Thermal response of lithium titanate battery during cycling under adiabatic condition
2014-11-15ZHAOXuejuanWANGQingsongPINGPingSUNJinhua
ZHAO Xue-juan,WANG Qing-song,PING Ping,SUN Jin-hua
(State Key Laboratory of Fire Science,University of Science and Technology of China,Hefei 230026,China)
1 Introduction
Lithium ion batteries are widely used in portable consumer electronics because of their high voltage,no memory effect and slow self-discharge rate when not in use.Through years of development of research technology,lithium ion batteries began advancing towards diversified directions including power grids,automotive and aerospace applications,etc.[1].Hereinto,the application of lithium ion battery in energy storage system of smart power grid is becoming a hot spot of current research[2].
Nowadays,in order to meet the requirements ofgreen economy and sustainable development,the smart power grid has become the optimal choice of energy technology to promote large scale use of clean energy.In particular,the wider range promotion of electric vehicle will drive the development of energy storage system in smart grid.For instance,according to the technology roadmap of European Commission(EC)[3],it is expected that a large number of EVs will be using the electric grid by year 2020.Above all,the energy link which can most effectively combine current electric vehicles and smart grid is lithium ion battery.In fact,power grid with energy storage in the form of lithium ion battery is being used and constructed in large scale around world.
Nevertheless,the widely-publicized fire and explosion incidents of lithium ion battery,which endangered personal safety of consumers,has raised safety concerns and long been blamed on exothermic reactions between battery components[4].In smart grid energy storage system,large amounts of battery was placed and used intensively,safety issue became a serious problem.Therefore,it is extremely important to use a safer kind of battery in energy storage system.Lithium titanate battery is a popular choice and was adopted in some energy storage systems.
Nevertheless,most commercial lithium ion batteries used currently take graphite as anode material,and corresponding study of thermal safety also focused on battery with graphite anode.Research on lithium titanate battery mainly carried out to improve its electrochemical performance[5-7],study of thermal safety is relatively scarce.In addition,traditional research of battery thermal stability was carried out in a static way focusing on a specific component,material mixture and a single battery[8-11].However,the actual battery fire and explosion accidents mostly happened when in use.Consequently,it is quite necessary to study the thermal behavior of a whole piece of battery under cycling,which is the research significance of our work,while research effort in this respect is far from adequate at present.
2 Experimental
The battery cell sample tested in this study was a soft pack battery with nominal capacity of about 900mAh.The voltage and specific heat capacity of the sample is 2.8Vand 1.0Jg-1K-1,respectively,with weight of 33.0g.The battery cell has a lithium nickel manganese cobalt(NMC)cathode,a lithium titanate anode,and electrolyte of 1.0MLiPF6/EC+DEC(1∶1,wt.%).
An accelerating rate calorimeter(ARC)combined withmulti-channel battery cycler was used in this work to study the heat release mechanism of a lithium ion battery during cycling.Hereinto,the multi-channel battery cycler was used to finish the charging and discharging process of battery according to the parameters set by user.And the ARC was employed to track the temperature change of the cell in an adiabatic environment.
In order to verify the reliability of the tested cell,cell sample firstly cycled outside the ARC by operating the battery cycler.After the cycle outside the ARC completed,cell sample was removed and fixed inside the ARC cavity to test its temperature changeunder charging and discharging with start temperature of 25°C.
The charge-discharge system for battery cell sample was set as follows.The cell sample was first discharged from 2.8Vto 1.5Vat specific rate current,after a standing period of 1minute,the cell was then charged from 1.5Vto 2.8Vat the same rate current.When cell voltage reached 2.8V,a constant voltage charge process was conducted with cutoff current of 20mA.The cell sample cycled 3times outside the ARC and 5times inside the ARC.The specific current was set at five different rates of 0.1C,0.2C,0.5C,1.0Cand 1.5C,respectively.
3 Results and discussion
3.1 Thermal response of lithium titanate battery when cycling inside the ARC
Thebattery sample was first tested at rate of 0.1Coutside the ARC and its cycle curve plot was plotted in Fig.1.It can be figured out that,both the current and voltage curves are very smooth,which indicates that the battery works properly outside the ARC.Fig.2shows the cycle curve of battery tested inside the ARC cavity.Its cycle condition was almost the same as that outside the ARC,which also indicated that this cell works normally when being tested.
The plots of temperature and voltage change over time of the tested battery are shown in Fig.3.It can be seen that,there is an obvious temperature rise in both discharging and charging process.This trend continues to the fifth cycle when temperature of battery finally reaches 65°C.The tested battery only swelled slightly during experiment without occurrence of thermal runaway.Further-more,the plot of temperature rise rate versus temperature was demonstrated in Fig.4.The values of temperature rise rate fluctuate between 0.001°C min-1and 0.03°C min-1,which signifies very low heat production rate.
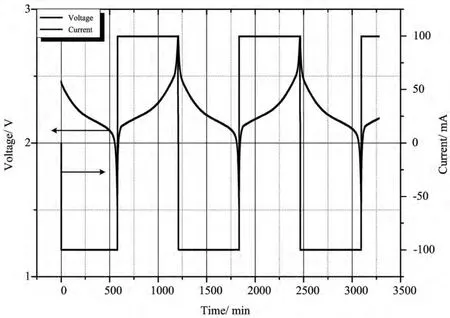
图1 钛酸锂电池在0.1C倍率下的循环曲线图(在ARC外部)Fig.1 Cycle curve for battery sample tested at rate of 0.1Coutside the ARC

图2 钛酸锂电池在0.1C倍率下的循环曲线图(在ARC内部)Fig.2 Cycle curve for battery sample tested at rate of 0.1Cinside the ARC
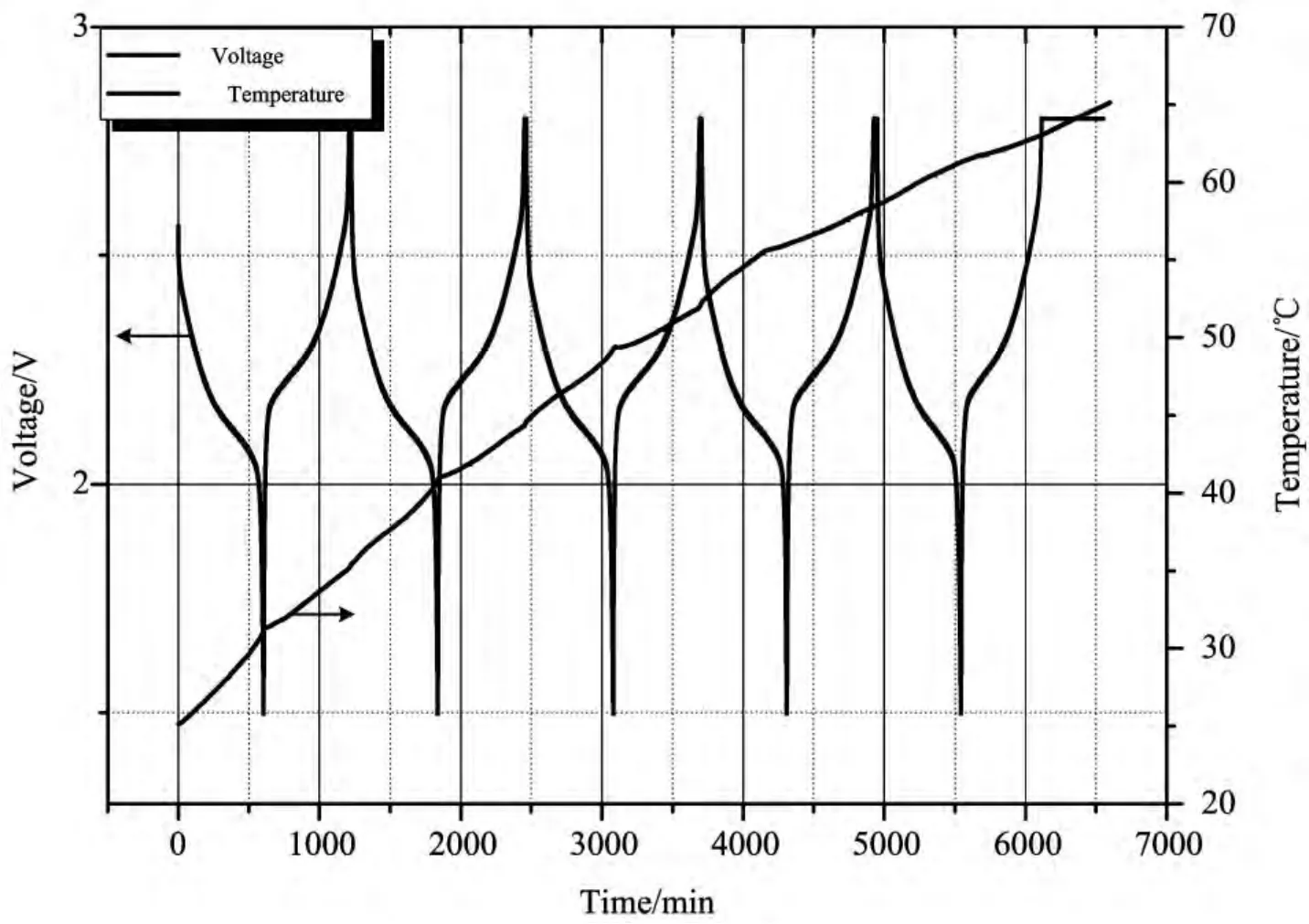
图3 钛酸锂电池在0.1C倍率下循环的温度/电压-时间曲线图Fig.3 Temperature and voltage versus time for lithium titanate battery tested at rate of 0.1C
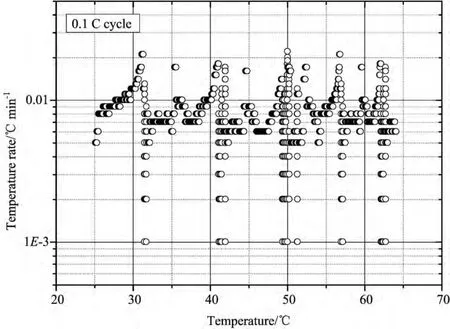
图4 钛酸锂电池在0.1C倍率下循环的温升速率-温度曲线图Fig.4 Temperature rise rate versus temperature for battery tested at rate of 0.1C
3.2 Heat generation in one cycle

The adiabatic heat generation is important for practical discussion of safety because it represents the thermal stability characteristic for a lithium ion battery.The simple thermal analytical equation describing the heat generation of a battery cell can be expressed as follows:where Qadis adiabatic heat generation(J),mis the mass of battery cell sample(g),cpis the specific heat capacity(J g-1K-1),andΔTadis the adiabatic temperature rise (K).Temperature data has been obtained from the ARC test,thus adiabatic heat generation can be calculated based on Eq.(1).
Heat generationper capacity for the lithium titanate battery at 0.1Cwas calculated and the results were listed in Table 1.It can be figured out from the table that,heat generation per capacity of each cycle was close to each other.In these cycle processes,irreversible Joule heat,reversible entropy change heat and over potential heat played a key role in battery heat production.In addition,total heat production was calculated as 1262JAh-1,which was far smaller than that of the battery system using graphite as the anode[1].All of the above proved high thermal stability for lithium titanate battery.
3.3 Cycle rate effect on thermal response of battery
In order to research the cycle rate effect on battery thermal response,tests with different cycle rate of 0.2C,0.5C,1.0Cand 1.5Cwere carried out.All of the tested samples cycled normally both outside and inside the ARC.
However,it's worth pointing out that,compared to the 0.1Ccase,when cell cycled at higher rates,the constant current charge process became shorter and constant voltage charge process lasted longer.Higher charge current results in larger cell polarization.When battery charged at high current,nominal voltage of the cell was easy to be reached,but its nominal capacity actually did not yet keep in the level.This was particularly apparent for 1.5Ccase,in which the constant current charge process was almost skipped.
Temperature and voltage changeof battery at higher rates were illustrated in Figs.5-9.Similar to case of 0.1C,battery temperature increased during both discharging and charging processes.Nevertheless,difference exists in the aspect of temperature change and calculated heat production rate for each cycle rate,as demonstrated in Figs.5-8and Table 1.It can be figured out that,although temperature rise of different cycle rate shows some disorder,distinct regularity can be found in heat production rate.It was found that,with the increase of cycle rate,heat production rate augments correspondingly for lithium titanate battery as seen in Fig.9.

表1 钛酸锂电池在不同倍率下各循环阶段产热量及产热速率对比表Table 1 Heat production and heat production rate of each cycle for lithium titanate battery at different cycle rates
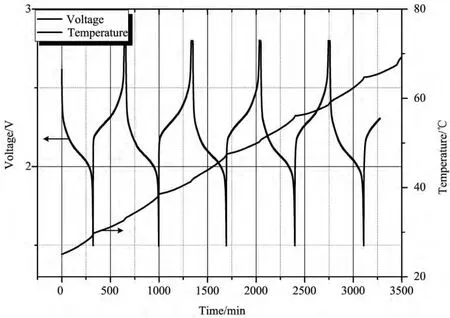
图5 钛酸锂电池在0.2C倍率下循环的温度/电压-时间曲线图Fig.5 Temperature and voltage versus time for lithium titanate battery tested at rate of 0.2C

图6 钛酸锂电池在0.5C倍率下循环的温度/电压-时间曲线图Fig.6 Temperature and voltage versus time for lithium titanate battery tested at rate of 0.5C
When batterycycled at lower cycle rate,heat production was determined by both reversible entropy change heat and irreversible Joule heat.However,with the increase of cycle rate,heat generation of the irreversible heat generated by o-vercoming the internal resistance increased rapidly.Therefore,total heat production rate was predominated by irreversible heat at higher rate and increased correspondingly with cycle rate[12].
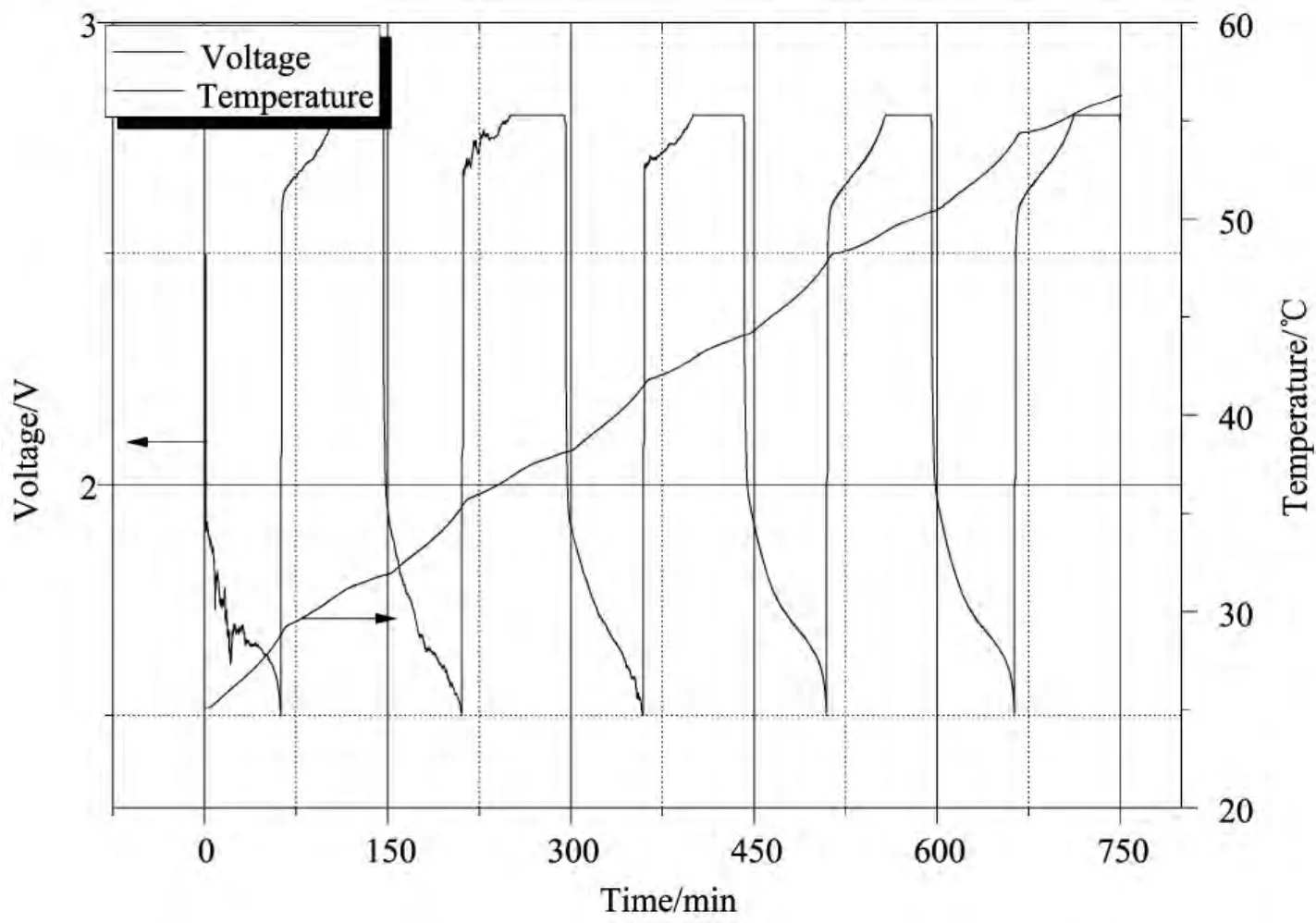
图7 钛酸锂电池在1.0C倍率下循环的温度/电压-时间曲线图Fig.7 Temperature and voltage versus time for lithium titanate battery tested at rate of 1.0C

图8 钛酸锂电池在1.5C倍率下循环的温度/电压-时间曲线图Fig.8 Temperature and voltage versus time for lithium titanate battery tested at rate of 1.5C
3.4 Thermal runaway characteristic for lithium titanate battery
In order to study the thermal runaway characteristic of lithiumtitanate battery,a new test was carried out at cycle rate of 1.5Cwith more cycle times of 30,and the result is shown in Fig.10.The battery worked normally during the first 15 cycles,however,the constant voltage charge process in the 16thcycle lasted slightly longer,and even longer process was found in that of the 17thcycle.Subsequently,this battery failed and could not work normally any more.

图9 钛酸锂电池在不同循环倍率下的产热速率对比图Fig.9 Plot of heat production rate at different cycle rates for lithium titanate battery
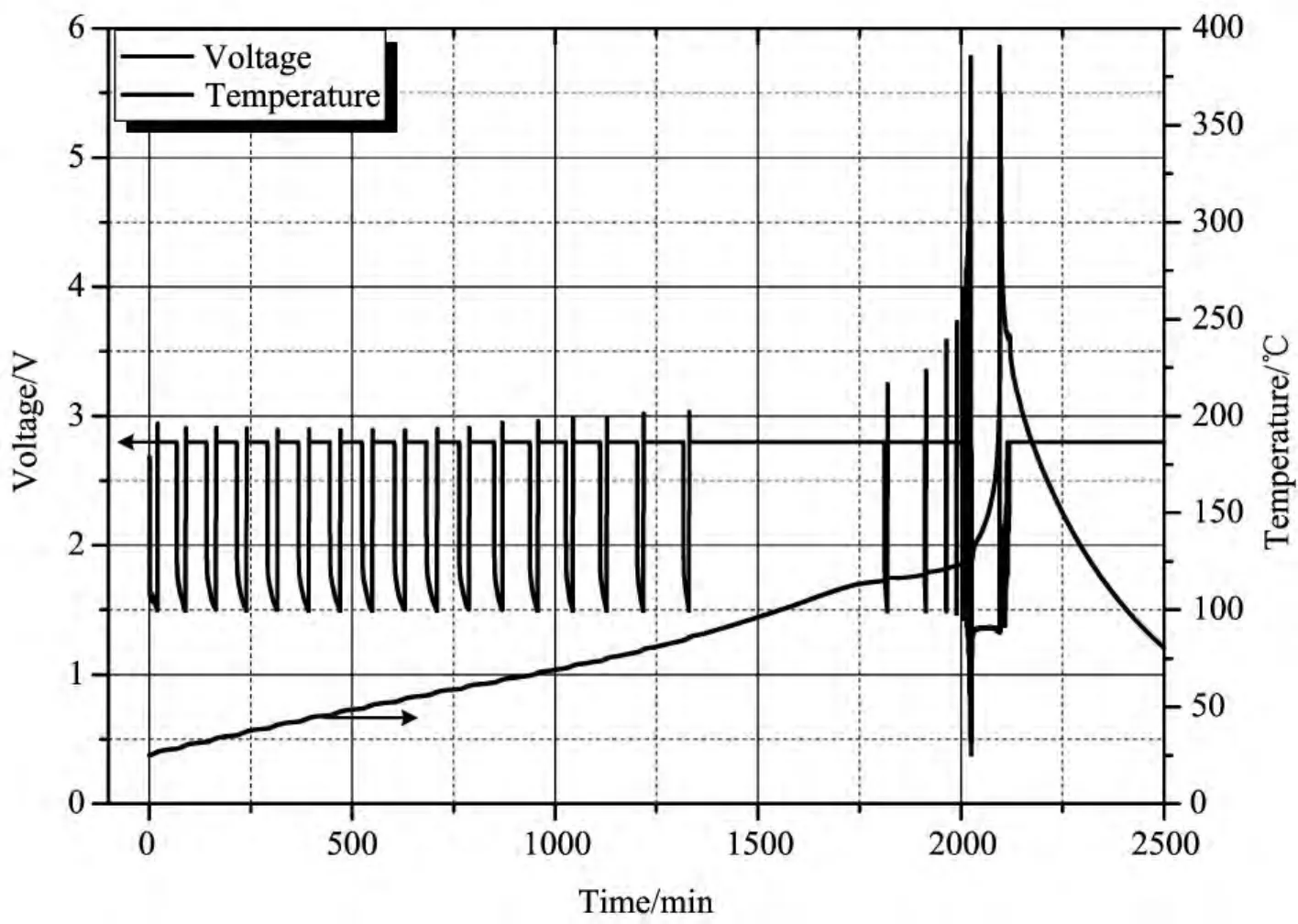
图10 钛酸锂电池在1.5C倍率下循环的温度/电压-时间曲线图(循环次数为30次)Fig.10 Temperature and voltage versus time plot of lithium titanate battery tested at for 1.5Cwith cycle times set at 30
Correspondingly,temperatureof battery kept rising all the way.After the thermal runaway starting point of about 125°C,high temperature caused reactions inside the battery occurred subsequently including reactions between negative active material and electrolyte,positive active material and electrolyte,the negative active and binder,etc.These reactions may occur simultaneously,leading to immediate thermal runaway,causing surface temperature of battery increasing sharply to the peak of 366.0°C.The whole process released a large amount of heat of 15243.5JAh-1.Even though the heat released was great,lithium titanate battery was still relatively safer than lithium cobalt oxides battery,which ran to thermal runaway when cycled at most 5times[13].
4 Conclusions
Thermal response of lithiumtitanate battery during cycling was studied by employing an accelerating rate calorimeter combined with multi-channel battery cycler.It was found that temperature rise existed in both discharging and charging processes.However,the magnitude of battery temperature rise and heat generation under adiabatic condition was small,and no thermal runaway occurred after cycled 5times.
To investigate the cycle rate effect on thermal characteristics oflithium titanate battery,tests with different cycle rates of 0.1C,0.2C,0.5C,1.0Cand 1.5Cwere carried out.Experimental results show that,with the increase of cycle rate,heat production rate augments correspondingly.Moreover,thermal runaway characteristic of lithium titanate battery was researched by testing a battery at cycle rate of 1.5Cwith cycle time of 30.The battery worked normally in the first 15cycles and thermal runaway happened soon afterwards,causing surface temperature of battery increasing sharply to the peak of 366.0°C with heat production rate of 436.8JAh-1h-1,which was less than that of cobalt oxides battery.
The above results were obtained under adiabatic condition,which corresponds to the worst case,proving outstanding thermal stability for lithium titanate battery.In the actual energy storage system of power grid,lots of batteries are packed together for use in series or parallel way.When fire happened,heat could not dissipate in time,causing quasi-adiabatic condition.Study in this paper can provide theoretical support for thermal safety design of lithium titanate battery.
[1]Wang QS,et al.Thermal runaway caused fire and explosion of lithium ion battery[J].Journal of Power Sources,2012,208:210-224.
[2]Horiba T,et al.Applications of high power density lithium ion batteries[J].Journal of Power Sources,2005,146(1):107-110.
[3]Commission of the European Communities[EB/OL],http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri1/4SEC:2009:1295:FIN:EN:PDF,2013-02.
[4]Leadbetter J,Swan LG.Selection of battery technology to support grid-integrated renewable electricity[J].Journal of Power Sources,2012,216:376-386.
[5]Wang YQ,et al.Rutile-TiO2nanocoating for a high-rate Li4Ti5O12anode of a lithium-ion battery[J].Journal of the American Chemical Society,2012,134 (18):7874-7879.
[6]Yi TF,et al.Structural and thermodynamic stability of Li4Ti5O12anode material for lithium-ion battery[J].Journal of Power Sources,2013,222:448-454.
[7]Zaghib K,et al.Electrochemical and thermal characterization of lithium titanate spinel anode in C–LiFePO4//C–Li4Ti5O12cells at sub-zero temperatures[J].Journal of Power Sources,2014,248:1050-1057.
[8]Wang QS,et al.Improved thermal stability of lithium ion battery by using cresyl diphenyl phosphate as an electrolyte additive[J].Journal of Power Sources,2010,195(21):7457-7461.
[9]Wang QS,et al.Enhancing the thermal stability of Li-CoO2electrode by 4-isopropyl phenyl diphenyl phosphate in lithium ion batteries[J].Journal of Power Sources,2006,162(2):1363-1366.
[10]Wang QS,et al.Improved thermal stability of graphite electrodes in lithium-ion batteries using 4-isopropyl phenyl diphenyl phosphate as an additive[J].Journal of Applied Electrochemistry,2009,39(7):1105-1110.
[11]Yao XL,et al.Comparisons of graphite and spinel Li1.33Ti1.67O4as anode materials for rechargeable lithium-ion batteries[J].Electrochimica Acta,2005,50(20):4076-4081.
[12]Li Q,et al.Investigation of the heat production of Liion batteries during cycling[J].Power Source Technol-ogy,2008,32(9):606-610.
[13]Zhao XJ,et al.Thermal response of lithium ion battery during charging and discharging using adiabatic calorimetry methodology[J].Under Review,2014.
