Effect of seasons on semen production, effect of melatonin on the liquid storage (5 ℃) with correlated study of birth rate in mithun (Bos frontalis)
2015-12-22PerumalJKChamuahAKNahakRajkhowa
P Perumal, JK Chamuah, AK Nahak, C Rajkhowa
National Research Centre on Mithun (ICAR), Jharnapani, Nagaland – 797 106, India
Effect of seasons on semen production, effect of melatonin on the liquid storage (5 ℃) with correlated study of birth rate in mithun (Bos frontalis)
P Perumal*, JK Chamuah, AK Nahak, C Rajkhowa
National Research Centre on Mithun (ICAR), Jharnapani, Nagaland – 797 106, India
ARTICLE INFO
Article history:
Received 5 August 2014
Received in revised form 10 September 2014
Accepted 10 December 2014
Available online 20 March 2015
melatonin (MT)
Mithun
Seminal parameters
Antioxidant profiles
Biochemical parameters
Breeding seasons
Calf birth rate
Objective: To assess the effect of melatonin (MT) at different seasons of the year on sperm motility, viability, total sperm abnormality, acrosomal membrane, plasma membrane and nuclear integrity, antioxidant profiles such as superoxide dismutase (SOD), catalase (CAT), glutathione (GSH) and total antioxidant capacity (TAC), enzymatic profiles such as aspartate amino transaminase (AST), alanine amino transaminase (ALT) and biochemical profiles such as malondialdehyde (MDA) production. Methods: Total numbers of 80 ejaculates (20 ejaculates in each season) were collected twice a week from mithun bulls and semen was split into five equal aliquots, diluted with the tris egg yolk citrate (TEYC) extender. Group 1: semen without additives (control), group 2 to group 5: semen was diluted with 1 mM, 2 mM, 3 mM, and 4 mM of melatonin, respectively. These seminal parameters, antioxidant, enzymatic and biochemical profiles were assessed at liquid storage of mithun semen (5 ℃). Simultaneously, retrospective study was conducted on birth rate of calf at different seasons from 2002 to 2012 from farm birth register of mithun farm. Results: Inclusion of melatonin into diluent resulted in significant (P<0.05) decrease in percentages of dead spermatozoa, abnormal spermatozoa and acrosomal abnormalities at different seasons of the year as compared with control group. Additionally, spring season has highest seminal and antioxidant profiles followed by autumn and winter season, whereas lower values were observed in ejaculates collected from summer season. Similarly retrospective study revealed that highest birth rate was in winter followed by autumn, spring and summer season and breeding was occurred in spring, winter, summer and autumn season, respectively with gestation period of 270- 290 days. Conclusions: The result of present study indicates that the melatonin protects seminal and antioxidant profiles varied in different seasons, semen quality also varied from different seasons and was highest in spring and lowest in summer season. It was concluded from the present study that breeding takes place throughout year but the peak breeding season is from November to April (spring and winter season) in mithun.
1. Introduction
Mithun (Bos frontalis) is a semi-wild free–range; rare bovine species present in the North-Eastern Hill (NEH) region of India and has an important place in the social, cultural, religious and economic life of the tribal population in NEH region[1]. Recent statistics indicates that the mithun population is decreasing gradually due to lack of suitable breeding bulls, increase in intensive inbreeding practices and lack of suitable breeding management in this region. Greater efforts are required from all quarters to preserve the mithun population to enhance the socio-economic status of this region. Since mithuns are semi wild animal and natural breeding is practiced in this species with limitations like cost and disease transmission. Thus, use of artificial insemination for improvement of its pedigree is utmost essential.
Season is one of the important factors that have influenced the variation in semen quality and fertility[2]. Seminal and biochemical parameters are significantly influenced byseasons. Heat tolerance and disease resistance capacity of indigenous cattle (Bos indicus) are better than exotic cattle (Bos taurus) bulls characterized by lower values of sperm abnormalities[3]. Nearly in all the species higher semen quality is obtained during spring season. Humid hot season is not desirable for the production of highly motile and fertile semen. Buffalo bulls do better during winter and spring seasons, while zebu bulls produce semen with better motility during spring and summer seasons[4]. The seasonal variations in freezability of buffalo bull semen indicated that the season significantly affected the post-thaw motility, and values being highest in ejaculates frozen in the winter and lowest in summer season[5].
Cold storage of semen is used to reduce metabolism and to maintain sperm viability over an extended period of time. But the quality of semen is deteriorated during this extended storage period. One cause of this decline is due to the action of the reactive oxygen species (ROS) generated by the cellular components of semen, abnormal spermatozoa and by neutrophils, namely a superoxide anion radical (O2●−), hydrogen peroxide (H2O2) [6-8] as the sperm membrane has high poly unsaturated fatty acids (PUFA). It results in the inhibition of both sperm ATP production and sperm movement, particularly forward progression[9]. The effects of lipid peroxidation include irreversible a loss in motility, damage to the sperm DNA and fertility[10]. Glutathione, glutathione peroxidase, glutathione reductase, catalase, superoxide dismutase, vitamin C and E are the major antioxidants naturally present in mammalian semen against ROS to protect the sperm from lipid peroxidation and to maintain its integrity[11, 12]. The level of antioxidant is decreased during the preservation process by dilution of semen with extender and excessive generation of ROS molecules[13]. Natural and synthetic antioxidant systems have been described as a defense functioning mechanism against lipid peroxidation (LPO) in semen. Thus, supplementation with natural antioxidants or synthetic antioxidants[6-8, 10] or feeding of the antioxidants[14] could reduce the impact of oxidative stress during the sperm storage process, and thus improve the quality of chilled semen[7, 8].
Melatonin is an indole derivative endogenous compound secreted rhythmically by the pineal gland in the brain and plays a major role in regulating the circadian clock and seasonal reproduction in mammals[15]. More recent studies have demonstrated that, besides its multiple actions on different physiological processes, melatonin as well as its metabolites is indirect antioxidants and powerful direct scavengers of free radicals[16]. In contrast to the majority of other known radical scavengers, melatonin is multifunctional and universal[17]. It is soluble both in water and in lipids and hence, acts as a hydrophilic and hydrophobic antioxidant. Melatonin also stimulates the activities of enzymes involved in metabolising ROS and preserves cell membrane fluidity. Indeed, melatonin was shown to be twice as potent as vitamin E in removing peroxyl radicals[18] and it is more effective in scavenging hydroxyl radicals than glutathione and mannitol[19]. However, it has been reported recently that melatonin prevents in vitro sperm capacitation and apoptotic like changes, which can be explained by a direct action of this hormone on spermatozoa. The addition of antioxidant such as melatonin to ram sperm[20], boar sperm[21], bull sperm[22] and mithun[8] has been shown to protect sperm against the harmful effects of ROS and improve the sperm motility and membrane integrity during sperm liquid storage or in the unfrozen state.
In seasonally breeding mammals that use changes in the photoperiod to time their reproductive cycles, temporal signals to the reproductive system are controlled by the daily rhythm in melatonin production[23]. Seasonal effects on semen quality and biochemical profiles have been observed in several ruminant species including cattle[3], buffaloes[5], sheep[24], goat[25] and horse [26]. However, seasonal changes in semen quality have not been described for mithun. The seasonal effects on changes in testicular volume, hormonal profiles, sexual behaviour and semen quality that affect the reproductive performance of males have been reported[27].
Further, perusal of literatures revealed paucity of information on the effect of addition of this anti-oxidant melatonin, on the maintenance of sperm viability during low temperature liquid storage of mithun semen and effect of season on the semen quality and calving rate in mithun species at different seasons or months. Hence, the objective of this study was to assess the effect of this additive on the seminal parameters of mithun semen at different seasons and correlate with calving rate to pursuit future semen collection and preservation protocols.
2. Materials and methods
2.1. Experimental animals
Eight apparently healthy mithun bulls of approximately 4 to 6 yr of age with good body condition (score 5-6) were selected from the herd derived from various hilly tracts of the NEH region of India and maintained under uniform feeding, housing and lighting conditions in National Research Centre on Mithun (ICAR), Jharnapani, Nagaland, India. Semen was collected from the animals through rectal massage method. Oxytocin (5 IU, intra muscular) was injected just prior to rectal palpation. Briefly, seminal vesicles were massaged centrally and backwardly for 5 min followed by the gentlemilking of ampullae one by one for 3-5 min, which resulted into erection and ejaculation[28]. An assistant collected the semen as it was emitted from the preputial orifice into a plastic bag suspended in a thermos containing warm water at the bottom, resulting in a temperature of 35-38 ℃ in the plastic bag. During collection, the initial transparent secretions were discarded and neat semen drops were collected in a graduated test tube with the help of a funnel. During the study, all the experimental protocols met the Institutional Animal Care and Use Committee regulations.
2.2. Semen collection and processing
The collection seasons were split into four viz. spring (February to April), summer (May to July), autumn (August to October) and winter (November to January). Total numbers of 80 ejaculates (20 ejaculates from each season) were collected from the mithun. Immediately after collection, the samples were kept in a water bath at 37 ℃ and evaluated for volume, colour, consistency, mass activity and pH. After the preliminary evaluations, samples were subjected to the initial dilution with pre-warmed (37 ℃) Tris egg yolk citrate extender (TEYC). The partially diluted samples were then brought to the laboratory in an insulated flask containing warm water (37 ℃) for further processing.
Each ejaculate was split into five equal aliquots and diluted with the TEYC extender with melatonin. Group 1: semen without additives (control), group 2 to group 5: semen with 1 mM, 2 mM, 3 mM and 4 mM of melatonin, respectively. However, pH of diluents was adjusted to be 6.8 – 7.0 by using phosphate buffer solution. Diluted semen samples were kept in glass tubes and cooled from 37 ℃to 5 ℃, at a rate of 0.2–0.3 ℃/min in a cold cabinet and maintained at 5 ℃ during liquid storage. The percentage of sperm motility, viability, total sperm abnormality[29], acrosomal integrity[30], plasma membrane integrity by hypo-osmotic swelling test (HOST) and nuclear integrity by Feulgen staining technique[31] were determined as per standard procedure in samples during storage of semen at 5 ℃.
2.3. Biochemical assays
An aliquot of semen from each sample was centrifuged at 800 × g for 10 min; sperm pellets were separated and washed by resuspending in PBS and recentrifuging (thrice). After the final centrifugation, 1 mL of deionised water was added to the spermatozoa and the spermatozoa and seminal plasma were snap-frozen and stored at −70 ℃ until further analysis. The antioxidant profiles such as CAT, SOD, GSH and TAC, intra cellular enzymes such as AST, ALT activity were estimated by commercial available kit. Lipid peroxidation level of sperm and seminal plasma was measured by determining the malonaldehyde (MDA) production, using thiobarbituric acid (TBA) as per the method of Buege and Aust[32] and modified by Suleiman et al. [33].
2.4. Retrospective study
The calving data from 2002 to 2012 was collected from calving register, National Research Centre on Mithun (ICAR), Jharnapani, Nagaland, India, analysed and presented in graph and table as month wise and season wise. The breeding was occurred in the jungle through natural breeding when the animal was in grazing.
2.5. Statistical analysis
The results were analysed statistically and expressed as the Mean ± S.E.M. Means were analyzed by one way analysis of variance (ANOVA), followed by the Tukey’s post hoc test to determine significant differences between the different seasons with additives or without additive and between the treatment and control groups on different seasons on the sperm parameters using the SPSS/PC computer program (version 15.0; SPSS, Chicago, IL). Differences with values of P<0.05 were considered to be statistically significant after arcsine transformation of percentage data by using SPSS 15.
3. Results
The effects of melatonin on sperm motility (Table 1), viability (Table 2), total sperm abnormality (Table 3), acrosomal (Table 4) plasma membrane (Table 5) and nuclear integrity (Table 6) in liquid storage (5 ℃) from different seasons were presented in tables. Results revealed that inclusion of melatonin into diluent resulted in decreases (P<0.05) in percentages of dead spermatozoa, abnormal spermatozoa and acrosomal abnormalities when semen samples were examined at different seasons compared with the control group. Semen quality and effect of melatonin on seminal parameter were significantly (P<0.05) differed between the seasons and was higher in spring season followed by autumn, winter and summer season. Averaged over time, mean total sperm abnormalities were (6.92 ± 0.61), (6.07 ± 0.78), (5.60 ± 0.80), (5.27 ± 0.94) and (6.99 ± 0.65), respectively for control, 1 mM, 2 mM, 3 mM and 4 mM of melatonin treated mithun semen in spring season. Additionally, melatonin at 1 mM, 2 mM and 4 mM were inferior to melatonin 3 mM treatments for these characteristics, and there was a significant (P<0.05)difference between melatonin at 1 mM, 2 mM, 4 mM and 3 mM for these response at different seasons. Similarly the antioxidant enzymatic profiles revealed that highest mean SOD (Figure 4) and CAT (Figure 5), GSH (Figure 6) and TAC (Figure 7) were recorded in melatonin treated semen than control group and differed significantly (P<0.05) between groups and between seasons and was higher in spring season followed by autumn, winter and summer season in this precious species. Intracellular enzymes such as AST (Figure 1) and ALT (Figure 2) were decreased (P<0.05) in melatonin treated semen in different seasons and were lower in 3 mM treated semen and semen from spring season. Similarly MDA production (Figure 3) was differed significantly between the melatonin treated and control groups and was lower in the melatonin treated group (3 mM) in different seasons and was significantly (P<0.05) higher in summer season. It was obvious from the data of this experiment that addition of melatonin, especially at 3 mM to the semen diluent resulted in significant improvement in quality, seminal parameters, antioxidant, enzymatic activity and reduction of MDA production of mithun semen stored in in vitro at 5 ℃ in different seasons. Moreover, semen from spring season was higher quality and effect of melatonin on semen quality was higher in spring season followed by autumn and winter season whereas lower quality was observed in summer season.
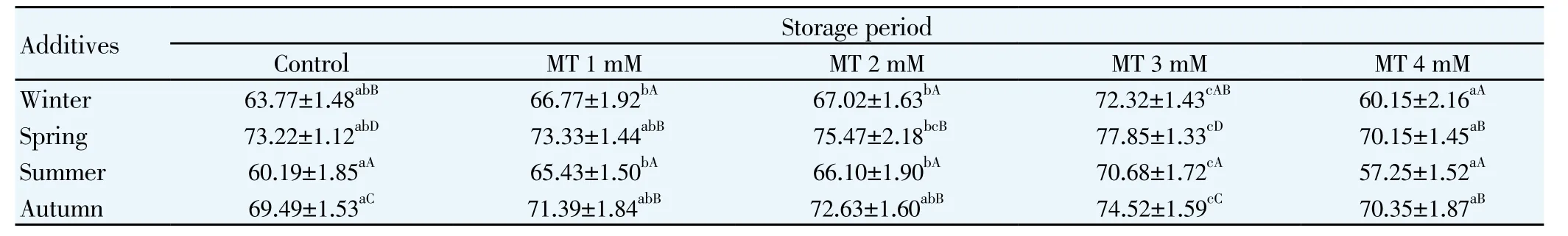
Table 1 Motility percentage for mithun semen following storage at 5℃for different seasons (Mean ± S.E.).
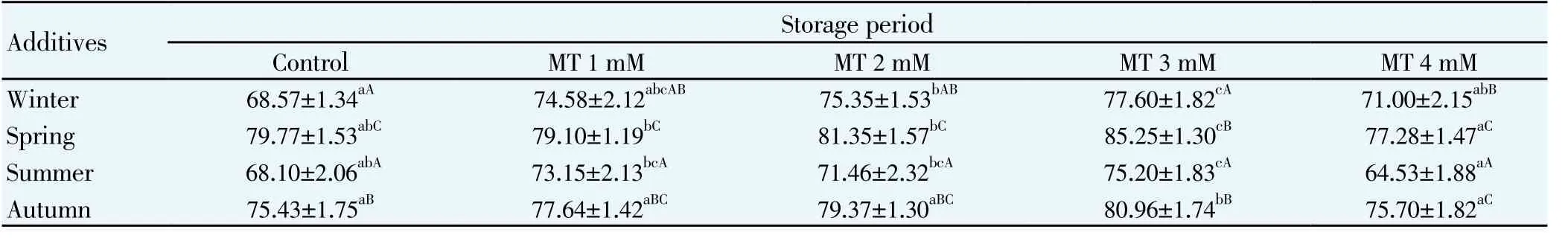
Table 2 Viability percentage for mithun semen following storage at 5℃for different seasons (Mean (±S.E.).
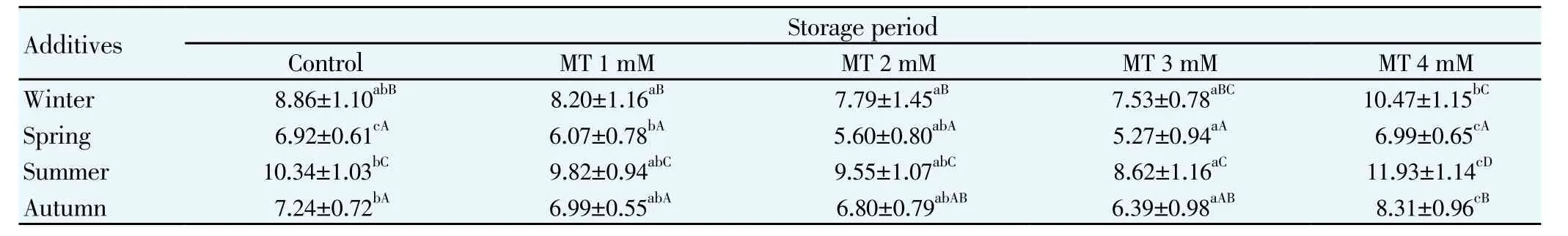
Table 3 Total abnormal sperm percentage for mithun semen following storage at 5℃for different seasons (Mean±S.E.).
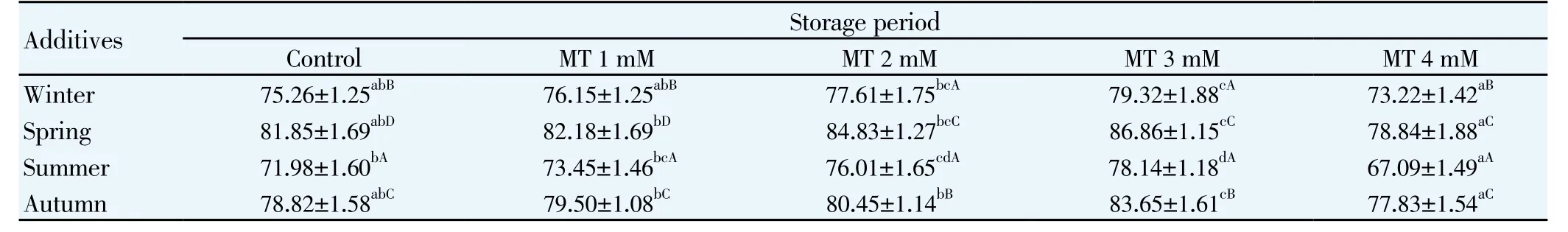
Table 4 Acrosomal Integrity (%) in semen of mithun for different seasons at 5℃(Mean (±S.E.).

Table 5 Plasma membrane integrity (HOST) (±S.E.) percentage for extended mithun semen containing additive at different seasons.

Table 6 Nuclear integrity (±S.E.) percentage for extended mithun semen containing additive at different seasons.
The result of the retrospective study was revealed that maximum calving takes place in November to February (winter season), it means, most of the breeding might have taken place in the months from February to April (Spring season) and similarly lowest calving rate was recorded during the month from May to July (summer season) (Figure 8), means very less number of animal had been in estrus in the month of August to October (autumn season) (Figure 9) considering the gestation length of mithun varies from 270 to 290 days. This study indicates that breeding takes place throughout year but the peak breeding season is from November to April (spring and winter season) in mithun.
4. Discussion
In the present study, the results revealed that addition of melatonin in semen was collected at different seasons has improved the seminal parameters, enzymatic and biochemical profiles of semen and was higher in spring followed by autumn, winter and summer season. Thus it protects the structures and functions of spermatozoa efficiently at different seasons. The semen quality was varied between the seasons, higher in spring followed by autumn, winter and lower in summer season. Thus, melatonin has enhanced the quality of semen by preserving efficiently during artificial insemination procedure in mithun species at different seasons.
There was paucity of report on effect of addition of melatonin on seminal parameters in mithun at different seasons and to the best of our knowledge this is the first report of the effect of melatonin on routine seminal, antioxidative, enzymatic and biochemical profiles in mithun semen, collected at different seasons. But many authors reported that melatonin has beneficial effects on preservation of mammalian sperm and improves the functional parameters of spermatozoa[20-22]. In the present study, melatonin supplementation on these seminal parameters revealed significant difference between the treatment groups in different seasons. The beneficial effects of melatonin in semen preservation are due to it is a very potent antioxidant[20-22]. Season is one of the important factors that have influenced the variation in semen quality and fertility[2]. Seminal and biochemical parameters are significantly influenced by seasons. Heat tolerance and disease resistance capacity of indigenous cattle (Bos indicus) are better than exotic cattle (Bos taurus) bulls characterized by lower values of sperm abnormalities[3].
Because of the mammalian sperm membrane has high polyunsatured fatty acids; it renders the sperm very susceptible to LPO, which occurs as a result of the oxidation of the membrane lipids by partially reduced oxygen molecules, such as superoxide, hydrogen peroxide and hydroxyl radicals[9]. Lipid peroxidation of the sperm membrane ultimately leads to the impairment of sperm function due to the attacks by ROS, altered sperm motility and membrane integrity and damage to sperm DNA and fertility through oxidative stress and the production of cytotoxic aldehydes[34]. In addition, the antioxidant system of seminal plasma and spermatozoa is compromised during semen processing[35]. The levels of antioxidant decreased during the preservation process by dilution of semen with extender and excessive generation of ROS molecules[13]. Natural and synthetic antioxidant systems have been described as a defense functioning mechanism against lipid peroxidation (LPO) in semen[13]. Therefore, inclusion of exogenous antioxidants with natural antioxidants could reduce the impact of oxidative stress during thesperm storage process, and thus improve the quality of chilled semen[7, 20]. Seasons are also influenced on the LPO production and antioxidant concentration as higher antioxidant was reported in winter as compared to summer season and higher LPO in summer season than winter season in semen of Tharparkar bull[3].
The summer season causes thermal stress to the animal and also affect the leydig cell function as chronic heat treatment has to reduce testosterone production and function of accessory sex glands especially epididymis and seminal vesicles because as these antioxidants are derived from epididymis especially cauda epididymis[36] and seminal vesicle[37] into the semen because the epididymis, accessory sex glands are thermo sensitive and androgen dependent[38]. So that, during summer season, the production of antioxidants was lower and LPO production was higher than spring or autumn or winter season in mithun.
The results of the present study showed that addition of 3 mM of melatonin improve the keeping quality of mithun semen preserved at 5 ℃ in different seasons and was higher in spring season. But the sperm motility was declined in summer season. Similar reports were observed in indigenous Tharparkar cattle[3] that winter season favours more sperm motility than summer season. Similar report was also observed in mountain animal yak as semen from autumn season was better than summer season[39]. The motility of sperm cell develops during their passage through the epididymis. Anaphylactic stress caused by summer heat stress, as depicted by the significant rise in body and testes temperature, causes derangement in epididymal functions and spermatogenesis and could lead to summer stress -mediated declined sperm motility and with similar feature of testicular hypoplasia and degeneration[40]. Temperature could give rise to secondary abnormalities as well with increase in sperm tail and mid-piece abnormalities as in testicular degeneration or partial hypoplasia of testes[41].
Similarly, decline rate in the motility percentage was higher in semen samples treated with 4 mM melatonin or without melatonin. But inclusion of 3 mM melatonin, the motility and viability parameters were increased as compared to control group[42]. The different effects of the different levels of melatonin might be explained according to the report of Ashrafi et al. [20] showed that the excessive amount of antioxidants caused changes the osmotic pressure of the extender and high fluidity of plasma membrane above the desired point, making sperm more prone to acrosomal damages and may be harmful to spermatozoa that lead to decrease in spermatozoa performance after liquid storage. In addition, the concentration of antioxidants added to extender should be considered since high dosage of antioxidants may be harmful to spermatozoa due to the change in physiological condition of semen extender. In mithun, survival of spermatozoa will increase when the dosage of antioxidant added to extender increases. However, the antioxidant dosage higher than required amount was toxic to spermatozoa[43]. The over expression of melatonin may reflect a defect in the development or maturation of spermatozoa, as well as sperm cellular damage, resulting in decreased sperm fertilization potential[22]. Similarly, in the present study, increasing dosage of melatonin, at 4 mM affected the seminal as well as biochemical parameters in mithun semen TEYC extender. At the same time, less dosage rate also affected the sperm parameters. But as per the dosage, the parameters were increased upto 3 mM then decreased to 4 mM. Differences in preservation protocols and extender formulations among laboratories, the time of addition/exposure of sperm with antioxidant, concentration of antioxidants and between species may explain, at least in part, this variability. The improvement of semen quality due to addition of exogenous melatonin recorded in the present study was previously reported in the form of motility and intact acrosomal membrane in ram sperm[20], boar sperm[21], bull sperm[22] and mithun[8]. Moreover, the addition of exogenous melatonin was significantly improving the percentages of DNA morphology, sperm viability and intact plasma membrane (swelling tails) especially at a level of 3 mM of melatonin[42]. The highest percentages of intact plasma and acrosomal membranes which were found in the present experiment due to 3 mM melatonin may be the reason for better motility in these samples[22]. Mitochondria in sperm cells encase the axosome, connect with dense fibres in the middle pieces and produce adenosine triphosphate (ATP). It has been reported[44] that the axoneme and mitochondria in sperms may be damaged by a high level of ROS. The studies have shown that melatonin can stabilise and protect mitochondria via several mechanisms[45].
Melatonin helps maintaining the integrity of normal acrosome and stabilizes the plasmalemma of spermatozoa and so increase motility[46]. Melatonin, in sperm cells is able to react with many ROS directly for protecting mammalian cells against oxidative stress, and hence maintaining sperm motility[22]. Therefore, as seen by this study, attempts to improve the motility and viability of the sperm cells by incorporating melatonin in liquid storage[20] and frozensemen form have been investigated[22]. Similar report was observed in Tharparkar cattle as semen ejaculates from winter season have higher sperm acrosomal integrity than semen from summer season[3].
Since, plasma membrane integrity reflects the biochemical integrity of sperm plasma membrane, and it is involved in the process of capacitation, acrosome reaction and ultimately binding of spermatozoa to the oocyte[47]. So, this test is able to assess the fertilizing ability of spermatozoa, the ultimate aim of which is to reduce the incidence of repeat breeding. Antoine and Pattabiraman[48] reported decrease in HOST reacting spermatozoa following scrotal insulation in bucks due to rise in testicular temperature as in summer stress. Similarly, in a study on bulls, HOS test reacting spermatozoa were reduced after heat treatment[49] similar to summer season. Thus it can be proposed that summer heat stress affects the HOS percentage by affecting the biochemical integrity of the sperm plasma membrane. Similar report was observed in indigenous (Bos indicus) cattle[3], buffaloes[5], sheep[24], goat[25] and horse[26] as season has influence on sperm acrosomal integrity.
Sperm morphology usually returns to normal value within approximately 8 weeks of the thermal insult[50]. However, a prolonged and (or) severe increase in testicular temperature will increase the interval for recovery in summer season. It appears that the decrease in semen quality associated with increased testicular temperature is ultimately related to the severity and the duration of the increased testicular temperature.
A recent report suggested that semen quality is deteriorated[51] by which DNA damage is induced in the male gamete by oxidative stress and spermatozoa are particularly vulnerable to this because they generate ROS and are rich in targets for oxidative attack. The authors also draw attention to the fact that, because spermatozoa are transcriptionally inactive and have little cytoplasm, they are deficient in both antioxidants and DNA-repair systems. Oxidative stress may be a cause of male infertility and contribute to DNA fragmentation in spermatozoa[52]. There are few studies on the effects of antioxidant addition to extenders during cooling and/or freezing mammalian spermatozoa[22]. In mithun semen, ROS are generated mainly by damaged and abnormal spermatozoa and by contaminating leukocytes. ROS damage cells by changes to lipids, proteins and DNA. Spermatozoa are potentially susceptible to peroxidative damage caused by ROS excess due to high amounts of polyunsaturated fatty acids in membrane phospholipids and to sparse cytoplasm. In the present study, addition of melatonin has reduced the DNA fragmentation/nuclear integrity especially at 3 mM in mithun semen preservation at 5 ℃ in different seasons. It similar to reports of Succu et al.[42] that the addition of melatonin preserved DNA integrity in cryopreserved ram spermatozoa.
Moreover, it maintains plasma and mitochondrial membrane integrity and cytoskeleton structure of flagella of sperm as cell protecting effects. Melatonin has also protects and stimulates the activities of antioxidant enzymes such as SOD, GSH and CAT[53], which helps to maintain membrane transportation[35] and fertility of the spermatozoa. It results indirectly reduces the number of free radicals, ROS and also may increase the production of molecules protecting sperm cells against oxidative stress. Indeed, melatonin was shown to be twice as potent as vitamin E in removing peroxyl radicals[18] and it is more effective in scavenging hydroxyl radicals than glutathione and mannitol[19].
SOD, CAT and GSH are important parts of antioxidant enzyme defence systems in sperm that convert superoxide (O2●−) and peroxide (H2O2) radicals into O2and H2O. GSHPx excludes peroxyl radicals from various peroxides. CAT and SOD also eliminate O2●−produced by nicotinamide adenine dinucleotide phosphate-reduced (NADPH) oxidase. In our study, the activity of CAT, SOD and GSH increased by inclusion of various concentrations of melatonin in the semen extender[22] and was higher in 3 mM added semen and in spring season than summer season in mithun species.
Glutathione antioxidant system consists of reduced glutathione (GSH), oxidised glutathione (GSSG), glutathione reductase (GSHRx), glutathione peroxidase (GSHPx), glutathione –s– transferase. GSHRx stimulates the reduction of GSSG to GSH. This ensures a steady supply of the reductive substrate (NADPH) to GSHPx. It is affected by thermal stress[54] due to summer heat stress. In the present study, GSH was reduced in the seminal plasma of mithun semen as they are positively correlated with sperm profiles suggests that this enzyme is high in seminal plasma and low in spermatozoa in ejaculated bovine semen[55].
Catalase is a tetramer of four polypeptide chain antioxidant is found in nearly all living organisms exposed to oxygen. This antioxidant is derived from the epididymis, seminal vesicle and detoxifies both intracellular and extracellular hydrogen peroxide by reduces H2O2to H2O and O2, by eliminating the potential ROS toxicity and it can reduce the loss of motility caused by leukocyte generated ROS[56]. In the present study, the concentration of SODdecreased in ejaculates of summer season because the thermo stress affects the normal function of testes and accessory sex glands as similar in cryptorchid testes[54].
Similarly superoxide dismutase (SOD) is an antioxidant that catalyzes the dismutation of superoxide into oxygen and hydrogen peroxide. It scavenges both extracellular and intracellular superoxide anion and prevents lipid peroxidation of the plasma membrane. SOD spontaneously dismutase anion to form O2and H2O2. SOD also prevents premature hyper activation and premature capacitation induced by superoxide radicals before ejaculating[56]. In the present study, the concentration of SOD decreased in ejaculates of summer season because the thermo stress affects the normal function of testes and accessory sex glands as similar in cryptorchid testes[54]. The findings of the present study, impaired detoxication of reactive oxygen species and concomitant oxidative stress, may be implicated in biochemical mechanisms responsible for testicular dysfunction in summer stressed animals.
The enzyme such as AST and ALT levels in seminal plasma are very important for sperm metabolism as well as sperm functions, provide energy for survival, motility and fertility of spermatozoa and these transaminase activities in semen are good indicators of semen quality because they measure sperm membrane stability[57]. Thus, increasing the percentage of abnormal spermatozoa in the preservation causes high concentration of transaminase enzyme in the extra cellular fluid due to sperm membrane damage and ease of leakage of enzymes from spermatozoa. Moreover, increase in AST and ALT activities of seminal plasma and semen in liquid storage stage may be due to structural instability of the sperm[58] or fragile nature of sperm membrane. In the present study, AST and ALT levels were lower in semen preserved at 3 mM of melatonin at liquid storage period as it stabilises the membrane integrity of acrosome, plasma, mitochondria and flagella of the sperm[45] and was lower in spring and autumn season than summer season as the summer hot and winter cold stress affect the function of accessory glands, seminal vesicle and epididymis that leads to poor plasma membrane and acrosomal membrane stability leads to more leakage of intra cellular enzymes in summer season. Moreover functions of these glands depend upon the androgen, summer stress cause production and secretion of androgen is affected. Thus the sperm from summer season has poor quality and poor stability in the present study. Similar report was observed in indigenous Tharparkar (Bos indicus) cattle[3] as spring and winter season have higher sperm acrosomal and plasma membrane integrity and lower extra cellular enzymatic profiles than semen from summer season.
It also prevents MDA production in diluents indicates it prevents premature capacitation and acrosomal reaction as acts as an antioxidant[22]. In the present study, MDA production was decreased in melatonin treated group as compared to the control untreated group[22]. However, it has been reported recently that melatonin prevents in vitro sperm capacitation and apoptotic like changes, which can be explained by a direct action of this hormone on spermatozoa. The effect of melatonin in preventing apoptotic like changes may be related to its antioxidant and free radical scavenging activities also increases fertility rate. So the semen samples treated with melatonin will have high cryoresistance power than untreated control group. In the present study, it was observed that sperm parameters that received at 3 mM of melatonin were significantly higher than those of the other and control group. These results are basically consistent with the results previously reported. Moreover, inclusion of melatonin in the semen extender increased the TAC. This may be due to the stimulatory effects of melatonin on the activity of enzymes involved in antioxidant defence[22]. In the present study, based on the result, the effect of melatonin on the seminal and biochemical parameters are dose dependent[20, 22, 42] and at 3 mM melatonin was optimum dose for mithun semen preservation at liquid storage stage. Moreover, 1 mM and 2 mM of melatonin were low dosage and 4 mM of melatonin was over dosage for mithun semen preservation at liquid storage.
The result of the retrospective study was revealed that maximum calving takes place in November to February (winter season), it means, most of the breeding might have taken place in the months from February to April (Spring season) and similarly lowest calving rate was recorded during the month from May to July (summer season), means very less number of animal had been in estrus and or bred in the month of August to October (autumn season) considering the gestation length of mithun varies from 270 to 290 days. This indicates that spring season is more favourable season for breeding of mithun bulls. But most of the breeding is occurred in spring and winter season and less breeding was occurred in autumn season as during this season there is higher temperature and humidity might be affecting the sexual desire of the animal as comparatively less number of animal bred during this season of heavy rain fall i.e. August to October that might scarce the animal to exhibit estrusbehaviour as it is observed in cattle in other part of India, though these are the flush season they get abundant of green grass, fodder, herbs and shrubs in jungle. Maximum number of animal bred in spring season (February to April) i.e. months of low rain fall and there is bit paucity of the fodder but that might have compensated with concentrate ration fortified with salt and mineral mixture in semi-intensive condition and the seasonal environment is more suitable for breeding in mithun.
Though the mithun is polyoestrus animal and adult female shows repeated estrus cycle after every 19-24 days interval with silent oestrous without bellowing and having standing heat period ranging from 4-16 hours. The expression of estrus behaviour is silent in mithun unlike cattle it is difficult to detect heat in female mithun by clinical symptoms especially in summer season. Moreover the semen quality during the summer season is poor as this high temperature affecting the semen production and sexual behaviour in mithun bulls.
Melatonin is produced and secreted during the night (dark). As days become shorter, the exposure to melatonin increases; this hormone, through a complex action on the hypothalamus-pituitary-gonads axis, exerts a stimulating effect on GnRH secretion by the hypothalamus and modulating gonadotropin and testosterone production[59] in short-day breeders. In the present study, semen production, semen quality and breeding were higher in spring and winter seasons, the seasons have short day light. This indicates that during this short day season, there was more secretion of melatonin in mithun and protected the seminal, biochemical and antioxidant parameters along with stimulation of hypothalamus to secrete more GnRH followed by high semen production. Moreover, there may be high variation in melatonin secretion and concentration throughout the year in mithun seminal plasma that could partly explain the differences in sperm quality and fertility observed between the breeding and non-breeding seasons[22] in mithun species. Similarly, in mithun cows, short day may induce secretion of melatonin, stimulation of GnRH followed by expression of heat, breeding, conception and calving. But in the present study, we didn’t measure the melatonin and other reproductive hormones concentration in different season in both male and female animal.
In this study, improvements was observed in sperm quality may be attributed to prevention of excessive generation of free radicals, produced by spermatozoa themselves, by means of their antioxidant property of melatonin. It indicates that the possible protective effects of melatonin supplementation are it enhances the antioxidant enzymes content and preventing MDA production in dose dependent manner in different seasons. Thus it may protect the spermatozoa during preservation and enhancing the fertility in this species at 3 mM. Further, semen production and effect of melatonin on seminal parameters were differed among the seasons and was higher in spring season followed by autumn and winter season whereas lowest in the summer season. The result of semen quality was positively correlated with result of calving rate of retrospective study in mithun. From this it was concluded that collection and preservation of semen should be conducted during spring, autumn and winter season for artificial insemination but more suitable breeding season for mithun from winter to spring season (November to April). Future, ultra low temperature sperm preservation/cryoprotective studies and endocrinological profiles at different seasons are warranted to confirm the present findings.
Conflict of interest statement
The authors declare that they have no conflict of interest.
[1] Simoons FJ. Gayal or mithun. In: Evolution of domesticated animals. (Manson IL. ed.). London: Longman; 1984,p.34-36.
[2] Mathevon M, Buhr MM, Dekkers JCM. Environmental, management, and genetic factors affecting semen production in Holstein bulls. J Dairy Sci 1998; 81: 3321-3330.
[3] Rajoriya JS, Prasad JK, Ghosh SK, Perumal P, Kumar A, Kaushal S, et al. Effects of seasons on enzymatic changes and cholesterol efflux in relation to freezability in Tharparkar bull semen. Asian Pac J Reprod 2013; 2(4): 280 -288.
[4] Kushwaha NS, Mukherji DP, Bhattacharya P. Seasonal variation in reaction time and semen quality of buffalo bulls. Indian J Vet Sci Anim Husband 1955; 25: 317-328.
[5] Sagdeo LR, Chitins AB, Deshmukh SN, Kaikini AS. Seasonal variations in relation to freezability of semen of Jersey and crossbred bulls with varying level of exotic inheritance. Indian J Anim Reprod 991; 12(2): 117-121.
[6] Perumal P, Selvaraju S, Selvakumar S, Barik AK, Mohanty DN, Das S, et al. Effect of pre-freeze addition of cysteine hydrochloride and reduced glutathione in semen of crossbred Jersey bulls on sperm parameters and conception rates. Reprod Domest Anim 2011a;46(4): 636-641.
[7] Perumal P, Vupru K, Rajkhowa C. Effect of addition of taurine on the liquid storage (5 ℃) of mithun (Bos frontalis) semen. Vet Med Int 2013; Article Id. 165348, http://dx.doi.org/10.1155/2013/165348.
[8] Perumal P, Vupru K, Khate K. Effect of addition of melatonin on the liquid storage (5 ℃)of mithun (Bos frontalis) semen. Int J Zool 2013; Article id. 642632, http://dx.doi.org/10.1155/2013/642632.
[9] Dandekar P, Nadkarni GD, Kulkarni VS, Punekar S. Lipid peroxidation and antioxidant enzymes in male infertility. J Postgrad Med 2002; 48: 186-189.
[10] Perumal P, Selvaraju S, Barik AK, Mohanty DN, Das S, Mishra PC. Role of reduced glutathione in improving post-thawed frozen seminal characters of poor freezable Jersey crossbred bull semen. Indian J Anim Sci 2011b; 81(8): 807-810.
[11] Bilodeau JF, Blanchette S, Gagnon C, Sirard MA. Thiols prevent H2O2-mediated loss of sperm motility in cryopreserved bull semen. Theriogenology 2001; 56: 275-286.
[12] Gadea J, Selles E, Marco MA, Copy P, Matas C, Romar R, et al. Decrease in glutathione content in boar sperm cryopreservation. Effect of the addition of reduced glutathione to the freezing and thawing extenders. Theriogenology 2004; 62(3): 690-701.
[13] Kumar R, Jagan Mohanarao G, Arvind R, Atreja SK. Freeze-thaw induced genotoxicity in buffalo (Bubalus bubalis) spermatozoa in relation to total antioxidant status. Mol Biol Rep 2011; 38(3): 1499-1506.
[14] Jayaganthan P, Perumal P, Balamurugan TC, Verma RP, Singh LP, Pattanaik AK, et al. Effects of Tinospora cordifolia supplementation on semen quality and hormonal profile of ram. Anim Reprod Sci 2013; 140(1): 47-53.
[15] Reiter RJ. Pineal melatonin: Cell biology of its synthesis and of its physiological interactions. Endocr Rev 1991; 12(2): 151-180.
[16] Reiter RJ, Tan DX, Kim SJ, Qi W. Melatonin as a pharmacological agent against damage to lipids and DNA. Proc West Pharmacol Soc 1998; 41: 229-236.
[17] Tomás-Zapico C, Coto-Montes A. A proposed mechanism to explain the stimulatory effect of melatonin on antioxidative enzymes. J Pineal Res 2005; 39(2): 99-104.
[18] Pieri C, Marra M, Moroni F, Recchioni R, Marcheselli F. Melatonin: A peroxyl radical scavenger more effective than vitamin E. Life Sci 1994; 55(15): 271-276.
[19] Hardeland R, Reiter RJ, Poeggeler B, Tan DX. The significance of the metabolism of the neurohormone melatonin: Antioxidative protection and formation of bioactive substances. Neurosci Biobehavioral Rev 1993; 17(3): 347-357.
[20] Ashrafi I, Kohram H, Naijian H, Bahreini M, Poorhamdollah M. Protective effect of melatonin on sperm motility parameters on liquid storage of ram semen at 5 ℃. Afr J Biotechnol 2011; 10(34): 6670-6674.
[21] Hyun-Yong J, Sung-Gon K, Jong-Taek K, Choon-Keun P, Hee-Tae C, Hak-Kyu L, et al. Effects of Antioxidants on Sperm Motility During in Vitro Storage of Boar Semen. Korean J Gerontol 2006; 16(6): 47-51.
[22] Ashrafi I, Kohram H, Ardabili FF. Antioxidative effects of melatonin on kinetics, microscopic and oxidative parameters of cryopreserved bull spermatozoa. Anim Reprod Sci 2013; 139(1-4): 25-30.
[23] Stetson MH, Elliot JA, Menaker M. Photoperiodic regulation of hamster testis: circadian sensitivity to the effects of light. Biol Reprod 1975; 13: 329-339.
[24] Colas G. Seasonal variations of the quality of sperm in the Ilede-France ram. I. Study of the cellular morphology and ‘massal’motility. Reprod, Nutr & Dev 980; 20: 1789-1799.
[25] Zarazaga LA, Guzman JL, Dominguez C, Perez MC, Prieto R. Effects of season and feeding level on reproductive activity and semen quality in Payoya buck goats. Theriogenology 2009; 71: 1316-1325. [26] Pickett BW, Faulkner LC, Voss JL. Effect of season on some characteristics of stallion semen. J Reprod & Fertil 1975; 23: 25-28.
[27] Cardozo J, Fernández-Juan M, Forcada F, Abecia A, Muiño-Blanco T, Cebrián-Pérez JA. Monthly variations in ovine seminal plasma proteins analyzed by two-dimensional polyacrylamide gel electrophoresis. Theriogenology 2006; 66: 841-850.
[28] Palmer C, Amundson S, Brito L, Waldner C, Barth A. Use of oxytocin and cloprostenol to facilitate semen collection by electroejaculation or transrectal massage in bulls. Anim Reprod Sci 2004; 80: 213-223.
[29] Tomar NS. Artificial insemination and reproduction of cattle and buffalos. Allahabad: Sarojprakashan;1997.
[30] Watson PF. Use of giemsa stain to detect change in acrosome of frozen ram spermatozoa. Vet Record 1975; 97(1): 12-15.
[31] Barth AD, Oko RJ. Preparation of semen for morphological examination in Abnormal morphology of bovine spermatozoa. Ames: Iowa State University Press; 1998,p. 8 -18.
[32] Buege JA, Aust SD. Microsomal lipid peroxidation. Method Enzymol 1978; 52: 302-310.
[33] Suleiman SA, Ali ME, Zaki MS, Malik EMEA, Nast MA. Lipid peroxidation and human sperm motility: protective role of vitamin E. J Androl 1996; 17(5): 530-537.
[34] Gavella M, Lipovac V, Vucic M, Rocic B. Relationship of sperm superoxide dismutase-like activity with other sperm specific enzymes and experimentally induced lipid peroxidation in infertile men. Andrologia 1996; 28(4): 223-229.
[35] Alvarez JG, Storey BT. Evidence for increased lipid peroxidative damage and loss of superoxide dismutase activity as a model of sub lethal cryo damage to human sperm during cryopreservation. J Androl 1992; 13: 232-241.
[36] Fouchecourt S, Metayer S, Locatelli A, Dacheux F, Dacheux JL. Stallion epididymal fluid proteome: qualitative and quantitative characterization; secretion and dynamic changes of major proteins. Biol Reprod 2000; 62:1790-1803.
[37] Tramer F, Rocco F, Micali F, Sandri G, Panfili E. Antioxidant systems in rat epididymal spermatozoa. Biol Reprod 1998; 59: 753-758.
[38] Saeed S, Khan FA, Rehman SB, Khan DA, Ahmad M. Biochemical parameters in evaluation of oligospermia. J Pakistan Med Assoc 1994; 44: 137-140.
[39] Sarkar M, Dutta Borah BK, Bandopadhayay S, Meyer HHD, Prakash BS. Season of the year influences semen output and concentrations of testosterone in circulation of yaks (Poephagus grunniens L.). Anim Reprod Sci 2009; 115: 300-305.
[40] Arthur GH, Noakes DE, Pearson H. Veterinary reproduction and obstetrics. 6th ed. London: Bailliere Tindal; 1989, p. 509-584.
[41] Sullivan JJ. Morphology and motility of spermatozoa. In: Salisbury GW, Van Demark NL, Lodge JR. (eds). Physiology of reproduction and artificial nsemination of cattle. 2nd ed. San Francisco: W.H. Free man;1978, p. 286-328.
[42] Succu S, Berlinguer F, Pasciu V, Satta V, Leoni GG, Naitana S. Melatonin protects ram spermatozoa from cryopreservation injuries in a dose-dependent manner. J Pineal Res 2011; 50 (3): 310-318.
[43] Maxwell WMC, Stojanov T. Liquid storage of ram semen in the absence or presence of some antioxidants. Reprod, Fertil & Develop 1996; 8: 1013-1020.
[44] Aitken RJ, Clarkson JS. Cellular basis of defective sperm function and its association with the genesis of reactive oxygen species by human spermatozoa. J Reprod & Fertil 987; 81 (2): 459-469.
[45] Lopez A, Garcia JA, Escames G, Venegas C, Ortiz F, Lopez LC, et al. Melatonin protects the mitochondria from oxidative damage reducing oxygen consumption, membrane potential, and superoxide anion production. J Pineal Res 2009; 46(2): 188-198.
[46] Sonmez M, Yuce A, Turk G. The protective effects of melatonin and Vitamin E on antioxidant enzyme activities and epididymal sperm characteristics of homocysteine treated male rats. Reprod Toxicol 2007; 23(2): 226-231.
[47] Argov N, Sklan D, Zeron Y, Roth Z. Association between seasonal changes in fatty-acid composition, expression of VLDL receptor and bovine sperm quality. Theriogenology 2007; 67: 878-885.
[48] Antoine D, Pattabiraman SR. Spermatozoa response to hypoosmotic swelling and freezability tests after testicular degeneration in bucks. Indian J Anim Reprod 999; 20: 54-56.
[49] Sivaramalingam K. Studies on hypo-osmotic swelling test (HOST) for evaluation of bull spermatozoa. MVSc Thesis, Madras Veterinary College, Madras. India; 1994.
[50] Kastelic JP, Cook RB, Coulter GH, Saacke RG. Insulating the scrotal neck affects semen quality and scrotal/testicular temperatures in the bull. Theriogenology 1996; 45: 935-942.
[51] Aitken RJ, De Luliis GN, Finnie JM, Hedges A, McLachlan R. Analysis of the relationships between oxidative stress, DNA damage and sperm vitality in a patient population: development of diagnostic criteria. Hum Reprod 2010; 25(10): 2415-2426.
[52] Aitken J, Fisher H. Reactive oxygen species generation and human spermatozoa: the balance of benefit and risk. Bioassays 1994; 16(4): 259-267.
[53] Karbownik M, Reiter RJ. Antioxidative effects of melatonin in protection against cellular damage caused by ionizing radiation. Proceedings of the Society for Experimental Biology and Medicine 2000; 225(1): 9-22.
[54] Ahotupa M, Huhtaniemi I. Impaired detoxification of reactive oxygen and consequent oxidative stress in experimentally cryptorchid rat testis. Biol Reprod 1992; 46: 1114-1118.
[55] Brown DV, Senger PL, Stone SL, Froseth JA, Becker WC. Glutathione peroxidase in bovine semen. J Reprod & Fertil 1977; 50: 117-118.
[56] De Lamirande E, Gagnon C. Impact of reactive oxygen species on spermatozoa: A balancing act between beneficial and detrimental effects. Hum Reprod 1995; 10: 15-21.
[57] Corteel JM. Effects du plasma séminal sur la survie et la fertilité des spermatozoids conservés in vitro. Reprod Nutri Develop 1980; 20: 1111-1123.
[58] Gundogan M. Some reproductive parameters and seminal plasma constituents in relation to season in Akkaraman and Awassi Rams. Turkish J Vet & Anim Sci 2006; 30: 95-100.
[59] Gavella M, Lipovac V. Antioxidative effect of melatonin on human spermatozoa. Archives Androl 2000; 44(1): 23-27.
*Corresponding author: Scientist, Animal Reproduction Lab., National Research Centre on Mithun (ICAR), Jharnapani, Nagaland – 797 106 (India).
E-mail: perumalponraj@gmail.com
杂志排行
Asian Pacific Journal of Reproduction的其它文章
- Molecular dysregulation of renal development: Congenital anomalies of the kidney and urinary tract
- Variations in semen characteristics rams of Ouled Djellal breed have received an important dietary supplement after regular and intensive collection
- Effects of long term storage of semen in liquid nitrogen on the viability, motility and abnormality of frozen thawed Frisian Holstein bull spermatozoa
- Effect of different thawing procedures on the quality and fertility of the bull spermatozoa
- Naloxone affects reproductive system in a rat model with polycystic features
- Effects of different concentrations of sucrose or trehalose on the postthawing quality of cattle bull semen
