Case-control resting-state fMRI study of brain functioning among adolescents with first-episode major depressive disorder
2014-12-08YunGONGLiliHAOXiyanZHANGYanZHOUJianqiLIZhiminZHAOWenqingJIANGYasongDU
Yun GONG, Lili HAO, Xiyan ZHANG, Yan ZHOU, Jianqi LI, Zhimin ZHAO, Wenqing JIANG, Yasong DU*
•Original article•
Case-control resting-state fMRI study of brain functioning among adolescents with first-episode major depressive disorder
Yun GONG,1#Lili HAO,1#Xiyan ZHANG,1Yan ZHOU,2Jianqi LI,3Zhimin ZHAO,1Wenqing JIANG,2Yasong DU1*
major depressive disorder, magnetic resonance imaging, case-control studies, adolescents, China
1. Introduction
According to the World Health Organization, depression will become the second largest cause of global disease burden by 2020.[1]Three-quarters of adults with depression have their initial episode during childhood or adolescence.[2]Low mood, decreased interests, and lack of happiness are the core symptoms of adolescent depression. This disabling condition has a high prevalence and frequently relapses after treatment. Adolescent depression can lead to serious consequences, such as poor academic performance and increased risks of substance abuse and suicide.According to the National Institute of Mental Health(NIMH) of the United States, depression is the third leading cause of death among adolescents.[3]Moreover,longitudinal research has shown that individuals with a history of depression in childhood or adolescence were four times more likely to experience depression during early adulthood than those who did not experience depression during childhood or adolescence.[4]In summary, adolescent depression results in severe suffering for the affected individuals and a substantialhealth burden for family members and the society at large. Despite the public health importance of this condition, the etiology of adolescent depression remains unclear.
Resting-state magnetic resonance imaging (MRI)refers to MRI scanning without any stimulation. Binderd and colleagues[5]found that the human brain has organized activity even under the resting state. The amplitude of low frequency fluctuations (ALFF) is one of the most common methods for measuring changes in blood oxygen level dependent (BOLD) signals within the low frequency range (0.01-0.08 Hz) during the resting state. Irregular patterns in ALFF can reveal abnormalities in spontaneous brain activities. This method has previously been used to assess brain activity in Attention Deficit/Hyperactivity Disorder (ADHD).[6]
There are several reports of studies that used resting-state MRI to assess brain activity – primarily in the frontal lobe and limbic system – in adults with depression. Most of these studies found abnormalities in the dorsolateral prefrontal cortex (DLPFC),[7]medial frontal gyrus,[8]anterior cingulate cortex (ACC),[9,10]amygdala, medial thalamus, and striatum.[11,12]These areas are located in the frontal-limbic circuit, which plays an important role in the etiology of depression.
There are far fewer functional brain imaging studies on adolescent depression than on adult depression. The mechanisms of adolescent depression are unclear; given that the brain regions which participate in emotional regulation mature during adolescence, the brain regions involved in adolescent depression may be different from those identified for adult depression. The current study hypothesizes that the occurrence of adolescent depression is associated with abnormal activities in the resting-state functioning of the brain networks related to emotional regulation. We used the ALFF method to compare resting-state brain activities between firstepisode (drug-naïve) adolescents with major depressive disorder and matched controls.
2. Methods
2.1 Sample
The process of recruiting subjects for the study is shown in Figure 1.
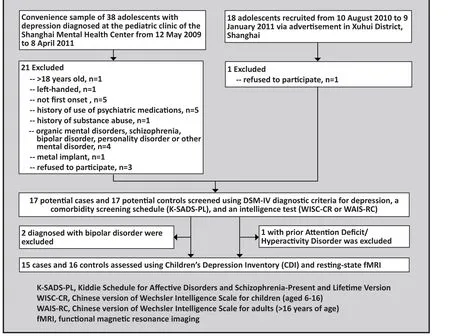
Figure 1. Flowchart of the study
2.1.1 Adolescent depression group
All participants were recruited from the pediatric outpatient clinic at the Shanghai Mental Health Center,Shanghai Jiao Tong University School of Medicine.Inclusion criteria were: (a) under 18 years of age; (b)right-handed; (c) met diagnostic criteria for major depressive disorder specified in the Diagnostic and Statistical Manual of Mental Disorders, fourth edition(DSM-IV) (the reliability and validity of DSM-IV in China is excellent[13]); (d) no previous depressive episode; (e)no history of taking antidepressants or other psychiatric medications; (f) normal intelligence (Wechsler Intelligence Test for child [WISC-CR] or for adults [WAISRC] score ≥85); and (g) the adolescent and his or her legal guardian provided written informed consent to participate in the study. Exclusion criteria were: (a)a history of dependence or abuse of psychoactive substances; (b) a history of organic mental disorder,schizophrenia, bipolar disorder, personality disorder, or other mental disorder; (c) a history of serious physical illness; (d) a history of degenerative neurological diseases, brain trauma, or cerebrovascular disease;(e) not suitable for magnetic resonance examinations because of metal implant; or (f) pregnancy.
In total, 17 patients were recruited. Two patients who were diagnosed with bipolar disorder in followup visits were excluded. Therefore, there were 15 adolescents in the case group including 10 males and 5 females. Their duration of illness ranged from 1 to 48 months (median=12 months).
2.1.2 Control group
The control group included adolescents who had never had depressive symptoms and never met DSMIV diagnostic criteria for depression or other mental disorders. Recruited via community advertisements,they were matched with cases who had completed the assessment on age, gender, and level of education.Inclusion criteria were: (a) under 18 years old; (b)healthy; (c) right-handed; (d) Wechsler Intelligence Test(for children or adults) score ≥85; (e) in good health during the week prior to the study without taking any medications; and (f) the adolescent and his or her legal guardian provided written informed consent to participate in the study. Exclusion criteria were:(a) a family history of depression or other mental or neurological disorder (in parents, sibs, or grandparents);(b) a history of dependence or abuse of psychoactive substances; (c) a history of mental disorders or recently taking medications that influence the central nervous system; (d) a history of degenerative neurological conditions, brain trauma, or cerebrovascular disease; (e)not suitable for magnetic resonance examinations due to metal implant; or (f) pregnancy.
Seventeen adolescents were initially recruited for the control group; one boy was excluded because of a history of Attention Deficit/Hyperactivity Disorder(ADHD). The 16 adolescent controls who completed the evaluation included 10 males and 6 females.
2.2 Procedures
2.2.1 Diagnosis and assessment
All participants in the study were independently evaluated by two attending pediatric psychiatrists using DSM-IV diagnostic criteria to confirm the diagnosis of major depressive disorder and the Children’s Depression Inventory (CDI) to assess the severity of depressive symptoms.[14]The Kiddie Schedule for Affective Disorders and Schizophrenia-Present and Lifetime Version(K-SADS-PL, kappa in China is 0.87[15]) was administered to evaluate the present and past history of mental disorders. Participants also completed the Wechsler Intelligence Test for children [WISC-CR] or, if over 16 years of age, for adults [WAIS-RC] for the evaluation of intelligence.
2.2.2 Magnetic resonance imaging
We used the 3.0T magnetic resonance imaging system (General Electric) for MRI scans. Routine imaging included T1WI and T2WI to screen for organic pathologies. Resting-state MRI was used to collect BOLD and echo-planar imaging (EPI).
The parameter settings for the MRI scans were:TR=2000 ms, TE=30 ms, FOV=230x230 mm2, and matrix=64x64 mm2. Thirty-three layers of 4 mm each were continuously scanned. During the resting state scanning, no tasks were assigned to participants and participants laid awake on their back with eyes closed.Participants were asked not to actively think about anything in particular during the procedure.
2.2.3 Processing of resting-state images
Data Processing Assistant for Resting-State fMRI(DPARSF2.0) software (Beijing Normal University) was used to analyze resting-state functional brain images.DPARSF was developed based on Statistical Parametric Mapping (SPM8) and Resting-State fMRI Data Analysis Toolkit (REST).
The following procedures were carried out to eliminate the effects of excessive head movements during the procedure. (a) Due to the instability of the signals at the beginning of the evaluation, data for the first ten time points were eliminated. (b) Subject motion was determined in rotation and translation; the maximum motion of any analyzed subject was 2.5 mm or 2.5°. (c) Spatial normalization (SN) was applied to the data; given anatomical differences in subjects’ brains,the fMRI results had to be standardized based on the SPM standard EPI template. (d) The data were smoothed using a Gaussian kernel of full width at half maximum of 4 mm. The main goals of these adjustments were to ensure that the data approximates the Gaussian random field (GRF) so it meets the statistical assumptions required to conduct the SPM, and to improve the signal to noise ratio. Alignment of images and spatial standardization changes the correlation between voxels, and Gaussian smoothing can ensure that neighboring voxels share more information. After the above procedures, ALFF results were assessed. Before calculating the ALFF, we performed linear drift correction and band-pass filtering to the smoothed images. The frequency range was 0.01-0.08 Hz.[6]The SPM images obtained through the estimation step were used for the final analysis.
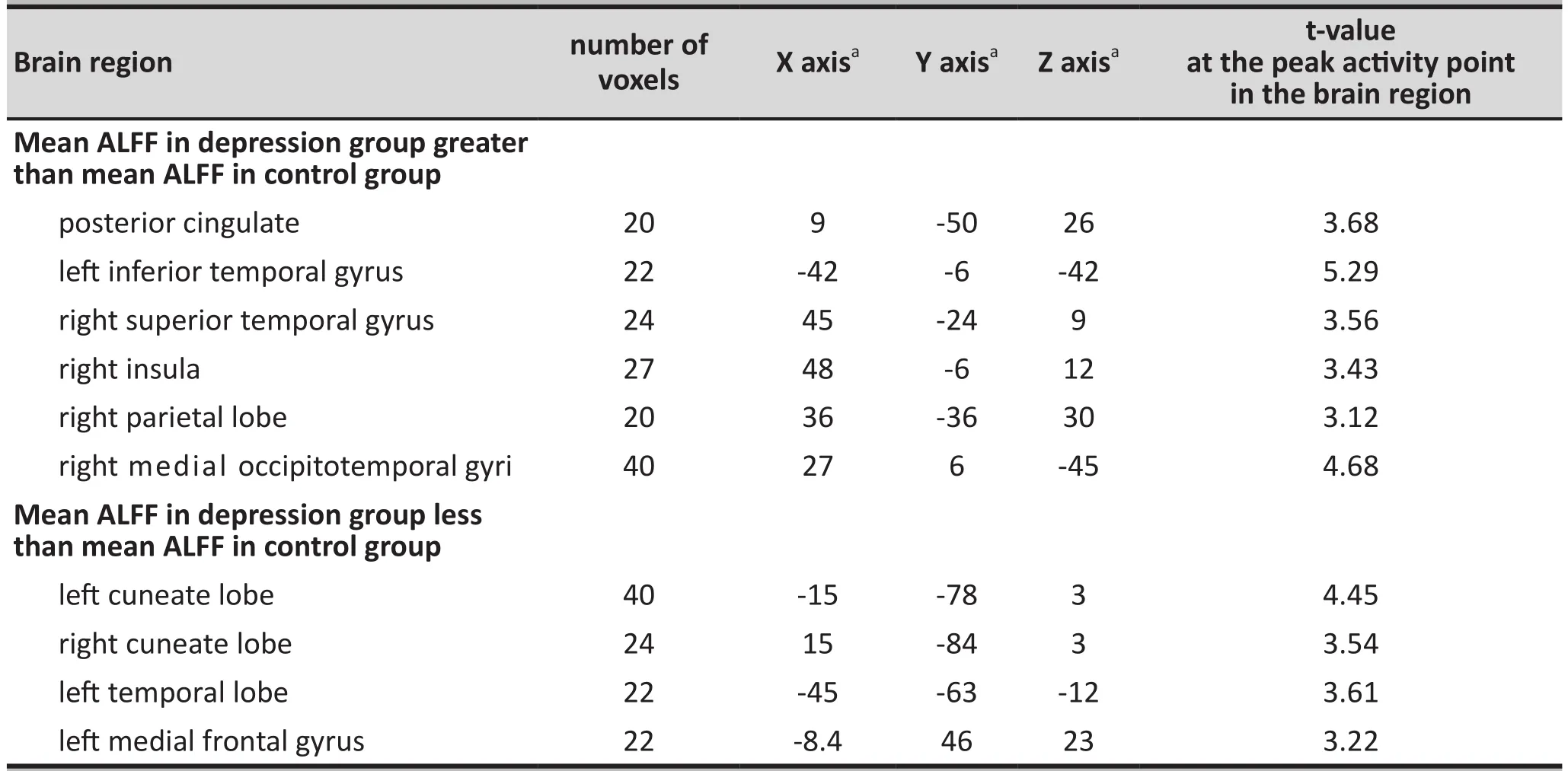
Table 1. List of brain areas ≥20 voxel in size with significant differences (p<0.005) in the amplitude of low frequency fluctuation (ALFF) during the resting state between the depression group and the control group
2.2.4 Statistical analyses
The standardized ALFF images from the two groups were compared using the SPM8 tool kit for MATLAB. Next,the statistical atlas of the brains was superimposed onto the averaged images of MNI T1 to devise the resting-state ALFF brain images that showed statistical differences between the two groups. The threshold for statistical differences in stimulated images werep<0.005(before correction) and the minimum size of the tested brain regions was 20 voxel. In brain regions that showed differences, boxes were used to highlight regions with higher ALFF, and arrows for regions with lower ALFF.
3. Results
Participants in the depressed group were 11 to 18 years of age while those in the control group were 10 to 17 years of age. The mean (sd) age in both groups was 15 (2) years. There were 10 males and 5 females in the depression group, and 10 males and 6 females in the control group. Adolescents in the depression group had 4 to 13 (median=9) years of education;those in the control group had 3 to 12 (median=9.5)years of education. The differences in age, gender,and education level between the two groups were not statistically significant. The mean (sd) scores of the Children Depression Inventory (CDI), were 22.1 (9.2) in the depression group and 9.4 (5.7) in the control group(t=4.68,p<0.001).
The amplitude of low frequency fluctuations(ALFF) measure derived from analysis of the fMRI was compared between the case group and the control group in all brain regions 20 voxel or larger. The ten regions in which there was a significant difference between cases and controls (specified as p-values for the independent t-tests of <0.005) are shown in Table 1. During the resting state, the depression group had higher mean ALFF than the control group in the posterior cingulate gyrus, left inferior temporal gyrus,right superior temporal gyrus, right insula, right parietal lobe, and right fusiform gyrus. However, the depression group had a lower mean ALFF than the control group in the right cuneate, left cuneate, left occipital lobe, and left medial frontal lobe.
The brain regions that showed between-group differences in resting-state ALFF were superimposed on a single T1 template. As shown in Figure 2, arrows mark areas where the depression group had higher ALFF activation than the control group and rectangles mark areas where the depression group had lower ALFF activation than the control group.
4. Discussion
4.1 Main findings
The depressed patients participating in this study were drug naïve adolescents who had just experienced their first depressive episode. This unique sample made it possible to exclude three factors that may confound assessments of the relationship between brain functioning and depression: age, number of depressive episodes, and use of antidepressant medications.
The development of the neurological system is a complex, ongoing process with different characteristics at different stages in the life cycle. In children and adolescents, using resting-state fMRI to assess brain function has many advantages over other neuroimaging techniques;[16]for example, the data collection process does not involve task performance so it can be completed within five minutes. Supekar and colleagues[17]found that compared to brain functioning in adults, adolescent resting-state brain functioning shows more connection between the sub-cortical areas and major sensory regions, and more connections between the corpus callosum and the paralimbic structure. In contrast, the adult brain shows more cortexcortex connections between the paralimbic structure,limbic structure, and corpus callosum. The adolescent brain shows higher levels of separation in functioning while the adult brain shows a greater integration of high-level functioning. A previous study compared resting-state brain network connections among children,adolescents, and adults using the anterior cingulate as the seed and found variations in patterns of functional connectivity in children and adults.[16]Specifically,children had a more diffuse pattern of functional connectivity with the voxel proximal to the seed region of interest while adults had more focal patterns of functional connectivity and a greater number of significantly correlated voxels at long distances from the seed. Adolescents demonstrated intermediate patterns of functional connectivity between these two patterns.[18]Findings from this study showed both similarities and differences in brain imaging results between adolescents and adults. This highlights the importance of conducting separate studies among adolescents in the assessment of brain development and abnormal neuropsychological conditions.
According to a previous study,[19]resting-state left brain functioning differs between individuals during the first depressive episode (incidence cases) compared to that of individuals during subsequent depressive episodes. Individuals with multiple episodes had a higher amplitude of low-frequency waves in the left putamen, left middle frontal gyrus, and left insula.In fact, there is a positive correlation between the number of depressive episodes and the amplitude of low-frequency waves in the left brain and in the left putamen (r=0.450p=0.021;r=0.535p=0.004,respectively). These findings support hypotheses about a relationship between duration of depression and brain functioning.
Use of antidepressants may also influence brain functioning in individuals with depression. For example,Sun and colleagues[20]found increased BOLD in the front right anterior cingulate, the bilateral dorsolateralfrontal lobe, the right orbital frontal cortex, the temporal cortex, the bilateral precuneus, the back anterior cingulate, and the right occipital eye field after eight weeks of treatment among a group of individuals with first-episode depression who were responsive to antidepressant treatment. They also found reversion to abnormal brain functioning after stopping antidepressant treatment.

Figure 2. Comparisons of resting state amplitudes of low frequency fluctuations (ALFF) between the depression group and the control group
This current study eliminated the influence of age, multiple relapses, and medications use and, thus,provided direct evidence about the characteristics of brain functioning during the resting state in adolescents with major depression. We found that compared to matched controls, depressed adolescents in the resting state had lower ALFF in the bilateral cuneate lobe, leftoccipital lobe, and left medial frontal lobe, andhigherALFF in the posterior cingulate, left inferior temporal gyrus, right superior temporal gyrus, right insula, right parietal lobe, and the right gyri fusiformis.
The medial frontal gyrus is part of the medial prefrontal cortex (MPFC). The MPFC and posterior cingulate are both part of the default network which includes the medial prefrontal cortex, the posterior cingulate, the inferior parietal lobule, the lateral temporal cortex, and the hippocampus.[21]Damage in this circuit can induce disturbance in mood and cognition, as seen in depressive disorders. The medial frontal gyrus plays a key role in the default network in terms of the processing, recognition and regulation of emotions. Abnormal activities of neurons in the medial frontal gyrus can lead to dysfunctional mood regulation,which is the pathological basis of the changes in mood, behavior, cognition, and endocrinology seen in depression.[22]Jin and colleagues[23]also reported dysfunctional medial prefrontal cortex among adolescents with first-episode depression. Guo and colleagues[8]found lower ALFF in the right medial frontal cortex among treatment responsive individuals with depression compared to controls. There is also evidence about volume change of the medial prefrontal cortex in depression.[24]Drevets[25]found that endophenotypic changes of depression included reduced volume of the left anterior hemisphere and increased volume in the right cingulate gyrus and the orbital frontal cortex. The anterior hemisphere includes the medial prefrontal cortex, which participates in the regulation of emotions. This evidence supports our finding of a relationship between abnormal activities of the spontaneous neurons in the medial frontal gyrus and the development of mood and cognitive symptoms in depression.
The current study also found lower mean ALFF in the left temporal lope and in the bilateral cuneate nucleus among the adolescents in the depression group. A previous study found lower concentrations of GABA in the occipital cortex – which includes the cuneus – among individuals with depression.[24]Guo and colleagues[8]found lower ALFF in the cuneate nucleus and lingual gyrus among individuals with treatment resistant depression and lower ALFF in the occipital lobe among individuals responsive to antidepressant treatment. This is in line with our finding of abnormal spontaneous neuronal activities under the resting state in depression. Chantiluke and colleagues[26]found lower activities in the occipital lobe during attention tasks among adolescents with depression. Together with findings from previous studies, our results support the involvement of the occipital lobe (including the cuneate nucleus) in the development of depression.
The posterior cingulate is related to sensory functioning and participates in visual memory, the processing of spatial information, proprioceptive sensibility, and the processing of emotions.[27]Yao and colleagues found decreased functional connectivity between the posterior cingulate, the middle prefrontal cortex, and the frontal cuneate nucleus among individuals with depression. They postulated that these changes can reinforce memories of negative experiences while reducing memories of positive circumstances and, thus, produce sustained negative emotions.[28]Structural imaging studies have documented smaller volume of the posterior cingulate among patients with depression.[29]PET and single photon emission computerized tomography (SPECT) studies also found increased metabolism or blood flow in the posterior cingulate.[7]Peterson and colleagues[30]found thinning of multiple cortexes including the posterior cingulate among individuals with a family history of depression.The cingulate is a key structure of the limbic system.Various areas of the posterior cingulate are closely connected to the amygdala, hypothalamus, dorsolateral prefrontal cortex, and the brain stem. These structures play important roles in emotion, cognition, autonomic nervous system functions, and mobile functions. One possibility is that a dysfunctional posterior cingulate causes connection failures with other related brain areas and subsequently gives rise to depression. This is consistent with our finding of higher ALFF in the right posterior cingulate in the depression group.
The insula plays an important role in the regulation of emotions and pain in coordination with the amygdala, interior cingulate, prefrontal cortex, and hippocampus.[31]In this study, we found higher ALFF in the insula among adolescents with depression. One possible mechanism that could explain this result is that the dysfunctional insula contributes to the negative interpretation of physical symptoms and interpersonal interactions. Our finding is consistent with Ding’s study that documented increased ALFF in the right insula among drug naïve adolescents with depression.[32]A previous study reported decreased mobility and vigor– symptoms of depression – among individuals with stroke in the right insula compared to those with stroke in the left insula or to those without insula stroke.Therefore, disconnection of the insula with the interior cingula and the frontal cortex can contribute to the occurrence of these symptoms.[33]PET studies found boosted bonding of 5-HTT (which is critical for 5-HTT reuptake) in the insula, striatum, and thymus among individuals experiencing a depressive episode compared to controls.[34]Takahashi and colleagues[35]found smaller insula in individuals with current depression (cMDD)and in individuals with a history of major depression who were currently in remission (rMDD) compared with controls. Jin and colleagues[23]also found dysfunctional insula among drug naïve adolescents with first-episode depression. Functional imaging studies found that the insula, especially the front insula, prefrontal lobe,and other parts of the limbic system participate in the regulation of emotions (e.g., guilt and sadness). Our findings are in line with findings from these studies.
The medial occipitotemporal gyrus is believed to play a part in the reading of facial expressions. Correct reading and interpretation of facial expressions is important for social interactions and can influence emotions. Surguladze and colleagues[36]found that individuals with depression showed positive reactions to sad facial expressions and neutral reactions to happy ones, while the controls showed positive reactions to happy facial expressions. Unlike our findings, Guo and colleagues foundlowerALFF in the left medial occipitotemporal gyrus among individuals who responded to antidepressant treatment. A possible reason for the different findings is the influence of antidepressants on measures of brain functioning.[8]In brief, abnormal activities in the medial occipitotemporal gyrus may lead to social withdrawal and negative cognition in depression.
The inferior temporal gyrus is located below the middle temporal gyrus; it participates in vision and in various cognitive processes.[37]Dysfunction of the inferior temporal gyrus has been linked to disrupted working memory.[38]Another study found that the inferior temporal gyrus is an important component of the network which connects the frontal, temporal,parietal, and occipital lobes; a network that has been associated with the outcome of antidepressant treatment.[23,39]Jin and colleagues[23]found dysfunctions of the temporal cortex in drug naïve adolescents with first-episode depression. And Guo and colleagues[8]found higher ALFF in the inferior temporal cortex among individuals with depression. Results from our study confirmed the results of these studies which highlight the role of the inferior temporal lobe in depression.
4.2 Limitations
Small sample size and the relatively large age range of the participating adolescents are limitations of the current study. Due to differential dropout rates between groups, there were 15 cases and 16 controls in the final analysis so we did not achieve perfect 1:1 matching of cases and controls. Future studies with larger sample sizes are needed to explore potential differences across age groups and across different types of depression.
4.3 Implications
This study among adolescents with drug-naïve, firstepisode depression identified abnormal brain functions during the resting state in several brain regions related to emotions. This helps to confirm the biological basis of depression. Further studies comparing depressionrelated brain changes in children, adolescents, adults,and elder individuals with depression will help to clarify how the biological characteristics of depression vary over the different stages of the life cycle.
Conflict of interest
The authors declare no conflict of interest related to this manuscript.
Funding
The study was supported by the National Science Foundation (grant number: 30970901) and the East China Normal University MRI key laboratory.
Ethics approval
This study was approved by the institutional review board of the Shanghai Mental Health Center.
Informed consent
All the participants and their legal guardians provided written informed consent to participate in the study.
1. Holden C. Global survey examines impact of depression.Science. 2000; 288(5463): 39-40. doi: http://dx.doi.org/10.1126/science.288.5463.39
2. Kim-Cohen J, Caspi A, Moffitt TE, Harrington H, Milne BJ, Poulton R. Prior juvenile diagnoses in adults with mental disorder: developmental follow-back of a prospectivelongitudinal cohort.Arch Gen Psychiatry.2003; 60(7): 709-717. doi: http://dx.doi.org/10.1001/archpsyc.60.7.709
3. National Institute of Mental Health [Internet]. Suicide in the U.S.: statistics and prevention. Atlanta: Center for Disease Control and Prevention; 2011 [cited 2011 May 2]. Available from: http://www.cdc.gov/nchs/fastats/suicide.htm
4. Rosso IM, Cintron CM, Steingard RJ, Renshaw PF, Young AD, Yurgelun-Todd DA. Amygdala and hippocampus volumes in pediatric major depression.Biol Psychiatry.2005; 57(1): 21-26. doi: http://dx.doi.org/10.1016/j.biopsych.2004.10.027
5. Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Rao SM, Cox RW. Conceptual processing during the conscious resting state.A functional MRI study.J Cogn Neurosci.1999; 11(1): 80-95
6. Zang YF, He Y, Zhu CZ, Cao QJ, Sui MQ, Liang M, et al. Altered baseline brain activity in children with ADHD revealed by resting-state rune functional MRI.Brain Dev.2007; 29(2): 83-91. doi: http://dx.doi.org/10.1016/j.braindev.2006.07.002
7. Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatmentresistant depression.Neuron.2005; 45 (5): 651-660. doi:http://dx.doi.org/10.1016/j.neuron.2005.02.014
8. Guo WB, Liu F, Xue ZM, Xu XJ, Wu RR, Ma CQ, et al.Alterations of the amplitude of low-frequency fluctuations in treatment-resistant and treatment-response depression:a resting-state fMRI study. Prog Neuropsychopharmacol Biol Psychiatry. 2012; 37(1): 153-160. doi: http://dx.doi.org/10.1016/j.pnpbp.2012.01.011
9. Drevets WC, Bogers W, Raichle ME. Functional anatomical correlates of antidepressant drug treatment assessed using PET measures of regional glucose metabolism. Eur Neuropsychopharmacol. 2002; 12(6): 527-544. doi : http://dx.doi.org/10.1016/S0924-977X(02)00102-5
10. Drevets WC, Price JL, Simpson JR, Todd RD, Reich T, Vannier M, et al. Subgenual prefrontal cortex abnormalities in mood disorders.Nature.1997; 386(6627): 824-827. doi: http://dx.doi.org/10.1038/386824a0
11. Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS.Can’t shake that feeling: event-related fMRI assessment of sustained amygdale activity in response to emotional information in depressed individuals.Biol Psychiatry.2002;51(9): 693-707. doi: http://dx.doi.org/10.1016/S0006-3223(02)01314-8
12. Drevets WC. Neuroimaging studies of mood disorders.Biol Psychiatry.2000; 48(8): 813-829. doi: http://dx.doi.org/10.1016/S0006-3223(00)01020-9
13. Wang ZQ, Yang SJ, Zhang YP, Phillips MR. [Use of a structured questionnaire to assess the concordance of the diagnosis of depression based on DSM-IV and the Chinese classification of mental disorders(CCMD-3)].Zhongguo Xin Li Wei Sheng Za Zhi. 2008; 22(7): 497– 500. Chinese
14. David Y, Li X. [Preliminary use of the Children’s Depression Inventory in China].Zhongguo Xin Li Wei Sheng Za Zhi.2000;14(4): 225-227. Chinese
15. Liu YX, Liu J, Wang YF. [Reliability and validity of Chinese version of the Mini International Neuropsychiatric Interview for Children and Adolescents (Child Version)].Zhongguo Xin Li Wei Sheng Za Zhi. 2011; 25(1): 8-13.Chinese. doi: http://doi.med.wanfangdata.com.cn/10.3969/j.issn.1000-6729.2011.01.003
16. Van Dijk KRA, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization.J Neurophysiol. 2010; 103(1): 297–321. doi: http://dx.doi.org/10.1152/jn.00783.2009
17. Supekar K, Musen M, Menon V. Development of largescale functional brain networks in children.PLoS Biol. 2009;7(7): e1000157. doi: http://dx.doi.org/10.1371/journal.pbio.1000157
18. Kelly a MC, Di Martino A, Uddin LQ, Shehzad Z, Gee DG,Reiss PT, et al. Development of anterior cingulate functional connectivity from late childhood to early adulthood.Cereb Cortex. 2009; 19(3): 640–657. doi: http://dx.doi.org/10.1093/cercor/bhn117
19. Yan R, Yao ZJ, Lu Q, Wei MB, Tang H, Han L. [The difference of fractional amplitude of low frequency fluctuation between first-episode and recurrent depressed patients: a resting-state functional magnetic resonance imaging study].Lin Chuang Jing Shen Yi Xue Za Zhi. 2013; 23(3): 145–148.Chinese
20. Sun J, Liu HQ, Sun HP, Zhang JH, Feng XY, Guo Q, Shi SX.[Amplitude of low frequency fluctuation in first-onset major depressive disorder by antidepressant treatment investigated with resting-state functional MRI].Zhongguo Yi Xue Ji Suan Ji Cheng Xiang Za Zhi. 2011; 17:212–216.Chinese. doi: http://doi.med.wanfangdata.com.cn/10.3969/j.issn.1006-5741.2011.03.004
21. Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease.Ann N Y Acad Sci. 2008 ; 11(24):1-38. doi : http://dx.doi.org/10.1196/annals.1440.011
22. Anand A, Li Y, Wang Y, Wu J, Gao S, Bukhari L, et al.Activity and connectivity of brain mood regulating circuit in depression: a functional magnetic resonance study.Biol Psychiatry.2005; 57(10): 1079–1088. doi: http://dx.doi.org/10.1016/j.biopsych.2005.02.021
23. Jin C, Gao C, Chen C, Ma S, Netra R, Wang Y,et al.A preliminary study of the dysregulation of the resting networks in first-episode medication-naïve adolescent depression.Neurosci Lett. 2011; 503(2): 105-109. doi: http://dx.doi.org/10.1016/j.neulet.2011.08.017
24. Hasler G, Northoff G. Discovering imaging endophenotypes for major depression.Mol Psychiatry. 2011; 16(6): 604-619.doi: http://dx.doi.org/10.1038/mp.2011.23
25. Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: Implications for neurocircuitry models of depression.Brain Struct Funct.2008; 213(1-2): 93-118. doi: http://dx.doi.org/10.1007/s00429-008-0189-x
26. Chantiluke K, Halari R, Simic M, Pariante CM, Papadopoulos A, Giampietro V, et al. Fronto-striato-cerebellar dysregulation in adolescents with depression during motivated attention.Biol Psychiatry. 2012; 71(1): 59-67. doi: http://dx.doi.org/10.1016/j.biopsych.2011.09.005
27. Epperson CN, Gueorguieva R, Czarkowski KA, Stiklus S,Sellers E, Krystal JH, et al. Preliminary evidence of reduced occipital GABA concentrations in puerperal women: a 1H-MRS study.Psychopharmacology (Berl). 2006; 186(3):425-433. doi : http://dx.doi.org/10.1007/s00213-006-0313-7
28. Yao ZJ, Wang L, Lu Q, Liu HY, Teng GJ. [Altered default mode network functional connectivity in patients with depressive disorders: restingstate fMRI study]. Zhongguo Shen Jing Jing Shen Ji Bing Za Zhi.2008; 34(5): 278-282. Chinese. doi: http://doi.med.wanfangdata.com.cn/10.3969/j.issn.1002-0152.2008.05.007
29. Caetano SC,Kaur S,Brambilla P, Nicoletti M, Hatch JP, Sassi RB, et al. Smaller cingulate volumes in unipolar depressed patients.Biol Psychiatry.2006; 59(8): 702-706.doi: http://dx.doi.org/10.1016/j.biopsych.2005.10.011
30. Peterson BS, Warner V, Bansal R, Zhu H, Hao X, Liu J, et al.Cortical thinning in persons at increased familial risk for major depression.Proc Natl Acad Sci USA.2009; 106(15): 6273-6278. doi: http://dx.doi.org/10.1073/pnas.0805311106
31. Robinson MJ, Edwards SE, Iyengar S, Bymaster F, Clark M, Katon W. Depression and pain.Front Biosci. 2009 ; 14:5031-5051
32. Ding J. [Case control study on brain structure and function of first-episode drug-naive adolescents with major depressive disorder using magnetic resonance (Doctoral Dissertation)].Changsha: Central South University. 2010. Chinese
33. Manes F, Paradiso S, Robinson RG. Neuropsychiatric effects of insular stroke.J Nerv Ment Dis.1999; 187(12): 707-712
34. Cannon DM, Ichise M, Rollis D, Klaver JM, Gandhi SK, Charney DS,et al. Elevated serotonin transporter binding in major depressive disorder assessed using positron emission tomography and [11C]DASB; comparison with bipolar disorder.Biol Psychiatry.2007; 8(62): 870-877. doi:http://dx.doi.org/10.1016/j.biopsych.2007.03.016
35. Takahashi T, Yücel M, Lorenzetti V, Nakamura K, Whittle S, Walterfang M, et al. Midline brain structures in patients with current and remitted major depression.Prog Neuropsychopharmacol Biol Psychiatry.2009; 33(6): 1058-1063. doi: http://dx.doi.org/10.1016/j.pnpbp.2009.05.020
36. Surguladze S, Brammer MJ, Keedwell P, Giampietro V, Young AW, Travis MJ, et al. A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder.Biol Psychiatry.2005; 57(3): 201-209.doi: http://dx.doi.org/10.1016/j.biopsych.2004.10.028
37. Onitsuka T, Shenton ME, Salisbury DF, Dickey CC, Kasai K,Toner SK, et al. Middle and inferior temporal gyrus gray matter volume abnormalities in chronic schizophrenia: an MRI study.Am J Psychiatry. 2004; 161(9): 1603-1611. doi:http://dx.doi.org/10.1176/appi.ajp.161.9.1603
38. Zeng LL, Shen H, Liu L, Wang L, Li B, Fang P, et al. Identifying major depression using whole-brain functional connectivity:a multivariate pattern analysis.Brain. 2012; 135(Pt 5): 1498-1507. doi: http://dx.doi.org/10.1093/brain/aws059
39. Gong Q, Wu Q, Scarpazza C, Lui S, Jia Z, Marquand A,et al. Prognostic prediction of therapeutic response in depression using high-field MR imaging.Neuroimage.2011; 55(4): 1497-1503. doi: http://dx.doi.org/10.1016/j.neuroimage.2010.11.079
2014-01-29; accepted: 2014-06-10)

Yun Gong obtained a bachelor’s degree in clinical medicine from South-East University School of Medicine in 2004 and a master’s degree in psychiatry and mental health from Zhejiang University School of Medicine in 2009. She attained a doctoral diploma from Shanghai Jiao Tong University School of Medicine (doctoral degree pending). She has been working as a Medical Science Liaison for MSD (Merck) since 2013. (The research reported here was completed while a student at Shanghai Jiao Tong University.) Her research interests are in medical imaging of psychiatric conditions.

Lili Hao obtained a bachelor’s degree in clinical medicine from Tai Shan Medical College in 2009 and a master’s degree in clinical medicine (internal medicine) from Wan Nan Medical College in 2012. She is currently a doctoral candidate majoring in psychiatry and mental health at Shanghai Jiao Tong University School of Medicine (expected graduation: July 2015) where she works in the Department of Child Psychiatry. Her research interests include youth psychiatry, functional imaging,and neuropsychological assessment.
首发抑郁症青少年患者的大脑功能——静息态功能磁共振成像的病例对照研究
龚云, 郝丽丽, 张喜燕, 周滟, 李建奇, 赵志民, 江文庆, 杜亚松
重度抑郁症,磁共振成像,病例对照研究,青少年,中国
Background:Adolescent depression results in severe and protracted suffering for affected individuals and their family members, but the underlying mechanism of this disabling condition remains unclear.Objectives:Compare resting-state brain functioning between first-episode, drug-naïve adolescents with major depressive disorder and matched controls.Methods:Fifteen adolescents with major depressive disorder and 16 controls underwent a resting-state fMRI scan performed using a 3T magnetic resonance scanner. The amplitude of low frequency fluctuation(ALFF) was used to assess resting-state brain function.Results:Adolescents with depression had higher mean (sd) scores on the Children Depression Inventory(CDI) than controls (22.13 [9.21] vs. 9.37 [5.65]). Compared with controls, adolescents with depression had higher ALFF in the posterior cingulate gyrus, left inferior temporal gyrus, right superior temporal gyrus, right insula, right parietal lobe, and right fusiform gyrus; they also exhibited lower ALFF in the bilateral cuneus,the left occipital lobe, and the left medial frontal lobe.Conclusions:Adolescent depression is associated with significant changes in the functioning of several regions of the brain.
[Shanghai Arch Psychiatry. 2014;26(4): 207-215.
http://dx.doi.org/10.3969/j.issn.1002-0829.2014.04.004]
1Shanghai Mental Health Center, Shanghai Jiao Tong University School of Medicine, Shanghai, China
2Renji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
3Key Laboratory of Magnetic Resonance, East China Normal University, Shanghai, China
#Yun GONG and Lili HAO are joint first authors.
* correspondence: yasongdu@163.com
A full-text Chinese translation of this article will be available at www.saponline.org on September 25, 2014.
背景:青少年抑郁症对患者及其家庭成员会产生长期严重的痛苦,但这种致残状况的潜在机制仍不清楚。目的比较未经药物的青少年首发抑郁症患者和匹配的对照者之间的大脑功能静息状态。方法:使用3T磁共振扫描仪对15名青少年抑郁症患者和16名对照者进行功能磁共振静息状态扫描。采用低频振荡振幅(amplitude of low frequency fluctuation,ALFF)来评估脑功能静息态。结果青少年抑郁症患者的儿童抑郁量表评分的均值(标准差)高于对照组(22.13[9.21]与9.37[5.65])。与对照组相比,青少年抑郁症患者在扣带回后部、左颞下回、右颞上回、右岛叶、右侧顶叶和右侧梭状回具有较高的ALFF;而在双侧楔叶、左枕叶和左内侧额叶表现出较低的ALFF。结论青少年抑郁症与大脑多个区域的显著功能变化有关。
本文全文中文版从2014年9月25日起在www.saponline.org可供免费阅览下载
猜你喜欢
杂志排行
上海精神医学的其它文章
- Advances in neuroimaging research of schizophrenia in China
- Advances in molecular genetic studies of attention deficit hyperactivity disorder in China
- Cross-sectional study of use of electronic media by secondary school students in Bangkok, Thailand
- Comorbidity of depressive and anxiety disorders:challenges in diagnosis and assessment
- Case report of narcolepsy in a six-year-old child initially misdiagnosed as atypical epilepsy
- Structural zeroes and zero-inflated models
