Effects of long term storage of semen in liquid nitrogen on the viability, motility and abnormality of frozen thawed Frisian Holstein bull spermatozoa
2015-12-22AbdulMALIKMuhammadLAILYMuhammadIrwanZAKIR
Abdul MALIK, Muhammad LAILY, Muhammad Irwan ZAKIR
1Department of Animal Science, Faculty of Agriculture. Islamic University of Kalimantan, Banjarmasin, Indonesia
2Office Animal husbandry. District of Tanah Laut, Province South Kalimantan, Indonesia
Effects of long term storage of semen in liquid nitrogen on the viability, motility and abnormality of frozen thawed Frisian Holstein bull spermatozoa
Abdul MALIK1*, Muhammad LAILY2, Muhammad Irwan ZAKIR1
1Department of Animal Science, Faculty of Agriculture. Islamic University of Kalimantan, Banjarmasin, Indonesia
2Office Animal husbandry. District of Tanah Laut, Province South Kalimantan, Indonesia
ARTICLE INFO
Article history:
Received 16 October 2014
Received in revised form 18 Novermber 2014
Accepted 25 November 2014
Available online 20 March 2015
Spermatozoa
Cryopreservation
Storage
liquid nitrogen
Sperm quality
Objective: To evaluate effects of long term storage of semen in liquid nitrogen on the motility, concentration, viability, and abnormality of frozen-thawed. Methods: A total of four Friesian Holstein bulls were used for this study. One hundred forty semen straws with produced during period from 2008 to 2013 and stored in the liquid nitrogen at the AI center were used in the research. The sample straw was divided into six groups; each group consist 20 semen straws. For group one all straw semen was produced on the 2013 with storage in liquid nitrogen as long as one year, the group 2, 3, 4, 5, and 6 were produced on the 2012, 2011, 2010, 2009 and 2008 with storage in liquid nitrogen as long as 2, 3, 4, 5, and 6 year, respectively. Results: The viability of thawed sperm was not significantly different decreased (P>0.05) between storage on the 1 year and storage on the 2 years. Whereas, the viability was significantly different (P<0.05) with storage on the 3, 4, 5 and 6 years. The motility of thawed sperm was not significantly different decreased (P>0.05) on the storage 1, 2 and 3 years. Whereas, the motility was significantly different (P<0.05) with storage on the 4, 5 and 6 years. The abnormality of thawed sperm was not significantly different increased (P>0.05) on the storage 1, 2 and 3 years. Whereas, the abnormality was significantly increased (P<0.05) with storage on the 4, 5 and 6 years. On the other hand the concentration of thawed sperm was not significantly different decreased (P>0.05) during storage in liquid nitrogen as long as six years. Conclusions: Based on the results in these experiments, it may be concluded that concentration sperm during one year storage in liquid nitrogen resulted in similar concentration storage as long as six years. However, the viability and motility sperm thawed storage in liquid nitrogen during six years was lower than storage on the 1 and 2 years.
1. Introduction
Dairy cows in Indonesia can be found in 19 of the 33 provinces. However, ninety seven percent of all dairy cows are located on the Java Island in the three provinces including east Java (47 %), central Java (25 %) and west Java (25 %). One of strategy for development Indonesian’s dairy industry is increasing the populations and productivity of dairy livestock. Artificial insemination (AI) is the good available tool for genetic improvement of dairy cattle in the minimum possible time. One of the factors for success AI is semen quality after thawed as well as relation to biophysical and biochemical characteristics of sperm[1]. Furthermore, Hayashi and Ishobe[2] reported that semen quality frozen thawed including viability, motility, and abnormality is crucial factor for successful AI. High viability, motility and abnormality of spermatozoa frozen thawed are significant factor because the relationship between the post-thawing sperm viability and the subsequent conception rate has been reported[3, 4].
Cryopreservation in -196 ℃ (liquid nitrogen) is a method that makes long-term storage of spermatozoa[5]. However, for the success of that, it is necessary that spermatozoaare preserved for long periods without damaging their fertilizing ability. Hammerstedt et al[6] and Watson[7] revealed that storage period per time is not having an effect on the sperm viability. However, researches designed to identify a decrease on performance of cryopreserved semen as a function of storage time are deficient. In several cases, the assumption of no damage to spermatozoa during cryopreservation is made subject to the semen being stored unaffected in liquid nitrogen[5].
The objective of this study was to evaluate effects of long term storage of semen in liquid nitrogen on the motility, concentration, viability and abnormality of frozen-thawed Frisian Holstein bull spermatozoa.
2. Materials and methods
2.1. Collection of semen
This study was conducted at the artificial insemination center Singosari, Malang in Indonesia. A total of four Holstein frisian bulls of at least 3-4 years of age and average weight of 800-850 kg were used this study. The bulls were housed under controlled condition at the AI Center. The semen was collected during period from 2008 to 2013 and stored in the liquid nitrogen. The semen from several bulls was stored together in large containers containing liquid nitrogen. These containers were routinely filled with liquid nitrogen to protect that the AI straws were constantly covered by liquid nitrogen. Semen was routinely collected using artificial vagina once or twice weekly. Immediately, after the semen ejaculate was evaluated for volume, color, pH, viability, motility, abnormality and sperm concentration. The volume of each ejaculate was measured in a graduated tube. The sperm concentration was calculated with a hemocytometer.
2.2. Semen cryopreservation
Freshly collected semen was mixed with Tris- egg yolk extender according to Hong et al.[8]. Prior to cryopreservation, semen was diluted to obtain a final concentration of 25 x 106sperm /straw. Extended semen were loaded in 0.25 mL straws (Biovet, France) and maintained at 4 ℃ for 2 hours before freezing. Then they were then frozen at 4 cm above liquid nitrogen to achieve approximately -120 ℃ for 10 min before being immersed into liquid nitrogen, and stored for one until six year before thawing.
2.3. Evaluation of frozen-thawed semen
Straws were thawed in a water bath at 37 ℃ for 50 to 60 second. Thawing was done by placing the straws in a water bath at the proper temperature. Immediately after thawing, the content of each straw was emptied in a 5 mL Falcon tube at 37 ℃. The sperm suspension was kept at 37 ℃ during post-thaw incubation. A total of 140 semen straw from Friesian-Holstein bull produced during period from 2008 to 2013 and stored in the liquid nitrogen at the AI center was used to the study. Sample straw was divided into the six groups, each group consist 20 semen straws. The group one, all straw semen was produced on 2013 with storage in liquid nitrogen as long as one year, the group 2, 3, 4, 5, and 6 were produced on the 2012, 2011, 2010, 2009 and 2008 with storage in liquid nitrogen as long as 2, 3, 4, 5, and 6 year, respectively.
2.4. Assessment motility
The motile sperm estimate by mixing the semen gently and placing a 10 μL drop of diluted semen on a warm slide and covered with a glass cover slip (18 × 18 mm) from five selected representative fields. The mean of the five estimations was recorded as final motility score. Sperm viability was assessed using nigrosin-eosin stain[9]. Placing 10 μL drop of diluted semen on a slide and adds with 40 μL drop of nigrosin-eosin, and smears on a slide and drying quickly in heating stage (37 ℃). Microscopes were selected randomly from ten fields, with total of 200 cells. Individual sperm were recorded as being viable (unstained) or dead (stained).
2.5. Sperm viability
Eosin-nigrosin staining was used to evaluate sperm viability adopted from Felipe-Pérez et al [10]. After thawing, one drop of the semen was placed on a tempered glass slide, which was mixed with one drop of eosin-nigrosin solution (0.2 g of eosin and 2 g of nigrosin were dissolved in a buffered saline solution (153 mM NaCl and 9.65 mM NaH2PO4, pH=7.4), mixed for 2 hours at room temperature and filtered to obtain the staining media). The mixture was smeared on the glass slide and allowed to air dry. One hundred spermatozoa were evaluated in at least five different fields in each smear under a light microscope. Eosin penetrates in non-viable cells, which appear red, nigrosin offers a dark background facilitating the detection of viable, non-stained cells.
2.6. Abnormalities of spermatozoa
The abnormalities of sperm were evaluated based on classified morphological abnormalities into the categories such as loose spermatozoa head, abnormal spermatozoa head and tail formation, presence of proximal cytoplasmic droplet, or distal cytoplasmic droplet adopted from Hafez and Hafez[11].
2.7. Statistical analysis
Percentage of mean values (± SEM) for various parameters of semen quality during six years experimental were calculated. The statistical significances of the effects of Viability, motility and concentration after storage in liquid nitrogen were determined by ANOVA (S-PLUS Statistical Program, Insightful Corporation Seattle, WA, USA).
3. Results
The effect of long term storage of semen in liquid nitrogen on the motility, concentration, viability and abnormality of frozen-thawed Frisian Holstein bull Spermatozoa have been presented in Table 1 and 2. Based on the evaluation of fresh ejaculation are shown in Table 1, overall parameters of semen characteristics were considered as standard. The viability of thawed sperm (Figure 2) was not significantly different decreased (P>0.05) between storage on the 1 year and storage on the 2 years. Whereas, the viability was significantly different (P<0.05) with storage on the 3, 4, 5 and 6 years. The motility of thawed sperm was not significantly different decreased (P>0.05) on the storage 1, 2 and 3 years. Whereas, the motility was significantly different (P<0.05) with storage on the 4, 5 and 6 years. The abnormality of thawed sperm was not significantly different increased (P>0.05) on the storage 1, 2 and 3 years. Whereas, the abnormality was significantly increased (P<0.05) with storage on the 4, 5 and 6 years. On the other hand the concentration of thawed sperm was not significantly different decreased (P>0.05) during storage in liquid nitrogen as long as six years.
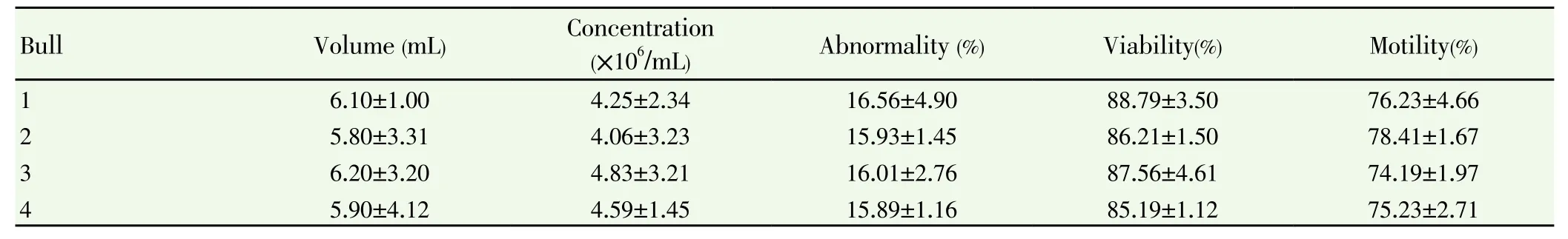
Table 1 Evarage of volume, viability, motility, abnormality and concentration semen evaluation just after collected using artificial vagina on the Frisian Holstein bull.
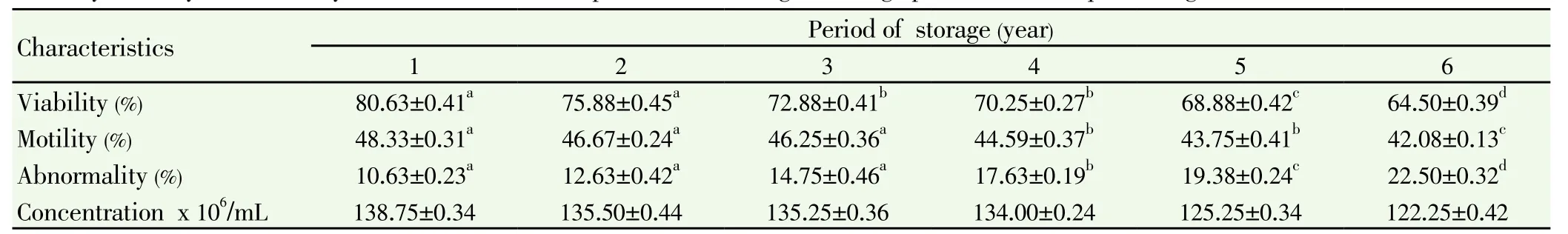
Table 2 Viability, motility, abnormality and concentration of sperm after thawing on storage periode in the liqiud nitrogen on the Frisian Holstein bull.
4. Discussion
The result of this study indicated that evarage volume of fresh ejaculate was ranged between 5.8 and 6.2 mL. This result strengthened findings reported by Shaha et al. [26] found the ejaculate volume 4.1 to 7.6 mL for Holstein Friesian cross Zebu cattle. Liquid nitrogen is used widely for the cryopreservation and long term storage of human and animal semen. This study reaffirmed previous study conducted by Hayashi and Ishobe[2] in which a similar concentration sperm of fresh ejaculate (455×106/mL) was reported in Frisian Holstein bull spermatozoa.
Liquid nitrogen is used widely for the cryopreservation and long term storage of human and animal semen. The present of this study was to investigate the effects of sperm viability after the long storage in the liquid nitrogen. The viability and motility of thawed sperm in liquid nitrogen were gradually decreased. Decreasing viability and motility after period of storage in liquid nitrogen in the present study were caused effect of genetic and cryopreservation. Chatterjee and Gagnon[12] reported that possible damage to sperm after a number of long storage at liquid nitrogen that have been suggested genetic damage, e.g. abnormalities of chromatin structure and DNA integrity. Meanwhile, reactive oxygen species (ROS) produced from cryopreservation can also induce of damage sperm thawed[13]. Cryopreservation also is a major cause of damage to the sperm thawed[6, 7, 14]. On the other hand Mazur[15]; Mazur and Kashimoto[16] revealed that in general, storage of sperm at the liquid nitrogen is better because it prevents thermal reactions and only slow background ionizing radiations could probably have a damaging effect on sperm function over a long period of time. Fraser et al. [17] demonstrated that the prolonged storage has effects to sperm motility, mitochondrial function and plasma membrane integrity. Perdesen and Lebech, [18]; Wolley and Richardson[19] found as long as freezing and thawing, mitochondria of spermatozoa undergo damages. Mitochondria are the source of sperm energy, and damage to their structure during the cryopreservation process is associated with reduced post-thaw including sperm viability and motility[20-22].
Another objective of this study was to determine the abnormality thawed sperm. Based on the data in Table 2 in this study, the abnormality was increase gradually during long term storage of semen in liquid nitrogen. These are probably due to deleterious effect of cryopreservation process, including cooling, freezing and thawing. Cryopreservation, freezing and thawing processes mayinduce spermatozoa damages especially to the plasma membrane and organelles[23, 24].
Accuracy of sperm concentration in the artificial insemination (AI) has impact to production efficiency of breeding stations, product quality, and fertility[25]. The results obtained in this study show that sperm concentration after thawed was not significantly different during six years storage in the liquid nitrogen.
Based on the results in these experiments, it may be concluded that concentration sperm during one year storage in liquid nitrogen resulted in similar concentration storage as long as six years. However, the viability and motility sperm thawed storage in liquid nitrogen during six years was lower than storage on the 1 and 2 years.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
This study was supported financially by fund of routine from Islamic University of Kalimantan. Banjarmasin, Indonesia with contract number: 041a/uniska-PP/X/2013. Authors are also grateful to Mr. Sarip Djaya and Mrs. Siti Darmawati.
[1] Medeiros CMO, Forell F, Oliveira ATD, Rodrigues JL. Current status of sperm cryopreservation: why isn’t it better? Theriogenology 2002; 57:327-344.
[2] Hayasi I, Isobe N. Characteristics of cryopreserved spermatozoa from a holstein-friesian bull thawed at different temperature. J Interl Develop & Cooperation 2005; 12 (1):107-110.
[3] Correa JR. Relationships among frozen-thawed sperm characteristics assessed via the routine semen analysis, sperm functional tests and fertility of bulls in an artificial insemination program. Theriogenology 1997; 48; 721-731.
[4] Linford E. The relationship between semen evaluation methods and fertility in the bull. J Reprod & Fertil 1976; 47; 283-291.
[5] Hammerstedt RH, Graham JK, Nolan JP. Cryopreservation of mammalian sperm: what we ask them to survive. J Androl 1990; 11; 73-88.
[6] Hong JHU, Wang QLI, Chen YL, Jlang ZL, Jia YH, Wang LQ, et al. Effects of addition of vitamin B12 to the extender on post- thaw motility, acrosome morphology, and plasma membrane integrity in bull semen. Turk J Veterinary & Anim Sci 2009; 35; 379-384.
[7] Haugana TYT, Gr¨ohn E, Kommisrud E, Ropstad O. Reksen. Effects of sperm concentration at semen collection and storage period of frozen semen on dairy cow conception. Anim Reprod Sci 2007; 97:1-11.
[8] Watson PF. Recent developments and concepts in the cryopreservation of spermatozoa and the assessment of their postthawing function. Reprod Fertil Dev 1995; 7: 871-891.
[9] Barth AD, Oko RJ. Abnormal morphology of bovine spermatozoa. Iowa: Iowa State University Press; 1989.
[10] Felipe-Pérez YE, Juárez-Mosqueda ML, Hernández- González EO, Valencia JJ. Viability of fersh and frozen bull sperm compared by two staining techniques. Acta Veterinaria Brasilica 2008; 2(4); 123-130.
[11] Hafez B, Hafez ESE. Reproductive behavior. In: Reproduction in farm animals. 7ed. New York: Lippincott Williams and Wilkens;2000, p. 293-306.
[12] Chatterjee S, Gagnon C. Production of reactive oxygen species by spermatozoa undergoing cooling, freezing, and thawing. Mol Reprod Dev 2001; 59; 451-458.
[13] Mammoto A, Masumoto N, Tahara M, Ikebuchi Y, Ohmichi M, Tasaka K, et al. Reactive oxygen species block sperm-egg fusion via oxidation of sperm sulfhydryl proteins in mice. Biol of Reprod 1996; 55: 1063-1068.
[14] Parks JE, Graham JK. Effects of cryopreservation procedures on sperm membranes. Theriogenology 1992 ; 38:209-222.
[15] Mazur P. Freezing of living cells: mechanisms and implications. Am J Physiol 1984; 247:C125-C142.
[16] Mazur P, Koshimoto C. Is intracellular ice formation the cause ofdeath of mouse sperm frozen at high cooling rates. Biol Reprod 2002; 66: 1485-1490.
[17] Fraser LJ, Strze˙zek W. Kordan. Post-thaw sperm characteristics following long-term storageof boar semen in liquid nitrogen. Anim Reprod Sci 2014 ; 147: 119 -127.
[18] Perdesen HS, Lebech PE. Ultrastructural changes in the human spermatozoa after freezing for artificialinsemination. Fertil Steril 1971; 22: 125-133.
[19] Wolley DM, Richardson DW. Ultrastructural injury to human spermatozoa after freezing and thawing. J Reprod Fert 1978; 53:389-394.
[20] Gadea J. Sperm factors related to in vitro and in vivo porcine fertility. Theriogenology 2005; 63: 431- 444.
[21] Fraser L, Strze˙zek J. Effect of different procedures of ejaculate col-lection, extenders and packages on DNA integrity of boar spermatozoafollowing freezing–thawing. Anim Reprod Sci 2007; 99: 317-329.
[22] Ortega Ferrusola C, Sotillo-Galán Y, Varela-Fernández E, Gallardo-Bola˜nos JM, González Fernández L, Tapia JA, et al. Detection of apoptosis like changes during the cryopreservation pro-cess in equine sperm. J Androl 2008 ; 29: 213-221.
[23] Pena FJ, Johannisson A, Wallgren M, Rodriguez-Martínez H. Assessment of fresh and frozen-thawed boar semen using an annexin-v assay: a new method of evaluating sperm membrane integrity. Theriogenology 2003; 60: 677-689.
[24] Munoz OVL, Amirat-Briand T, Diaz L, Va´squez E, Schmidt S, Desherces M, et al. Effect of semen dilution to low-sperm number per dose on motility and functionality of cryopreserved bovine spermatozoa using low-density lipoproteins (LDL) extender: Comparison to Triladyl and Bioxcell. Theriogenology 2009; 71: 895-900.
[25] Anzar AT Kroetsch, Buhr MM. Comparison of different methods for assessment of sperm concentration and membrane integrity with bull semen. J Androl 2009; 30: 661-668.
[26] Shaha SP, Alam MGS, Khatun M, Ahmed JU. Breeding soundness of study bulls. Bang. Veterinarian 2008; 25:51-61.
*Corresponding author: Abdul MALIK, Department of Animal Science, Faculty of Agriculture. Islamic University of Kalimantan. Banjarmasin. Indonesia.
E-mail: sidol_99@yahoo.com
Foundation project: This study was supported financially by fund of routine from Islamic University of Kalimantan. Banjarmasin, Indonesia with contract number: 041a/ uniska-PP/X/2013.
杂志排行
Asian Pacific Journal of Reproduction的其它文章
- Molecular dysregulation of renal development: Congenital anomalies of the kidney and urinary tract
- Effect of seasons on semen production, effect of melatonin on the liquid storage (5 ℃) with correlated study of birth rate in mithun (Bos frontalis)
- Variations in semen characteristics rams of Ouled Djellal breed have received an important dietary supplement after regular and intensive collection
- Effect of different thawing procedures on the quality and fertility of the bull spermatozoa
- Naloxone affects reproductive system in a rat model with polycystic features
- Effects of different concentrations of sucrose or trehalose on the postthawing quality of cattle bull semen
