Clinical probability and risk analysis of patients with suspected pulmonary embolism
2014-03-20GuldenOzerenYetginSuleAkkoseAydinOzlemKoksalFatmaOzdemirDilekKostakMertGokhanTorun
Gulden Ozeren Yetgin, Sule Akkose Aydin, Ozlem Koksal, Fatma Ozdemir, Dilek Kostak Mert, Gokhan Torun
1Goverment Hospital of Afyon, Afyon, Turkey
2Emergency Department, Uludag University Faculty of Medicine, Bursa, Turkey
Corresponding Author:Sule Akkose Aydin, Email: akkose@uludag.edu.tr
Clinical probability and risk analysis of patients with suspected pulmonary embolism
Gulden Ozeren Yetgin1, Sule Akkose Aydin2, Ozlem Koksal2, Fatma Ozdemir2, Dilek Kostak Mert2, Gokhan Torun2
1Goverment Hospital of Afyon, Afyon, Turkey
2Emergency Department, Uludag University Faculty of Medicine, Bursa, Turkey
Corresponding Author:Sule Akkose Aydin, Email: akkose@uludag.edu.tr
BACKGROUND:Pulmonary embolism (PE) is one of the most frequent diseases that could be missed in overcrowded emergency departments as in Turkey. Early and accurate diagnosis could decrease the mortality rate and this standard algorithm should be de fi ned. This study is to fi nd the accurate, fast, non-invasive, cost-effective, easy-to-access diagnostic tests, clinical scoring systems and the patients who should be tested for clinical diagnosis of PE in emergency department.
METHODS:One hundred and forty patients admitted to the emergency department with the fi nal diagnosis of PE regarding to anamnesis, physical examination and risk factors, were included in this prospective, cross-sectional study. The patients with a diagnosis of pulmonary embolism, acute coronary syndrome or infection and chronic obstructive pulmonary disease (COPD) were excluded from the study. The demographics, risk factors, radiological fi ndings, vital signs, symptoms, physicallaboratory fi ndings, diagnostic tests and clinical scoring systems of patients (Wells and Geneva) were noted. The diagnostic criteria for pulmonary emboli were: fi lling defect in the pulmonary artery lumen on spiral computed tomographic angiography and perfusion defect on perfusion scintigraphy.
RESULTS:Totally, 90 (64%) of the patients had PE. Age, hypotension, having deep vein thrombosis were the risk factors, and oxygen saturation, shock index, BNP, troponin and fi brinogen levels as for the biochemical parameters were signi fi cantly different between the PE (+) and PE (–) groups (P<0.05).The Wells scoring system was more successful than the other scoring systems.
CONCLUSION:Biochemical parameters, clinical findings, and scoring systems, when used altogether, can contribute to the diagnosis of PE.
Pulmonary embolism; Probability; Emergency department
INTRODUCTION
Pulmonary embolism (PE) is an obstructive disease of the pulmonary arterial system occurring in different stages and locations. It is commonly caused by the embolization of thrombus originating from the deep veins of the lower extremities.[1,2]PE is the third cause of cardiovascular related deaths after coronary arterial diseases and stroke.[3]The mortality rate can be decreased to 3% by appropiate diagnosis and medication.[4]
The differential diagnosis of PE consists of commonly seen diseases. Its symptoms and findings are non-spesific, and clinical diagnosis is not reliable. PE can be overlooked because of comorbidities, and the diagnosis can be delayed.[4]In recent years, new developments have been introduced in the diagnosis and treatment of PE. But standard approach for the diagnosis and treatment of PE is not available.
International and national guidelines have been published to ensure consensus on the diagnosis, treatment and prophylaxis of PE, which requires a multidisciplinary approach.[5,6]Pulmonary angiography, a de fi nitive diagnostic method for the diagnosis of PE, isinvasive and expensive.[7,8]Contemporarily, noninvasive diagnostic approaches are preferred such as lower extremity ultrasonography (USG), ventilation-perfusion (V/P) scintigraphy and spiral computed tomographic angiography (SCTA) with the combination of various clinical and laboratory findings rather than pulmonary angiography.
The patients with PE are commonly seen at emergency departments. In our country, 30% of health care provider applications are done by emergency departments. This rate shows that 90 millions of emergency department applications occur annually.[9]There are depatments of emergeny medicine having 2000 patients per day in our country.[9]PE could be overlooked in this kind of overcrowded emergency departments because of diagnostic delay instead of treatment failure. The diagnosis can be done with the combination of clinical suspection, risk scores and other diagnostic approaches. In addition, this combination should be as simple, accurate and reliable as possible.
The objective of the present study was to determine the probability of PE and the prognosis of patients suspected of having PE using basic laboratory and clinical variables that are noninvasive, inexpensive, easily accessible and that provide an accurate prognostic evaluation with clinical scoring systems.
METHODS
Patients
A total of 140 patients aged 18 years or over who presented to the Uludag University Medical Faculty Emergency Department between June 1, 2010 and June 1, 2011 who were diagnosed with PE were included in this prospective cross sectional study. The patients aged below 18 who had PE, acute coronary syndrome or infection simultaneously, chronic obstructive pulmonary disease (COPD) and right ventricular loading shown by echocardiography (ECHO) were excluded from the study. Demographic features, vital signs (blood pressure, heart rate, respiratory rate), shock ındex (SI), symptoms at admission (dyspnea,chest pain, pleuritic pain, cough, hemoptysis, edema in calfs and calf pain), and onset of symptoms, physical examination findings [tachypnea, tachycardia, cyanosis, sweating, fever, jugular venous distension, crepitan ralles, wheezing, ronchi, decreased respiratory sounds and deep vein thrombosis (DVT) signs], predisposing factors (age>60 years, immobilization, DVT, previous PE, family history, vacation, the history of santral venous catheter, stroke, systemic arterial hypotension, heart failure, malignancy, smoking, pregnancy, estrogen intake, surgery, COPD and clinical probability scorings for PE), laboratory outcomes [arterial blood gas, troponin, brain natridiuretic peptid (BNP), fibrinogen], posteroanterior (PA) chest X-ray, electrocardiography (ECG), ECHO and lower extremity Doppler USG outcomes were recorded.
Parameters
Arterial blood gas analyses were performed and partial oxygen pressure (PaO2) and oxygen saturation (SaO2) were determined. The diagnostic criteria for pulmonary emboli were as follows: filling defect in the pulmonary artery lumen in SCTA and perfusion defect in perfusion scintigraphy. Atelectasis, cardiomegaly, pleural effusion, in fi ltration and diaphragma elevation were investigated in the PA chest X-rays ordered in the emergency department. Likewise, in ECG, sinusal tachycardia, incomplete/ complete right bundle branch block, nonspeci fi c ST wave changes, S1Q3T3 pattern, T wave inversion in V1-V3 were also viewed.
Ventilation-perfusion scintigraphy and SCTA were used for fi nal diagnosis of the patients. SCTA was carried out using a 64-detector Siemens Somatom Definition device and filling defects observed in the pulmonary arterial lumen were taken as the criteria for PE. Macroaggregate (MAA) labeled with technetium-99 m was used for perfusion scintigraphy and perfusion defects on perfusion scintigraphy were also taken as the criteria for PE. The outcomes of perfusion scintigraphy were assessed together with PA chest X-rays of the patients ordered within 24 hours. The results were classified according to the PIOPED[10]criteria as high, medium, low and normal/close to normal. In this study, the main femoral, deep femoral, superficial femoral and crural veins were examined using a Toshiba XARIO USAP-77OA model USG device. First, vessel calibration and sonopathologic appearance in the lumen were studied with the B-mode. Then, fi lling with color, augmentation, spontaneous reflux and the presence of reflux with valsalva were examined through colored Doppler. Failure to receive a response with the device probe and lack of observed filling with colored Doppler was accepted as positive fi ndings for DVT.
The clinical probability for the patients was separately calculated with the Wells[11]and Geneva[12]clinical estimation scoring systems and the patients were divided into the low, medium and high probability groups.
Statistical analysis
The data were analyzed using SPSS13.0 statistical software. Normal distribution of the data was examined with the Shapiro-Wilk test. None normally distributed data were compared in the two groups using the Mann-Whitney U test. Pearson's product-moment correlation coefficient, Yates' correction for continuity and Fisher's exact test were used to analyze the categorical data. ROC analysis was carried out to compare the sensitivity and speci fi city in the Wells and Geneva clinical scorings. The signi fi cance level was set at P<0.05.
RESULTS
One hundred and forty patients were enrolled in this prospective study. Of these patients, 52.9% were female and 47.1% were male. Ninety (64%) of the patients were de fi ned as PE positive by SCTA or V/Q scintigraphy. PE (+) and PE (–) groups were compared for risk factors and older age (>60 age), hypotension, and DVT were found to be statistically significant for PE (P: 0.028, 0.008, 0.005).
PE (+) and PE (–) groups were compared for signs and symptoms, and dyspnea, calf pain, swelling of calf and tachypnea were found to be statistically significant for the PE (+) group (P: 0.00, 0.034, 0.010, 0.033).
Patient's mean blood pressures and SaO2ratios were compared and these parameters were statistically lower in the PE (+) group (P: 0.020, 0.002).
PE (+) and PE (–) groups were compared for SI, and cases in which SI> 1 were found to be statistically signi fi cant (0.022).
Right bundle branch block and S1Q3T3 pattern were found to be statistically significant in the PE (+) group (P=0.004, P=0.005).
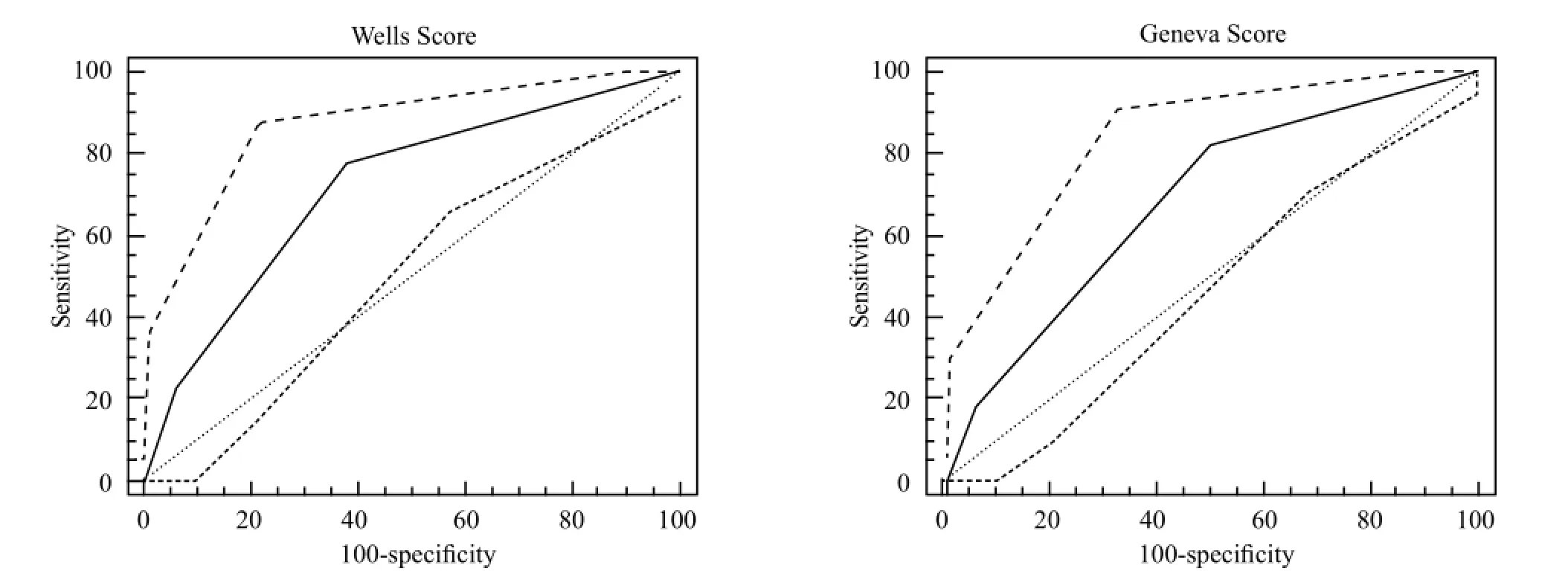
Figure 1. Comparison of Wells and Geneva probability classi fi cation diagnostic values with ROC curve.
In the PE (+) group, 30% of the patient's PA chest X-ray were evaluated as normal. Pleural effusion and in fi ltration were found to be statistically signi fi cant in the PE (+) group (P: 0.002, 0.042).
Troponin, BNP and fi brinogen levels were compared between the PE (+) and PE (–) groups (P=0.013, P<0.001, P=0.033).
PE clinical probability was calculated separately by Wells and Geneva clinical estimation scoring systems, and the patients were classified into low risk, medium risk and high risk probability groups. The incidence of PE decreased as the probability decreased both in the Wells and Geneva clinical estimation scoring systems, and there was a signi fi cant decreasing correlation (Table 1).
When both scoring systems were compared through ROC curve analysis, the AUC value was higher in the Wells than in the Geneva scoring system (Wells AUC=0.720 and Geneva AUC=0.681) (Figure 1).
The Wells scoring system identified only 1 (5%) patient with a low probability, which had a high clinical probability calculated with the Geneva scoring system. None of the patients with high scores according to the Wells scoring system had a low probability according tothe Geneva scoring system (Table 2).
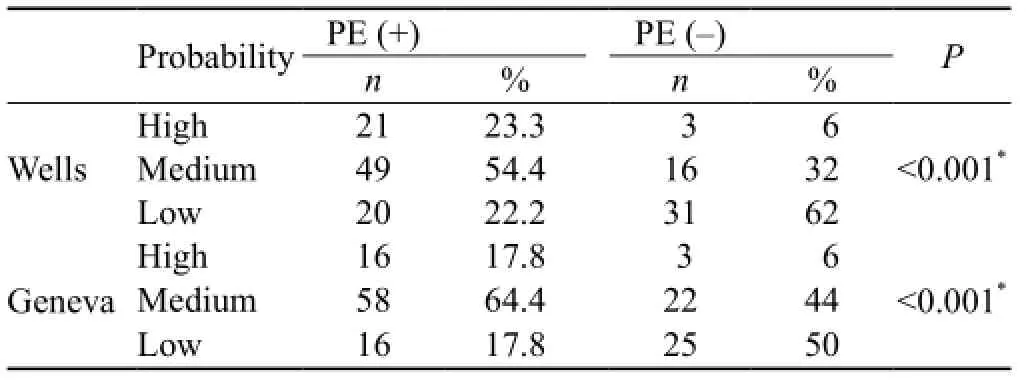
Table 1. The ef fi ciency of Wells and Geneva scores in PE

Table 2. The correlation between Wells and Geneva scores in PE (+) patients
The Wells and Geneva scoring systems were found to be correlated with each other. Three patients (5.9%) who were found to be in the low probability group by the Wells scoring system were evaluated as in the high probability group by the Geneva scoring system. However, 2 patients (8.3%) who were found to be in the high probability group by the Wells scoring system were evaluated as in the low probability group by the Geneva scoring system (Table 3).
DISCUSSION
The easily accessed, highly sensitive algoritm should be chosen for the diagnosis of PE. Risk factors, clinical situation, laboratory findings, and clinical scoring systems must be evaluated together. This study aimed to de fi ne the clinical probability of PE, make a risk analysis, and estimate the prognosis of patients with suspected acute PE using basic laboratory and clinical variables and clinical scoring systems in the emergency department.
In many studies,[13–15]the presence of risk factors was investigated in the patients who were considered to have PE. Miniati et al[16]de fi ned immobilization, history of thrombophlebitis, malignancy and lower extremity fractures as significant risk factors. In various studies conducted in this country, risk factors were defined by different rates. Kıral et al[17]found older age in 37%, surgical intervention in 18%, and cardiologic diseases in 18.5% of the patients with PE. Çakmak et al[18]and Kadakal et al[19]found that the most common risk factors were DVT, lower extremity fractures, and surgical intervention. Atikcan et al[20]found the most common risk factor was a history of DVT and abdominal surgery in the 42 patients followed up, whereas they did not find a risk factor in 38% of the patients. In the present study, advanced age (58%), immobilization (33%), a history of operation within the last three months (33%), and DVT (30%) were determined as the risk factors for the development of PE. Significant differences were foundbetween the patients with and without the diagnosis of PE in terms of advanced age, systemic hypotension, and DVT.
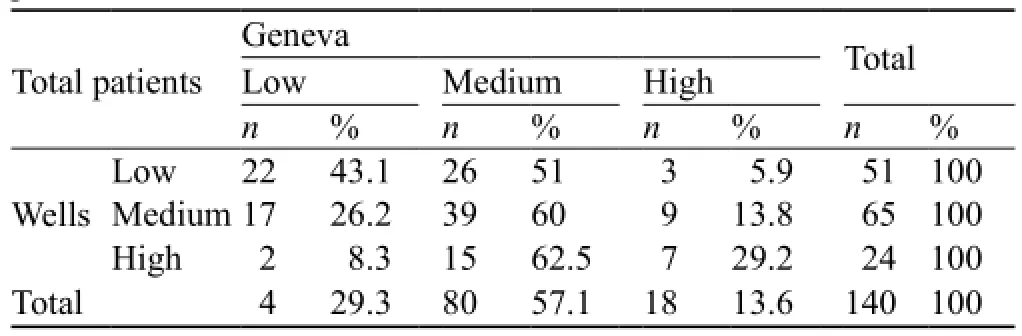
Table 3. The correlation between Wells and Geneva scores in all patients
In the International Cooperative Pulmonary Embolism Registry (ICOPER) study, shortness of breath was found in 82%, chest pain in 49%, coughing in 20%, syncope in 14% and hemoptysis in 7% of PE patients.[21]The study by Miniati et al[16]revealed that the most common distinctive clinical symptoms in PE patients were shortness of breath (78%), chest pain (44%) and syncope (26%). In our study, the most common symptom was dyspnea (83%) followed by calf edema, calf pain and pleuritic pain, respectively.
In our study, the most common clinical fi ndings were tachycardia, tachypnea, rales and decreased respiratory sounds. The incidences of tachypnea and DVT symptoms were signi fi cantly higher in PE (+) patients than in those without PE. These results are consistent with those reported elsewhere.[22]
Tachycardia is a common finding in patients with pulmonary embolism, while low SO2and hypotension are expected in massive pulmonary embolism. However, it should be remembered that these findings are not PE specific.[5,23]In our study, a significant difference was noted between the PE (+) and PE (–) groups in terms of SO2. The reason of the lack of significant difference in pulse rate was interpreted as the presence of an alternative cause of tachycardia such as COPD, pneumonia, congestive heart failure, anxiety, malignancy, etc. Indeed, both groups were tachycardic.
SI is calculated by the ratio of heart rate to systolic blood pressure. In case of a shock index >1, patients are defined as having hemodynamic instability.[24]SO2and shock index can help to determine the severity of PE. The risk of mortality increases with increase of the shock index.[25]Another study[26]showed that when SO2value decreased from 95% to 94%, the mortality rate increased from 1.8% to 19%. In our study, a signi fi cant difference was found between PE (+) and PE (–) patients in terms of SO2level. There was also a significant difference in shock index.
ECG findings are nonspecific for the diagnosis ofPE, but they are useful to rule out diseases like acute myocardial infarction (MI) and pericarditis. In a study[23]in which ECG findings were found to be abnormal in 70% of patients, the most common pathological fi ndings were sinus tachycardia, S1Q3T3 pattern, T wave inversion and atrial fibrillation. The ECG findings of sinus tachycardia and signs of right ventricular overload have been shown to be associated with poor prognosis in patients with PE.[27–28]
In our study, ECG findings detected in PE (+) patients were sinus tachycardia, complete/incomplete right bundle branch block (RBBB), S1Q3T3 and normal sinus rhythm. When patients with and without pulmonary embolism were compared, a significant difference was found for complete/incomplete RBBB and S1Q3T3, and the results were consistent with those reported previously. This condition emerged because of right ventricular loading in patients in the pulmonary embolism group.
Contrary to common belief, chest X-rays may be normal in PE patients. In the PIOPED study, 12% of 383 patients, and in the PISAPED study, 14% of the patients were considered to have a normal chest X-ray.[10,29]In our study, 30% of the patients had normal chest X-rays. In the PIOPED study, the most common findings were atelectasis and pulmonary paranchymal consolidation, whereas in the PISAPED study, Westermark findings and pulmonary consolidation were observed.[10,29]In our study, in order of the frequency, normal findings were detected in 30%, atelectasis in 24% and infiltration in 20% of the patients. When the chest X-ray findings of the PE (+) and PE (–) groups were compared, pleural effusion and infiltration were found to be significantly higher in the PE (–) group. This was thought to be due to the diagnosis of congestive heart failure and pneumonia.
In conclusion, when the chest X-ray is found to be normal in a patient with acute hypoxemia in which bronchial obstruction is not found, the likelihood of PE should be considered first. However, if there are abnormal fi ndings, none of them are pathognomonic for PE.
In the PIOPED study, the PaO2levels of the patients who did not have previous cardiopulmonary disease were not different from healthy people.[10]On the other hand, there are studies[6,23]reporting that at least 80% of PE patients are hypoxemic. In our study, 80% of the patients were found to be hypoxemic.
Troponin and BNP levels are found to be increased in right ventricule dilatation and right ventricule microinfarction.[30]Troponin and BNP increase shows the right ventricule disfunction and hemodynamic instability.[30]In our study, troponin and BNP levels were significantly higher in the PE (+) group and significant difference was observed between the PE (+) and PE (–) groups.
One of the objectives of this study was to compare the two scoring systems used to establish the diagnosis of PE. When all patients were evaluated by the Wells and Geneva scoring systems, the patients in the high, medium and low probability groups who were diagnosed with PE were found as 24%, 54% and 22% according to the Wells system, respectively. These rates were 17%, 64.4% and 17%, respectively according to the Geneva system. Both systems help to diagnose PE at a statistically significant level. The values of the Wells and Geneva systems in reaching a diagnosis of PE were classi fi ed as high, medium and low and compared through ROC curve analysis; the Wells system was found to be more valuable in reaching an accurate diagnosis of PE.
There are limitations in this study. First, the clinical probability score was defined by a single physician, and his score was not compared with that of another physician. Second, a small number of patients were included in the study. Third, this study was an unicentral hospital study.
We believe that the combined use of biochemical parameters, clinical fi ndings and clinical scoring systems would contribute to the diagnosis of PE.
Funding:None.
Ethical approval:This study was approved by the ethics committee under number 2009-12/14.
Con fl icts of interest:The authors declare that there is no con fl ict of interest relevant to the content of the article.
Contributors:Yetgin GO proposed the study, analyzed the data and wrote the fi rst draft. All authors contributed to the design and interpretation of the study and to further drafts.
REFERENCES
1 Dalen JE. Pulmonary embolism: what have we learned since Virchow? Natural history, pathophysiology, and diagnosis. Chest 2002; 122: 1440–1456.
2 Kearon C. Natural history of venous thromboembolism. Circulation 2003; 107 (23 Suppl 1): I22–I30.
3 Konstantinides S. Acute pulmonary embolism. N Engl J Med 2008; 359: 2804.
4 British Thoracic Society Standards of Care Committee Pulmonary Embolism Guideline Development Group. British Thoracic Society guidelines for the management of suspected acute pulmonary embolism. Thorax 2003; 58: 470–484.
5 Türk Toraks Derneği Pulmoner Tromboembolizm Tanı ve Tedavi Uzlaşı Raporu. Türk Toraks Dergisi 2009.
6 Hirsh J, Guyatt G, Albers GW, Harrington R, Schünemann HJ, American College of Chest Physician. Antithrombotic and thrombolytic therapy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133 (6 Suppl): 110S–112S.
7 Van Beek EJ, Brouwerst EM, Song B, Stein PD, Oudkerk M. Clinical validity of a normal pulmonary angiogram in patients with suspected pulmonary embolism- a critical review. Clin Radiol 2001; 56: 838–842.
8 Stein PD, Athanasoulis C, Alavi A, Greenspan RH, Hales CA, Saltzman HA, et al. Complications and validity of pulmonary angiography in acute pulmonary embolism. Circulation 1992; 85: 462–468.
9 www.saglik.gov.tr/Ekutuphane/kitaplar/istaturk.
10 PIOPED Investigators. Value of the ventilation/perfusion scan in acute pulmonary embolism. Results of the prospective investigation of pulmonary embolism diagnosis (PIOPED). JAMA 1990; 263: 2753–2759.
11 Wells PS, Ginsberg JS, Anderson DR, Kearon C, Gent M, Turpie AG, et al. Use of a clinical model for safe management of patients with suspected pulmonary embolism. Ann Intern Med 1998; 129: 997–1005.
12 Wicki J, Perneger TV, Junod AF, Bounameaux H, Perrier A. Assessing clinical probability of pulmonary embolism in the emergency ward: a simple score. Arch Intern Med 2001; 161: 92–97.
13 Stein PD, Hsiu Ling H, Afzal A, Noor HA. Incidence of acute pulmonary embolism in a general hospital. Chest 1999; 116: 909–913.
14 Miniati M, Monti S, Bottai M. A structured clinical model for predicting the probality of pulmonary embolism. Am J Med 2003; 114: 173–179.
15 Hansson PO, Welin L, Tibblen G, Eriksson H. Deep vein thrombosis and pulmonary embolism in the general population. Arch Intern Med 1997; 157: 1665–1670.
16 Miniati M, Prediletto R, Formichi B, Marini C, Di Ricco G, Tonelli L, et al. Accuracy of clinical assessment in the diagnosis of pulmonary embolism. Am J Respir Crit Care Med 1999; 159: 864–871.
17 Kıral N, Salepci B, Ozdoğan S. Retrospective analysis: high probability of clinical pulmonary embolism. Respiratory Diseases 2002; 13: 172–176.
18 Çakmak F, Isık C, Gündoğdu C. Retrospective analysis of patients diagnosed with pulmonary embolism at the center for chest diseases and thoracic surgery between the years 1987–1990. Respiratory Diseases 1992; 3: 53–62.
19 Karadal F, Cetinkaya E, Yıldız P, Soysal F, Tekin A, Yılmaz V. Diagnosis of clinical pulmonary embolism with high probability. Respiratory Diseases 2000; 11: 140–143.
20 Atikcan S, Atalay F, Turgut D, Ünsal E. Pulmonary thromboembolism: a retrospective evaluation of 42 cases. Respiratory Diseases 2002; 13: 87–93.
21 Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet 1999; 353: 1386–1389.
22 Yüksel EG, Turan F, Özyardımcı N. Retrospective analysıs of pulmonary embolism. Lung Archive 2001; 2: 79–84.
23 Torbicki A, Van Beek EJR, Charbonnier B. Guidelines on diagnosis and management of acute pulmonary embolism. Eur Heart J 2000; 21: 1301–1336.
24 Rady MY, Nightingale P, Little RA, Edwards JD. Shock index: a re-evaluation in acute circulatory failure. Resuscitation 1992; 23: 227–234.
25 Otero R, Trujillo-Santos J, Cayuela A, Rodriguez C, Barron M, Martin JJ, et al. Haemodynamically unstable pulmonary embolism in the RIETE Registry: systolic blood pressure or shock index? Eur Respir J 2007; 30: 1111–1116.
26 Kline JA, Miller DW. Risk stratification for acute pulmonary embolism. J Natl Compr Canc Netw 2011; 9: 800–810.
27 Escobar C, Jimenez D, Marti D. Prognositic value of echocardiographic findings in hemodynamically stable patients with acute symptomatic pulmonary embolism. Rev Esp Cardiol 2008; 61: 224–250.
28 Vanni S, Polidori G, Vergara R, Pepe G, Nazerian P, Moroni F, et al. Prognostic value of ECG among patients with acute pulmonary embolism and normal blood pressure. Am J Med 2009; 122: 257–264.
29 Worsley DF, Alavi A, Aronchick JM, Chen JT, Greenspan RH, Ravin CE. Chest radiografic findings in patients with acute pulmonary embolism: Observations from the PIOPED study. Radiology 1993; 133–136.
30 Wolde M, Tulevski II, Mulder JW. Brain natriuretic peptide as a predictor of adverse outcome in patients with pulmonary embolism. Circulation 2003; 107: 2082–2084.
Received April 19, 2014
Accepted after revision September 6, 2014
World J Emerg Med 2014;5(4):264–269
10.5847/wjem.j.issn.1920–8642.2014.04.004
杂志排行
World journal of emergency medicine的其它文章
- Current pre-hospital traumatic brain injury management in China
- Emergency bedside ultrasound for the diagnosis of pediatric intussusception: a retrospective review
- How to secure the connection between thoracostomy tube and drainage system?
- Thyroid hormone alterations in trauma patients requiring massive transfusion: An observational study
- The incidence of oxygen desaturation during rapid sequence induction and intubation
- A single subcutaneous dose of tramadol for mild to moderate musculoskeletal trauma in the emergency department
