大气CO2浓度升高对亚洲玉米螟生长发育及繁殖的影响
2015-03-10杨群芳解海翠王振营何康来
赵 磊, 杨群芳, 解海翠, 王振营, 何康来,*
1 四川农业大学, 成都 611130 2 中国农业科学院植物保护研究所, 植物病虫害生物学国家重点实验室, 北京 100193
大气CO2浓度升高对亚洲玉米螟生长发育及繁殖的影响
赵 磊1,2, 杨群芳1, 解海翠2, 王振营2, 何康来2,*
1 四川农业大学, 成都 611130 2 中国农业科学院植物保护研究所, 植物病虫害生物学国家重点实验室, 北京 100193
大气CO2浓度升高影响寄主植物的营养而间接影响节肢动物外,还直接影响许多昆虫的生长发育和繁殖。为探讨大气CO2浓度升高对亚洲玉米螟Ostriniafurnacalis(Guenée)生长发育和繁殖的影响,在CDCC- 1型密闭式动态CO2气室内研究了当前大气CO2浓度375 μl/L及大气CO2浓度分别升高0.5倍和1倍,即达到550 μL/L和750 μL/L条件下人工饲料饲养亚洲玉米螟实验种群生命表及其营养效应指标。结果表明,大气CO2浓度分别升高到550μL/L和750 μL/L时,亚洲玉米螟幼虫成活率分别降低3.0%和8.9%;幼虫、蛹和成虫体重则没有显著差异;在750 μL/L CO2浓度下幼虫和蛹历期分别显著延长13.1%和25.8%。虽然单雌产卵量和净增值率(R0)在大气高CO2浓度下有增加趋势,但未达到显著性差异。与当前大气CO2浓度相比,高CO2浓度下玉米螟的取食量分别增加9.1%和34.0%,排粪量分别增加42.3%和42.0%。
亚洲玉米螟 ;大气二氧化碳;直接影响;生命表
大气CO2浓度升高对昆虫的影响及其对这一变化的响应是全球气候变化领域研究的热点问题之一。一方面,CO2是植物光合作用的主要原料之一,其浓度升高对植物生长及生理特性产生显著影响[1- 2],如光合效率提高,促进植物生长,叶片中C/N升高,次生代谢及化学防御物质发生改变,影响植食性昆虫食物的质量,进而影响植食性昆虫的生长发育和繁殖等[3- 4]。另一方面,大气中高浓度CO2直接影响昆虫的呼吸和生理代谢以及某些与化学通讯相关的行为改变[5- 8]。高CO2浓度(750 μL/L)环境中生长的棉铃虫Helicoverpaarmigera(Hbn.) 幼虫体内营养物质显著下降,取食量、排粪量明显增加,生长发育延缓,体重减轻,内禀增长力和成活率下降[9]。禾谷缢管蚜在高浓度CO2直接影响下成蚜的繁殖力增强,生长速率明显加快[10],而麦长管蚜Sitobionavenae(F.) 体内乙酰胆碱酯酶活性与蚜虫对报警信息素的响应呈显著负相关,同时超氧化物歧化酶和乙酰胆碱酯酶活性提高[11]。显然,不同种类的昆虫对大气CO2浓度升高的响应各异。
亚洲玉米螟Ostriniafurnacalis(Guenée)是玉米上的主要害虫,常年造成玉米减产10%左右,且严重危害频发,导致玉米损失30%以上甚至绝收[12]。有关大气CO2浓度升高对玉米螟生长发育的直接影响还未见报道。本文研究了高CO2浓度下亚洲玉米螟实验种群的生长发育,测定其个体营养效应指标,旨在明确高CO2浓度下对玉米螟生长发育及繁殖的影响,为未来CO2浓度升高的环境下更深入了解亚洲玉米螟发生的生态学机制及其测报、综合治理等提供依据。
1 材料与方法
1.1 供试昆虫
实验所用亚洲玉米螟为采自山东夏津玉米田卵块,孵化后在室内(28±1)℃,80% RH,光照周期L∶D =16h∶8 h条件下用人工饲料饲养3代,建立室内种群。饲养技术及人工饲料参照新7号饲料配方及饲养方法[13]。
1.2 试验方法
1.2.1 试验处理
不同CO2浓度试验在密闭式动态CO2人工气候箱(CDCC- 1型,宁波赛福PRX- 450D-CO2)内进行。试验设置3个CO2浓度处理,即375 μL/L(当前大气CO2浓度)、(550±50) μL/L和(750±50) μL/L(模拟大气CO2浓度分别升高0.5倍和1.0倍)。人工气候箱内温度:白天(28±1)℃,夜间(27±1)℃;湿度75%—85%;光照周期L∶D=16h∶8h(L, 6:00—22:00; D, 22:00—6:00)。亚洲玉米螟幼虫饲养容器为直径9 cm的塑料培养皿,上盖有直径4 cm 圆孔,并以70目不锈钢纱网封闭圆孔。
1.2.2 亚洲玉米螟种群生命表参数及死亡率测定
将亚洲玉米螟初孵幼虫接入配好的人工饲料[13],遂即放入不同CO2浓度人工气候箱。每个CO2浓度处理中,用培养皿单头饲养60头,重复3次。每天定时更换人工饲料并记录亚洲玉米螟的龄期和死亡数。化蛹后将蛹置于相应CO2浓度人工气候箱中(温度(25±1) ℃;RH 85%—90%;光照周期L∶D=16h∶8 h),每天观察各CO2浓度下成虫的羽化情况,将当天羽化的成虫按1∶1配对置于养虫笼(11 cm× 8 cm× 8 cm)中,并给与3%的蔗糖水喂养。每天定时更换产卵纸并记录产卵量。根据饲养数据资料,计算亚洲玉米螟在不同CO2浓度下的实验种群生命表参数[14- 16](表1)。
1.2.3 亚洲玉米螟生长发育测定
将亚洲玉米螟初孵幼虫接入配好的人工饲料上,遂即放入不同CO2浓度人工气候箱。每个CO2浓度处理中,用培养皿单头饲养20头幼虫,重复3次,共在9个人工气候箱进行。每天观察记录幼虫的发育进度参数,即幼虫发育历期(幼虫孵化当天至化蛹前的天数)、幼虫体重(化蛹前最大体重);蛹历期(化蛹当天到羽化前的天数)、蛹重(化蛹后第2天称量蛹重);成虫历期(羽化后到成虫死亡的天数)、成虫体重(羽化当天取食或饮水前的体重)。同时记录各发育阶段及每天玉米螟的存活数。
1.2.4 亚洲玉米螟幼虫营养效应测定
在测定不同CO2浓度下亚洲玉米螟幼虫生长发育状况的同时,每天9:00定时更换人工饲料,并称量新鲜及取食后饲料的重量,收集幼虫每天排出的粪便和取食剩余饲料,用烘箱烘干(80 ℃,72 h)至恒重并称量干重。参照Waldbauer[17]氏昆虫食物消耗利用率方法,根据幼虫期取食量和排粪量计算近似消化率(AD)、毛转化率(ECI)、净转化率(ECD)、相对消耗率(PCR)、相对生长率(PGR)、平均相对生长速率(MRGR)(表2)。
表1 种群生命表参数计算公式
Table 1 Formulations for calculating population features from life table

种群参数Populationparameters计算公式*Formulationsforestimationofparameters净增殖率R0NetreproductiverateR0=Σlxmx平均世代周期TMeangenerationtimeT=Σxlxmx/Σlxmx内禀增长力rmInnaterateofincreaserm=lnR0/T周限增长率λFiniteincreaserateλ=erm种群加倍时间tDoublepopulationtimet=ln2/rm
*x为时间间隔(d);lx为在x时期开始时的存活率;mx为在x时期间平均每雌产雌数

表2 营养效应指标计算公式Table 2 The calculations for index of food utilization
**Q为幼虫期取食量(mg);F为幼虫期排粪量(mg);W1为初孵幼虫体重(mg),W2为幼虫最终体重(mg);P为幼虫每天平均重量;t1为幼虫历期(d)
1.2.5 数据处理
各处理间差异显著性采用方差分析,平均数比较采用LSD测验。数据分析前,先进行适当的数据转换(百分数进行反正弦转换,发育历期进行自然对数转换),以满足方差分析要求。统计分析计算应用SAS9.0软件进行。
2 结果分析
2.1 大气CO2浓度对亚洲玉米螟种群生命表参数及死亡率的影响
大气CO2浓度分别升高0.5倍和1.0倍处理下成虫的单雌产卵量分别为191.5粒和227.1粒,比对照177.4粒增加了7.9%和28.1%,但各浓度间并无显著差异(df=2,113;F=1.06;P=0.3483)。根据不同CO2浓度下亚洲玉米螟的实验种群生命表计算出各主要生命参数(表3),虽然在大气高CO2浓度(750 μL/L)处理下净增值率比对照上升10.2%,但各处理间差异不显著。由于平均世代周期显著延长5.3%和11.7%,而使内禀增长力和周限增长率显著降低,种群加倍天数显著延长9.1%。
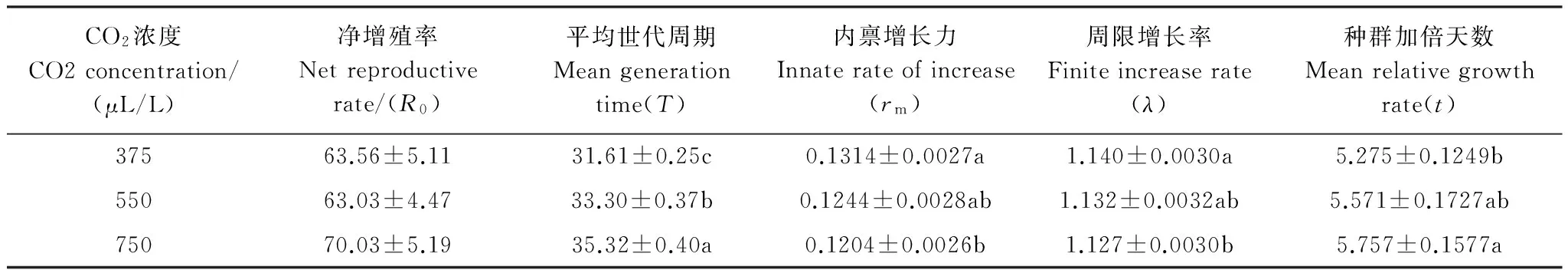
表3 不同大气CO2浓度下亚洲玉米螟种群生命参数Table 3 Population features calculated from life tables of Asian corn borer reared under different levels of atmospheric CO2
R0: df=2,110,F=0.61,P=0.5456;T: df=2,109,F=12.21,P<0.0001;rm: df=2,109,F=4.07,P=0.0197;λ: df=2,109,F=4.13,P=0.0187;t: df=2,109,F=3.19,P=0.0449; *同列数字后字母不同者表示处理间差异显著(LSD,P<0.05)

图1 不同大气CO2浓度下亚洲玉米螟累计死亡率 Fig.1 The cumulative mortality of Ostrinia furnacalis under different levels of atmospheric CO21龄:df=2,6, F=131.41, P<0.0001; 2龄:df=2,6, F=72.25, P<0.0001; 3龄:df=2,6, F=31.62, P=0.0007; 4龄:df=2,6, F=15.39, P=0.0043; 5龄:df=2,6, F=15.61, P=0.0042; 蛹:df=2,6, F=21.81, P=0.0018
根据亚洲玉米螟在各个发育阶段的死亡数,绘制了不同大气CO2浓度下的累计死亡率曲线(图1)。高CO2浓度环境下亚洲玉米螟各龄期的累计死亡率均显著高于当前大气CO2浓度。此外,大气CO2浓度升高对亚洲玉米螟死亡率的影响因其所处虫态不同而异。高CO2浓度750 μL/L浓度环境下3龄以前幼虫死亡率显著高于当前大气CO2浓度;3龄至蛹期各处理幼虫死亡率变化不大。说明亚洲玉米螟在3龄以后对高浓度CO2的耐受能力增强。
2.2 大气CO2浓度对亚洲玉米螟生长发育的影响
2.2.1 发育历期
随大气CO2浓度的增加,亚洲玉米螟幼虫、蛹和成虫的发育历期都有延缓趋势(表4)。与当前大气CO2浓相比,在550 μL/L大气CO2浓度下饲养亚洲玉米螟,其幼虫和蛹历期分别延长10.3%和7.6%,但没有显著差异,而当大气CO2浓度升高1.0倍时,幼虫和蛹历期分别显著延长了13.1%和25.7%,而成虫寿命与对照差异不显著。这说明大气CO2浓度升高对亚洲玉米螟幼虫和蛹的发育速度有抑制作用。
2.2.2 个体体重
随大气CO2浓度的增加,亚洲玉米螟幼虫、蛹和成虫的体重有下降的趋势(表5)。虽然在大气CO2浓度分别升高0.5倍和1.0倍下饲养的亚洲玉米螟与在当前大气CO2浓度相比,幼虫体重分别降低5.8%和7.5%,蛹重分别降低3.7%和4.8%,成虫体重分别降低6.7%和1.7%,但均差异不显著。然而,种群的幼虫平均相对生长率显著低于对照(df= 2,82;F= 4.22,P= 0.0187)(表6)。
表4 不同大气CO2浓度下亚洲玉米螟幼虫、蛹和成虫的发育历期
Table 4 Durations of larva, pupa, and adult development stages ofOstriniafurnacalisreared under different levels of atmospheric CO2

CO2浓度(μL/L)CO2concentration发育历期Developmenttime/d幼虫Larva蛹Pupa成虫Adult37514.5±0.2b6.6±0.1b7.4±0.255016.0±0.5ab7.1±0.2b7.2±0.175016.4±0.8a8.3±0.3a7.5±0.2
幼虫期: df= 2,147,F= 3.35,P= 0.0411; 蛹期: df=2, 143,F= 20.80,P<0.0001; 成虫期: df= 2,141,F= 0.93,P= 0.3996; 同列数字后字母不同者表示经LSD测验差异显著(P<0.05)
表5 不同大气CO2浓度下亚洲玉米螟幼虫、蛹和成虫体重
Table 5 Larva, pupa, and adult weights ofOstriniafurnacalisreared under different levels of atmospheric CO2

CO2浓度(μL/L)CO2concentration个体体重Bodyweight/mg幼虫Larva蛹Pupa成虫Adult37579.2±2.559.8±2.138.0±1.955074.6±2.257.5±1.935.5±1.475073.2±2.156.9±1.737.4±1.2
幼虫: df=2,153,F= 1.78,P= 0.1721; 蛹: df=2,148,F=0.59,P= 0.5538; 成虫: df= 2,146,F= 0.75,P= 0.4764
2.3 高CO2浓度对亚洲玉米螟幼虫营养效应
与当前大气CO2浓度相比,当大气CO2浓度分别升高0.5倍和1.0倍时,亚洲玉米螟幼虫的取食量和排粪量都显著增加,取食量分别增加9.1%和34.0%,排粪量分别增加42.3%和42.0%(表6)。但对食物的近似消化率、毛转化率、净转化率及相对消耗率、相对生长率与对照比较并无显著差异。

表6 不同大气CO2浓度下亚洲玉米螟幼虫的营养效应Table 6 The consumption and utilization of food by Ostrinia furnacalis under different levels of atmospheric CO2
取食量: df= 1, 57,F= 5.42,P= 0.0234; 排粪量: df= 2, 82,F=17.31,P<0.0001; AD: df=2, 82,F= 1.31,P= 0.2784; ECI: df=2, 82,F= 1.08,P= 0.3458; ECD: df= 2, 75,F= 1.27,P= 0.2932; RCR: df= 2, 82,F= 0.23,P= 0.7949; RGR: df= 2, 82,F= 0.90,P= 0.4071; *同列数字后字母不同者表示经LSD测验差异显著(P<0.05);
AD: Approximate digestibility,ECI: Efficiency of conversion of ingested food,ECD: Efficiency of conversion of digested food,RCR: Relative consumption rate,RGR: Relative growth rate,MRGR: Mean relative growth rate
3 讨论
目前研究大气CO2浓度升高对于昆虫的影响多数测试幼虫取食在设定未来大气CO2浓度升高0.5倍或1倍(通常约500—700 μL/L)环境下生长的寄主植物组织时的生长、发育和繁殖以及存活率的改变[18- 21],这必然受到寄主植物因大气CO2浓度升高而产生的营养代谢和次生防御代谢变化而引起其作为昆虫食物质量的改变的影响,且由于研究者所采用的昆虫种类及寄主植物的不同,其结果往往各异。如大气高CO2浓度下生长的棉铃对棉铃虫的适合度下降[22];蛱蝶幼虫Junoniacoenia取食大气高CO2浓度下长叶车前Plantagolanceolata的死亡率增加[23];麦长管蚜在大气高CO2浓度影响下产卵期提前,繁殖力显著提高[24];对5种蚜虫及其寄主在大气高CO2浓度的研究表明,Acyrthosiphonpisum(Harris)在ViciafabaL.上的种群下降,Myzuspersicae(Sulzer)在SolanumdulcamaraL.上的种群上升,而AphisneriiBoyer de Fonscolombe在AsclepiassyriacaL.、AphisoenotheraeOestlund 在OenotherabiennisL.和Aulacorthumsolani(Kaltenbach)在NicotianasylvestrisSpeg. & Comes上的种群没有变化[25]。以室外大气CO2浓度升高(700 μL/L)条件下种植的甜菜BetavulgarisL.在相同CO2浓度培养箱中饲喂甜菜夜蛾Spodopteraexigua(Hbn.) 幼虫,其存活率显著高于在当前大气CO2浓度(350 μL/L)培养箱中的饲喂处理[26];而当幼虫在大气高CO2浓度且高肥条件下生长的棉苗上取食,其存活率比对照下降50%,雌虫存活率是雄虫的2倍[27];将粉纹夜蛾Thichoplusiani(Hbn.)初孵幼虫接于生长在大气高CO2浓度条件下的菜豆Phaseoluslunata上取食,其存活率与正常大气环境下没有显著差异[28];用人工饲料在高CO2浓度下饲养棉铃虫,存活率没有影响[7]。然而,本研究结果显示,大气CO2浓度升高条件下取食人工饲料的亚洲玉米螟幼虫的存活率下降。此外,大龄幼虫对CO2的适应性要高于低龄幼虫。这可能与不同龄期幼虫的生活习性和栖息的微生态环境有关,即大龄幼虫尤其是4—5龄幼虫蛀茎危害[29],蛀孔内为相对密闭的环境,其CO2浓度应该高于外界大气CO2浓度。由此可见,大气CO2浓度升高对植食性昆虫生存率的影响因种类、不同发育时期及其寄主植物的不同而异。
CO2亦是昆虫的呼吸代谢产物,环境中高浓度CO2会直接影响昆虫的呼吸和生理代谢,对昆虫生长发育的各个阶段可能产生影响,不同昆虫及处理方式所产生的影响亦各异。短时间高浓度CO2处理可使Blatellagermanica幼虫发育历期延长[30];与CO2处理可使Achetadomesticus虫体重减轻相比,短暂的大气高浓度CO2处理对其生长和体重几乎没有不利影响[31- 32];与当前大气相比,CO2浓度升高1倍下棉铃虫幼虫发育历期延长,体重没有显著影响[9]。甜菜夜蛾幼虫在大气高CO2浓度且高肥条件下生长的棉苗上取食,其发育历期延长,体重显著下降[27]。将粉纹夜蛾幼虫在生长于大气高CO2浓度条件下的菜豆上取食,其蛹重比正常大气环境下显著降低28]。本研究显示随大气CO2浓度升高亚洲玉米螟幼虫期和蛹期延长,而成虫寿命与对照差异不显著;各虫态虫体重差异不显著,但幼虫平均相对生长率显著降低。
短时间反复CO2处理或麻醉对于昆虫繁殖力的影响已有多方面的研究报道,如延缓或阻碍交尾[33- 35]、降低雌虫受精频率或雄虫授精能力[36]、抑制卵巢管中卵母细胞的发育[37]、雌虫所产卵的雌雄比降低[38]等。一些昆虫在高CO2浓度处理0.5—1 h可使产卵量和孵化率降低,若反复处理甚或麻醉影响显著提高[39- 42]。模拟当前大气CO2浓度升高1倍条件下饲养的棉铃虫单雌产卵量增加7.9%[9]。本研究显示随大气CO2浓度升高亚洲玉米螟的产卵量有增加趋势,世代周期显著延长,内禀增长力显著下降。说明未来CO2浓度升高可能不利于亚洲玉米螟种群发展。
高CO2浓度对昆虫的影响具有长期的、多世代的作用,在长期的影响下存在着很大的不可知性[43],需要连续多代的持久观察研究。Awmack和Docherty等研究显示,高CO2处理一个世代,昆虫的历期几乎没有变化,而连续处理多个世代后,其生长发育受到极大的影响[44- 45];Brooks研究连续3个世代的叶甲Gasrophysauiridula对高浓度CO2(600 μL/L)的响应显示第二代雌虫的产卵量比第一代少30%[46];而Yin 等 研究在高CO2浓度下以人工饲料饲养连续3个世代的棉铃虫,结果显示CO2对其世代影响不明显[47]。而本实验进仅研究了高CO2浓度(550,750 μL/L)下,亚洲玉米螟一个世代的生长发育、繁殖及种群生命表对未来高CO2浓度的响应,对亚洲玉米螟连续多个世代是否对高CO2浓度存在同样或更大的响应还有待进一步研究。
致谢:感谢美国农业部农业研究处作物遗传育种研究室昆虫专家(USDA-ARS,Crop Genetics and Breeding Research Unit)、乔治亚大学教授Xinzhi Ni博士修改英文摘要。
[1] Dijkstra P, Hymus G, Colavito D, Vieglais D A, Cundari C M, Johnson D P, Hungate B A, Hinkle C R, Drake B G. Elevated atmospheric CO2stimulates aboveground biomass in a fire-regenerated scrub-oak ecosystem. Global Change Biology, 2002, 8(1): 90- 103.
[2] 马祥庆, 刘爱琴, 邵文, 康国庆. 大气CO2增加对森林生态系统影响研究综述. 福建林学院学报, 1996, 16(2): 177- 182.
[3] Lindroth R L, Kinney K K, Platz C L. Responses of deciduous trees to elevated atmospheric CO2: productivity, phytochemistry, and insect performance. Ecology, 1993, 74(3): 763- 777.
[4] Bazzaz F A. The response of natural ecosystems to the rising global CO2levels. Annual Review of Ecology and Systematic, 1990, 21: 167- 196.
[5] Stange G, Wong C. Moth response to climate. Nature, 1993, 365(6448): 699- 700.
[6] Stange G. Effects of changes in atmospheric carbon dioxide on the location of hosts by the moth,Cactoblastiscactorum. Oecologia, 1997, 110(4): 539- 545.
[7] 邓永学, 赵志模, 李隆术. 高浓度CO2气调防治谷蠹及杂拟谷盗的研究. 粮食储藏, 2002, 31(1): 43- 56.
[8] Nicolas G, Sillans D. Immediate and latent effects of carbon dioxide on insects. Annual Review of Entomology, 1989, 34: 97- 116.
[9] 吴刚, 陈法军, 戈峰. CO2浓度升高对棉铃虫生长发育和繁殖的直接影响. 生态学报, 2006, 26(6): 1732- 1738.
[10] Xing G M, Zhang J, Liu J, Zhang X Y, Wang G X, Wang Y F. Impacts of atmospheric CO2concentrations and soil water on the population dynamics, fecundity and development of the bird cherry-oat aphid. Entomology, 2003, 31(5): 499- 514.
[11] Sun Y C, Su J W, Ge F. Elevated CO2reduces the response ofSitobionavenae(Homoptera: Aphididae) to alarm pheromone. Agriculture, Ecosystems & Environment, 2010, 135(1/2): 140- 147.
[12] 王振营, 鲁新, 何康来, 周大荣. 我国研究亚洲玉米螟历史、现状与展望. 沈阳农业大学学报, 2000, 31(5): 402- 412.
[13] 周大荣, 王玉英, 刘宝兰, 剧正理. 玉米螟人工大量繁殖研究: I. 一种半人工饲料及其改进. 植物保护学报, 1980, 7(2): 113- 122.
[14] Laing J E. Life history and life table ofTetranychusurticaeKoch. Acarologia, 1969, 11(1): 32- 42.
[15] Carey J R. Applied Demography for Biologists: with Special Emphasis on Insects. Oxford: Oxford University Press, 1993.
[16] 张孝義. 昆虫生态及预测预报. 北京: 中国农业出版社, 2002.
[17] Waldbauer G P. The consumption and utilization of food by insects. Advance Insect Physiology, 1968, 5: 229- 288.
[18] Coviella C E, Trumble J T. Effects of elevated atmospheric carbon dioxide on insect-plant interactions. Conservation Biology, 1999, 13(4): 700- 712.
[19] Hunter M D. Effects of elevated atmospheric carbon dioxide on insect-plant interactions. Agricultural and Forest Entomology, 2001, 3(3): 153- 159.
[20] Whittaker J B. Impacts and responses at population level of herbivorous insects to elevated CO2. European Journal of Entomology, 1999, 96(2): 149- 156.
[21] Zavala J A, Nabity P D, DeLucia E H. An emerging understanding of mechanisms governing insect herbivory under elevated CO2. Annual Review of Entomology, 2013, 58: 79- 97.
[22] Chen F J, Wu G, Parajulee M N, Ge F. Long-term impacts of elevated CO2and transgenic Bt cotton on performance and feeding of three generations of cotton bollworm. Entomologia Experimentalis et Applicata, 2007, 124(1): 27- 35.
[23] Fajer E D, Bowers M D, Bazzza F A. The effects of enriched carbon dioxide atmospheres on the Buckeye butterfly,Junoniacoenia. Ecology, 1991, 72(2): 751- 754.
[24] Awmack S, Harrington R, Leather S R, Lawton J H. The impacts of elevated CO2on aphid-plant interactions. Aspects Applied Biology, 1996, 45: 317- 322.
[25] Hughes L, Bazza F A. Effects of elevated CO2on five plant-aphid interactions. Entomologia Experimentalis et Applicata, 2001, 99(1): 87- 96.
[26] Caulfield F, Bunce J A. Elevated atmospheric carbon dioxide concentration affects interactions betweenSpodopteraexigua(Lepidoptera: Noctuidae) larvae and two host plant species outdoors. Environmental Entomology, 1994, 23(4): 999- 1005.
[27] Akey D H, Kimball B A. Growth and development of the beet armyworm on cotton grown in an enriched carbon dioxide atmosphere. Southwestern Entomologist, 1989, 14(3): 255- 260.
[28] Osbrink W L A, Trumble J T, Wagner R E. Host suitability ofPhaseoluslunataforTrichoplusiani(Lepidoptera: Noctuidae) in controlled carbon dioxide atmospheres. Environmental Entomology, 1987, 16(3): 639- 644.
[29] 周大荣, 何康来, 王振营, 叶志华, 文丽萍, 高云霞, 宋彦英. 亚洲玉米螟综合防治技术. 北京: 金盾出版社, 1995.
[30] Brooks M A. Growth-retarding effect of carbon dioxide anesthesia on the German cockroach. Journal of Insect Physiology, 1957, 1(1): 76- 84.
[31] Edwards L J, Patton R L. Effects of carbon dioxide anesthesia on the house cricket,Achetadomesticus(Orthoptera: Gryllidae). Annals of the Entomological Society of America, 1965, 58(6): 828- 832.
[32] Woodring J P, Clifford C W, Roe R M, Beckman B R. Effects of CO2and anoxia on feeding, growth, metabolism, water balance, and blood composition in larval female house crickets,Achetadomesticus. Journal of Insect Physiology, 1978, 24(6/7): 499- 509.
[33] Kumar H, Saxena K N. Mating behavior of the cotton leafhopper,Empoascadevastans, in relation to its age, ovarian development, diurnal cycle, and CO2treatment. Annals of Entomological Society of America, 1978, 71(1): 108- 110.
[34] Lum P T M. Effect of carbon dioxide anesthesia at eclosion upon mating efficiency of malePlodiainterpunctella(Lepidoptera: Pyralidae). Journal of Stored Products Research, 1974, 10(1): 69- 71.
[35] Whisenant B R, Brady U E. Effects of anesthesia on the subsequent mating behavior ofPlodiainterpunctellamales. Journal of the Georgia Entomology, 1967, 2(1): 27- 30.
[36] Moloo S K, Kutuza S B. Effects of carbon dioxide anaesthetic onGlossina. Acta Tropica, 1975, 32(2): 159- 165.
[37] Press J W, Flaherty B R, Arbogast R T. Oöcyte maturation inTriboliumcastaneumafter repetitive sublethal carbon dioxide exposures. Annals of the Entomological Society of America, 1973, 66(2): 480- 481.
[38] Hodkowa M, Fuzeau-Braesch S. Effet du gaz carbonique sur la reproduction dePyrrhocorisapterussous deux régimes lumineux: inhibition de la reproduction en jours longs. Comptes Rendus de I′Académie des Sciences, 1988, 306(3): 121- 124.
[39] Aliniazee M T, Lindgren D L. Egg hatch ofTriboliumconfusumandTriboliumcastaneumcoleoptera: tenebrionidae in different carbon dioxide and nitrogen atmospheres. Annals of the Entomological Society of America, 1970, 63(4): 1010- 1012.
[40] Barrer P M, Jay E G. Laboratory observations on the ability ofEphestiacautella(Walker) (Lepidoptera: Phycitidae) to locate, and to oviposit in response to a source of grain odour. Journal of Stored Products Research, 1980, 16(1): 1- 7.
[41] Lum P T M, Flaherty B R. Effect of carbon dioxide on production and hatchability of eggs ofPlodiainterpunctella(Lepidoptera: Phycitidae). Annals of the Entomological Society of America, 1972, 65(4): 976- 977.
[42] Press J W, Flaherty B R. Hatchability ofPlodiainterpunctellaeggs exposed to a carbon dioxide atmosphere: relationship of egg age to exposure time. Journal of the Georgia Entomological Society, 1973, 8(3): 210- 213.
[43] Lawton J H. The response of insects to environmental change // Harrington R, Stork N E, eds. Insects in Changing Environment: Symposium of the Royal Entomological Society. London: Academic Press, 1995: 3- 26.
[44] Awmack C S, Harrington R, Leather S R. Host plant effects on the performance of the aphidAulacorthumsolani(Kalt.) (Homoptera: Aphiididae) at ambient and elevated CO2. Global Change Biology, 1997, 3(6): 545- 549.
[45] Docherty M W, Hurst F A, Whittaker D K. Responses of tree sap-feeding herbivores to elevated CO2. Global Change Biology, 1997, 3(1): 51- 59.
[46] Brooks G L, Whittaker J B. Responses of multiple generations ofGastrophysaviridula, feeding onRumexobtusifolius, to elevated CO2. Global Change Biology, 1998, 4(1): 63- 75.
[47] Yin J, Sun Y C, Wu G, Ge F. Effects of elevated CO2associated with maize on multiple generations of the cotton bollworm,Helicoverpaarmigera. Entomologia Experimentalis et Applicata, 2010, 136(1): 12- 20.
Direct effects of the elevated atmospheric carbon dioxide levels on the growth,development and reproduction ofOstriniafurnacalis(Guenée)
ZHAO Lei1,2, YANG Qunfang1, XIE Haicui2, WANG Zhenying2, HE Kanglai2,*
1SichuanAgriculturalUniversity,Chengdu611130,China2StateKeyLaboratoryforBiologyofPlantDiseasesandInsectPests,InstituteofPlantProtect,ChineseAcademyofAgriculturalSciences,Beijing100193,China
The level of atmospheric CO2has risen from 280μL/L to 360μL/L following the industrial revolution, engendering a critical shift in global biogeochemical cycles. This level of CO2is anticipated to double by the end of this century. By altering the chemical composition of foliage, the increase in atmospheric CO2levels may fundamentally alter the relationships between insect herbivores and their host plants. In addition to the elevated CO2levels affecting arthropods indirectly by altering chemical components of the host plants, many insects and arthropods respond directly to the increase in atmospheric CO2level. The Asian corn borer,Ostriniafurnacalis(Guenée) (Lepidoptera: Crambidae), is a key pest of maize production and causes 10%—30% yield losses of in most maze production areas in China. The response ofO.furnacalisto elevated CO2levels will affect the population dynamics and its damage to the maize plants. Direct effects of enriched atmospheric CO2levels on growth, development and fecundity of the Asian corn borer,O.furnacalis, were assessed the insects have been reared on the artificial diet. The effects were examined in the closed-dynamic CO2chamber (CDCC- 1) under either ambient (375 μL/L) and elevated CO2levels (i.e., 550 μL/L and 750 μL/L, respectively). When compared with ambient CO2level, the survival rates of the larvae were decreased by 3.0% and 8.9% under the two elevated CO2levels, respectively. In addition, the mortality was higher during the first and second instars reared under elevated CO2(750 μL/L) than ambient (375 μL/L). However, there were no significant differences among the mortalities of the third and later instar larvae reared either under ambient or the elevated CO2levels (550 μL/L and 750 μL/L). There were no significant differences in the larval, pupal, and adult weight among the ambient and the two elevated CO2treatments. However, the durations of larval and pupal development were significantly prolonged respectively by 13.1% and 25.8% at 750 μL/L level of elevated CO2when compared with the ambient CO2level, which led to the longer generation time. The mean generation time (T) significantly prolonged by 5.3% and 11.7%, respectively under the two elevated CO2level treatments. Therefore, the innate rate of increase (rm) and finite increase rate (λ) were significantly decreased, which led to the double population time (t) prolonged 9.1%. Although the number of eggs oviposited per female and the net reproductive rate (R0) increased under the treatments with the elevated CO2levels when compared with the ambient CO2level, the difference was not statistically significant among the three CO2treatments. When compared with the ambient CO2level, the larvae consumed significantly more artificial diet (9.1% and 34.0%) and or excreted significantly more frass (42.3% and 42.0%) under the two elevated CO2treatments, 550 μL/L and 750 μL/L, respectively. The results from this study indicate that the exposure to elevated CO2: a) significantly increase larval and pupal development time ofO.furnacalis, which result in the significantly decrease of the innate rate of increase (rm) for the population; b) increase larval mortality, but the third and latter instar larvae were more tolerant to the elevated CO2than the younger ones; and c) significantly increase food consumption, which may have led to more serious insect damage to the host plants in nature under elevated CO2levels than ambient CO2level.
Ostriniafurnacalis; atmospheric carbon dioxide; direct effect; life table
国家973项目《气候变化介导的农业灾变时空演化规律研究》(2010CB951503)
2013- 04- 17;
日期:2014- 04- 03
10.5846/stxb201304170732
*通讯作者Corresponding author.E-mail: klhe@ippcaas.cn
赵磊, 杨群芳, 解海翠, 王振营, 何康来.大气CO2浓度升高对亚洲玉米螟生长发育及繁殖的影响.生态学报,2015,35(3):885- 891.
Zhao L, Yang Q F, Xie H C, Wang Z Y, He K L.Direct effects of the elevated atmospheric carbon dioxide levels on the growth, development and reproduction ofOstriniafurnacalis(Guenée).Acta Ecologica Sinica,2015,35(3):885- 891.
