates to the NCCN Guidelines [J]. J Natl Compr Canc Netw, 2013, 11(5): 531-546.
[2]Fang WL, Huang KH, Wu CW, et al. Combined splenectomy does not improve survival in radical total gastrectomy for advanced gastric cardia cancer [J]. Hepatogastroenterology, 2012, 59(116): 1150-1154.
[3]Yao XX, Sah BK, Yan M, et al. Radical gastrectomy with combined splenectomy: unnecessary [J]. Hepatogastroenterology, 2011, 58(107-108): 1067-1070.
[4]Usui S, Tashiro M, Haruki S, et al. Spleen preservation versus splenectomy in laparoscopic total gastrectomy with D2 lymphadenectomy for gastric cancer: A comparison of short-term outcomes [J]. Asian J Endosc Surg, 2016, 9(1): 5-13.
[5]Nakata K, Nagai E, Ohuchida K, et al. Technical feasibility of laparoscopic total gastrectomy with splenectomy for gastric cancer: clinical short-term and long-term outcomes [J]. Surg Endosc, 2015, 29(7): 1817-1822.
[6]Noguchi K, Okada K, Kawamura H,et al. Operative procedure for pancreatoduodenectomy in a patient who had previously undergone total gastrectomy, distal pancreatectomy, and splenectomy [J]. Am Surg, 2012, 78(2): 103-105.
[7]Mizuno S, Isaji S, Ohsawa I, et al. Pancreaticoduodenectomy with resection of the splenic artery and splenectomy for pancreatic double cancers after total gastrectomy. Preservation of the pancreatic function via the blood supply from the posterior epiploic artery: report of a case [J]. Surg Today, 2012, 42(5): 482-488.
[8]Wang F, Chang YC, Chen TH, et al. Prognostic significance of splenectomy for patients with gastric adenocarcinoma undergoing total gastrectomy: a retrospective cohort study [J]. Int J Surg, 2014, 12(6): 557-565.
[9]Csesndes A, Burdilies P, Rojas J, et al. A prospective randomized study comparing D2 total gastrectomy versus D2 total gastrectomy plus splenectomy in 187 patients with gastric cancer [J]. Surgery, 2002, 131(4): 401-407.
[10]Toge T, Kameda A, Kuroi K, et al. The role of the spleen in inmunosuppression and the effects of splenectomy on prognosis in gastric cancer patients [J]. Nihon Geka Gakkai Zasshi, 1985, 86(9): 1120-1123.
[11]Yu W, Choi GS, Chung HY. Randomized clinical trial of splenectomy versus preservation in patients with proximal gastric cancer [J]. Br J Surg, 2006, 93(5): 559-563.
[12]Oh SJ, Hyung WJ, Li C, et al. The effect of spleen-preserving lymphadenectomy on surgical outcomes of locally advanced proximal gastric cancer [J]. J Surg Oncol, 2009, 99(5): 275-280.
[13]Sano T, Sasako M, Shibita T, et al. Randomized controlled trail to evaluate splenectomy in total gastrectomy for proximal gastric cancer(JCOG0110): analysis of operative morbidity, operation time , and blood loss [J]. J Clin Oncol, 2010, 28(15 Suppl): 4020.
[14]Aoyagi K, Kouhuji K, Miyagi M, et al. Prognosis of metastatic splenic hilum lymph node in patients with gastric cancer after total gastrectomy and splenectomy [J]. World J Hepatol, 2010, 2(2): 81-86.
[15]Hamabe A, Omori T, Oyama T, et al. A case of Helicobacter pylori infection complicated with gastric cancer, gastric mucosa-associated lymphoid tissue lymphoma, and idiopathic thrombocytopenic purpura successfully treated with laparoscopy-assisted total gastrectomy and splenectomy [J]. Asian J Endosc Surg, 2011, 4(1): 32-35.
[16]Yao XX, Sah BK, Yan M, et al. Radical gastrectomy with combined splenectomy: unnecessary [J]. Hepatogastroenterology, 2011, 58(107-108): 1067-1070.
[17]Zhang H, Pang D, Xu H, et al. Is concomitant splenectomy beneficial for the long-term survival of patients with gastric cancer undergoing curative gastrectomy? A single-institution study [J]. World J Surg Oncol, 2014, 12: 193.
[18]Wang F, Kang Y, Zu HL, et al. Clinicopathologic characteristics and prognosis of gastric cancer patients underwent gastrectomy combined with splenectomy [J]. Hepatogastroenterology, 2014, 61(136): 2434-2547.
[19]Hanaoka M, Shinohara H, Haruta S, et al. Successful distal gastrectomy after distal pancreatectomy combined with splenectomy by assuring the blood flow to the remnant stomach from the left inferior phrenic artery [J]. Hepatogastroenterology, 2014 , 61(135): 2156-2158.
[20]Erturk S, Ersan Y, Cicek Y, et al. Effect of simultaneous splenectomy on the survival of patients undergoing curative gastrectomy for proximal gastric carcinoma [J]. Surg Today, 2003, 33(4): 254-258.
(责任编辑:李 健)
Effect of radical surgery combined with splenectomy in treatment of gastric cancer: a Meta-analysis
LIU Qiling
Department of First General Surgery, Shanghai Public Health Clinical Center, Shanghai 201508, China
Objective To evaluate the effect of radical gastrectomy (RG) combined with splenectomy (SE) on long-term outcomes of patients with gastric cancer by a Meta-analysis. Methods A search of databases to identify randomized controlled trials (RCTs) in PubMed, Cochrane, Web of knowledge, Ovid, SpringerLink, CNKI, WanFang data were performed. Outcome measures were survival rate, operation-related events, postoperative mortality (30 days). The Meta-analysis was performed by RevMan 5.3. Results Five RCT studies met the inclusion criteria, including 588 patients in SE group and 756 patients in splean-preserving group. There was no significant difference in the 5-year overall survival rate between SE group and spleen-preserving group (OR=0.80, 95%CI: 0.60~1.06,P>0.05). Sensitivity analysis indicated the 5-year overall survival rate in SE group was lower than that in spleen-preserving group (OR=0.72, 95%CI: 0.53~0.97,P<0.05); The complications rate in SE group was higher than that in spleen-preserving group (OR=2.51, 95%CI: 1.90~3.33,P<0.05);There was no significant difference in postoperative mortality (30 days) between the two groups (OR=1.57,95%CI: 0.59~4.23,P>0.05). Conclusion SE did not show a beneficial effect on survival rate compared with splenic preservation. Routinely performing SE should not be recommended.
Advanced gastric carcinoma; Radical gastrectomy; Splenectomy; Randomized controlled trial; Meta-analysis
10.3969/j.issn.1006-5709.2016.08.022
R735.2
A
1006-5709(2016)08-0917-04
2015-08-13
