2-吡唑啉衍生物合成研究进展
2012-12-09蔺志平乔凤霞
蔺志平,乔凤霞
(保定学院 生化系,河北 保定 071000)
学科综述
2-吡唑啉衍生物合成研究进展
蔺志平,乔凤霞
(保定学院 生化系,河北 保定 071000)
2-吡唑啉类化合物是极为重要的含氮五元杂环化合物,在有机合成及相关领域有非常广泛的应用.本文按照吡唑啉环上不同位置的取代基种类,介绍近年来由α,β-不饱和羰基化合物与肼及其衍生物直接环化合成此类化合物的研究进展.引用参考文献83篇.
2-吡唑啉衍生物;有机合成;综述
2-吡唑啉衍生物是一类重要的五元氮杂环化合物,具有消炎、抗菌[1]、抗病毒[2]、和杀虫[3]等药理作用[4-8].此类化合物还具有良好的光学性质,被广泛用作纺织物和纸张等的荧光增白剂[9]以及光电材料和电致发光体系中的空穴传输媒介等[10].因此吡唑啉衍生物合成方法的研究日益成为有机合成中的热点,引起人们的重视.本文按照吡唑啉环上不同位置的取代基种类,介绍近年来由α,β-不饱和羰基化合物与肼及其衍生物直接环化合成此类化合物的研究进展(Scheme 1).
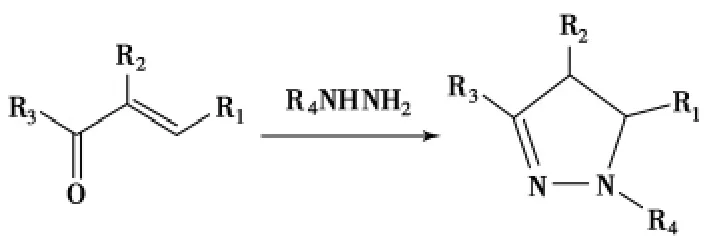
Scheme 1
1 1-苯基(或取代苯基)-2-吡唑啉的合成
早在19世纪,Fischer等制备腙时意外发现利用丙烯醛与苯肼反应得到的不是腙,而是1-苯基吡唑啉[11],这一现象引起了人们的极大兴趣.随后,在醛与肼的进一步研究中发现,某些醛与肼生成的腙不稳定,在热醋酸中易重排成吡唑啉.1918年,Straus[11]研究发现,无论是α,β-不饱和醛或酮还是肼,只要苯环上带有取代基,则得到稳定的产物腙.而Auwers和Voss[11]则发现,芳环上不带取代基的α,β-不饱和醛或酮与苯肼反应迅速生成腙,无需分离,低温下可迅速重排成吡唑啉.
Sachchar等[12]将1-取代苯基-3-呋喃基-2-丙烯-1-酮、1-噻吩基-2-丙烯-1-酮或1-吡啶基-2-丙烯-1-酮与苯肼在醋酸中回流4h,然后放置过夜即可得到吡唑啉,产率31%~82%.Palaska等利用同样的方法由1,3-二芳基-2-丙烯-1-酮与苯肼反应4h,合成了一系列的1,3,5-三芳基-2-吡唑啉,产率为81%~94%[13].
2006年,Ozdemir等将1-(2-呋喃)基-3-芳基-2-丙烯-1-酮与肼、苯肼、氨基硫脲在醋酸中回流4h合成了1-苯基-3-(2-呋喃)-2-吡唑啉、1-氨基硫羰基-3-(2-呋喃)-2-吡唑啉或1-N-取代的氨基硫羰基-3-(2-呋喃)-2-吡唑啉衍生物,产率58%~64%[14-15].
以醋酸为溶剂和催化剂,将1,3-二芳基-2-丙烯-1-酮和苯肼在90℃反应2h,即生成1,3,5-三芳基吡唑啉,不必分离直接加入适量的TPCD(二氢铬酸四吡啶合钴)可进一步生成1,3,5-三芳基吡唑[16].朴文香等[17]在醋酸中回流6h或与乙二醇单乙醚常温搅拌3h合成了9种吡唑啉,产率50%~85%.在醋酸中将1-芳呋喃基-3-对二甲胺基苯丙烯酮或1-芳呋喃基-5-对二甲胺基苯基戊二烯酮和苯肼加热溶解,回流状态下滴加无水乙醇合成了芳呋喃吡唑啉化合物(Scheme 2).结果表明:呋喃基的引入扩大了共轭体系,使其荧光发射光谱λmax大多都大于450nm,可作为荧光染料[18].
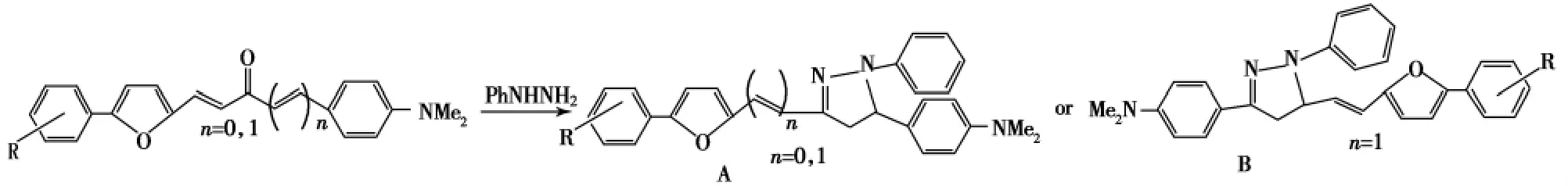
Scheme 2
通过产物的紫外可见吸收光谱和质谱数据表明:n=1时,反应缩合环化发生在戊二烯酮中羰基和不与呋喃基相连的双键上,生成的产物是3-位含有不饱和双键的吡唑啉A而不是B.可能的反应机理是苯肼的α氮原子与烯酮的β烯碳进行1,4-加成后,β氨基与羰基碳原子发生分子内关环,进而生成吡唑啉化合物[19].
徐峰等[20]则将三唑环引入吡唑啉的3位(Scheme 3),使得此类化合物具有了多种药理作用,如抗肿瘤、抗病毒、抗菌、舒张血管等作用.黎芳等[19]的研究结果表明:吡唑啉3位引入三唑环后有蓝色荧光;1位引入取代基也使化合物的荧光波长移至480nm左右.
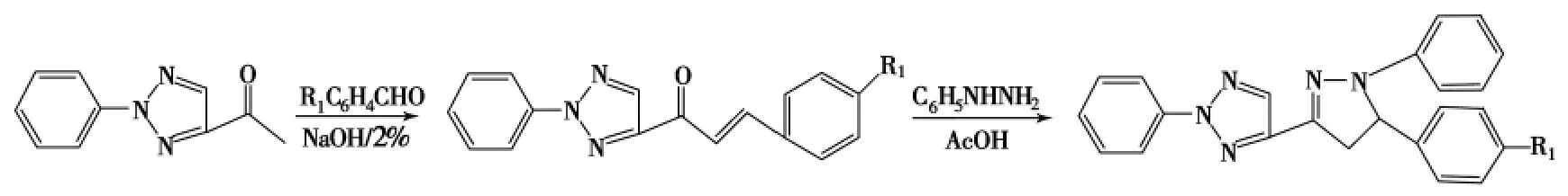
Scheme 3
传统方法中,吡唑啉环的合成均是在弱酸性条件下,如醋酸溶液中进行的,但这类反应大多需要在回流状态下进行;而改用乙二醇单乙醚为反应介质,反应可以在较低的温度如室温或水浴加热中进行.在氮气保护下,将1,3-二芳基-2-丙烯-1-酮和苯肼加入乙二醇单乙醚中,水浴加热搅拌2h,也可制得1,5-二苯基-3-对硝基苯基吡唑啉,产率可达79%[21].将苄叉氯苯乙酮溶于乙二醇单乙醚中,加入苯肼室温搅拌1h后即可制得吡唑啉,产率43%.研究发现此化合物可用于制备兰色防伪荧光材料,能实现彩色兰、绿、红三原色荧光防伪[22].同样条件下,韩秀英等[23]可将1,3-二苯基-5-(4-氯苯基)-2-吡唑啉的产率提高至80%.
张耀谋等[24]在无水乙醇中回流反应12h合成了1,3,5-三芳基-2-吡唑啉,产率52%~61%.把传统的酸性条件改为强碱如氢氧化钠催化,1,3-二芳基-2-丙烯-1-酮与苯肼在乙醇中加热至70℃回流8h,也得到了1,3,5-三苯基-2-吡唑啉[25].将萘基查尔酮与盐酸苯肼在乙醇中回流,相应吡唑啉的产率78%~91%[26].
以乙醇和甲苯的混合液作为反应介质,将双查尔酮与苯肼在酸性条件下(pH=4~5)加热回流6h,经缩合环化制得具有良好光电导性能的吡唑啉化合物,产率90%(Scheme 4)[27].
Scheme 4
El-Rayyes将对苯二甲醛与芳香甲基酮生成的查尔酮与肼、苯肼或甲基肼反应也可生成相应的吡唑啉衍生物,产率81%~87% (Scheme 5)[28].
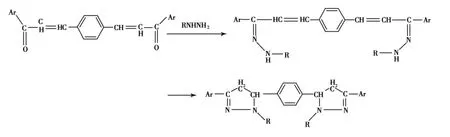
Scheme 5
在乙酸和乙醇混合液中,将1-(3’-吲哚基)-3-(4”-氰基苯基)-2-丙烯-1-酮和苯肼回流36h,可生成1-苯基-3-(3’-吲哚基)-5-(4’-氰基苯基)-2-吡唑啉,产率58%[29-30].同样条件下,王忠义等[31]也合成了一系列吡唑啉,产率37%~67%.
在吡唑啉环的4位上引入三唑基或咪唑基,具有明显的杀菌和植物激素活性.1,3-二芳基-2-三唑或咪唑基丙烯酮与(取代)苯肼反应,在三乙胺/乙醇中仅得到反式异构体,在醋酸中产物却几乎为等量的顺反异构混合体[32].在进一步的研究过程中发现了不同的实验结果:(Z)或(E)-1,3-二芳基-2-(1H-1,2,4-三唑-1-基)-2-丙烯-1-酮与苯肼在醋酸中反应均生成顺式和反式吡唑啉异构体混合物,但在三乙胺中却只生成反式异构体[33].可能的反应机理是:醋酸中苯肼的α氮原子与烯酮的β烯碳进行1,4-加成后,β氨基与羰基碳原子发生分子内的5-Exo-Trig方式关环而生成顺式和反式吡唑啉化合物,而在三乙胺中,1,4-加成更倾向于生成热力学稳定的过渡态反式吡唑啉化合物.
在三乙胺中,将盐酸苯肼和含有异恶唑环的丙烯酮于60℃反应10h,也可成功将异恶唑环引入吡唑啉的3位,产率51%~83%(Scheme 6)[34].
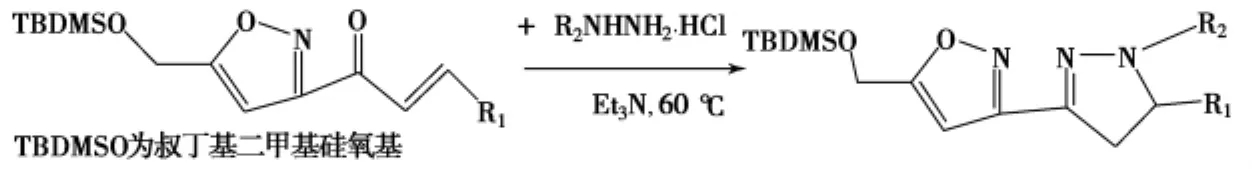
Scheme 6
微波技术应用在有机合成中可提高反应速率,简化操作步骤,增加反应选择性,提高产率,易纯化等,是实现绿色有机合成的一种重要手段[35-36].Kidwai[37]首次将微波辐射应用于2-吡唑啉的合成.以醋酸为溶剂微波辐射反应3min,1-苯基-3-苯基-5-(4’-甲氧基)苯基-2-吡唑啉和1-苯基-3-(4’-溴)苯基-5-(3’,4’-亚甲基二氧苯基)-2-吡唑啉产率由无微波辐射时的68%和48%提高到78%和72%,以碱性三氧化铝为载体微波辐射1.5min,产率可进一步提高到82%和80%.载体和微波辐射的配合使用不仅可以缩短反应时间,提高反应产率,还可以避免有机溶剂的使用.微波辐射下用K2CO3作为催化剂,吡唑啉的产率进一步提高到85%~95%[38].廖勇等[39]用微波辐射代替传统的回流条件,用取代苯肼盐酸盐和β-二甲胺基苯丙酮盐酸盐反应,合成了一系列1,3-二苯基吡唑啉类化合物,产率41%~89%.李记太等将超声辐射应用于1,3,5-三芳基-2-吡唑啉衍生物的合成,超声辐射反应1.5~3h,产率可高达83%~96%[40-41].双查尔酮与苯肼的环化反应,经超声辐射2h,相应的1,5-二芳基-3-芳乙烯基-2-吡唑啉的产率43%~80%[42].
2 1-苯并噻唑基-2-吡唑啉的合成
将2-肼基苯并噻唑和1,3-二芳基-2-丙烯-1-酮在乙二醇独乙醚溶液中回流2h,可生成具有较强荧光性的1-苯并噻唑基-3,5-二苯基吡唑啉,产率81%~84.5%(Scheme 7)[43-49].
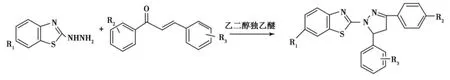
Scheme 7
将2-肼基苯并噻唑和呋喃亚甲基苯乙酮在氢氧化钠/无水乙醇中回流6h可制得5-呋喃基-1-苯并噻唑基吡唑啉,产率均为51%以上[50].在无水乙醇和10%氢氧化钠的等体积混合溶液中,加入2-肼基苯并噻唑与1-(3-吲哚基)-3-(2-呋喃基)-2-丙烯-1-酮回流5h可得5-(2-呋喃基)-3-(3-吲哚基)-1-(2-苯并噻唑)-2-吡唑啉,产率54%~62%(Scheme 8)[51].
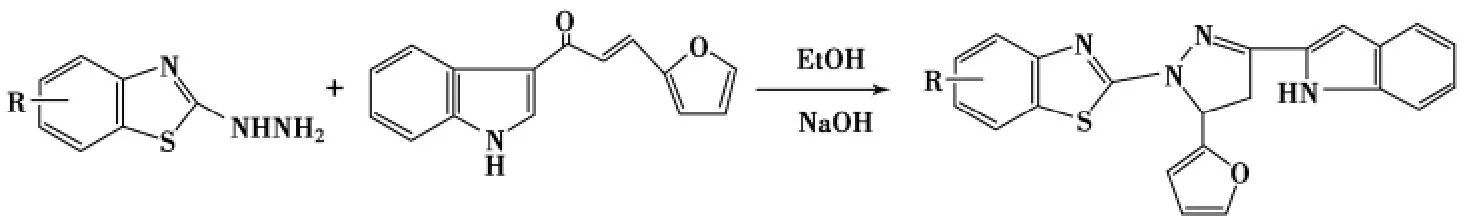
Scheme 8
同样条件下,用取代苯甲醛和3-吲哚基乙酮制得的烯酮与2-肼基苯并噻唑缩合环化可生成5-苯基-3-(3-吲哚基)-1-(2-苯并噻唑)-2-吡唑啉,产率46%~58%(Scheme 9)[52].
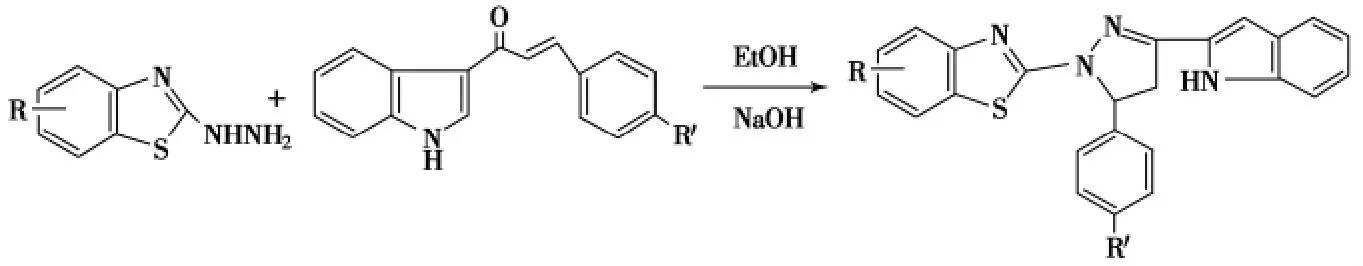
Scheme 9
将对氨基苄叉苯乙酮和2-肼基苯并噻唑或2-肼基苯并咪唑在乙二醇独乙醚溶液中回流,可得到3-苯环上带有-NH2的吡唑啉化合物[53].2-肼基苯并噻唑和烯酮在无水乙醇中,回流反应6~7h,可合成一系列2-吡唑啉,产率为51%~69%[54-55].同样条件下,2-肼基苯并噻唑和1-苯基-3-(3-噻吩基)-2-丙烯-1-酮或3-苯基-1-(2-噻吩基)-2-丙烯-1-酮回流反应6h,可合成1-(2-苯并噻唑)-3-苯基-5-(3-噻吩基)-2-吡唑啉(BPTP)和1-(2-苯并噻唑)-3-(2-噻吩基)-5-苯基-2-吡唑啉(BTPP),产率分别为62%和69%[56].总体来说,2-肼基苯并噻唑和烯酮在乙二醇独乙醚中比在无水乙醇或无水乙醇-氢氧化钠混合溶液中缩短一定的反应时间.
3 2-吡唑啉或1-乙酰基-2-吡唑啉的合成
将氯代芳基丙烯酮与肼在醋酸中回流反应3h,得到氯代3,5-二芳基-2-吡唑啉衍生物,产率为65%~93%[57].齐传民等将 -(对二甲氨基苯亚甲基)-环戊酮与肼在冰醋酸为溶剂的沸水浴中加热搅拌3h,然后常温放置14h,相应的双环吡唑啉的产率为61.5%(Scheme 10)[58].

Scheme 10
将肼逐滴加入1,3-二芳基-2-丙烯-1-酮的醋酸溶液然后回流8h,相应吡唑啉的产率为62%~82%[59-61].结果表明某些吡唑啉具有抑制单胺氧化酶等功能[59].3-位苯环上不含取代基的吡唑啉对P-糖蛋白有较好的亲和力[60].
1,3-二萘基-2-丙烯-1-酮与肼在90~100℃的无水醋酸中回流7h,得到3,5-二萘取代的-2-吡唑啉,产率为47%~85%(Scheme 11)[62].若改用微波辐射[63],相应吡唑啉的产率提高到82%~99%.
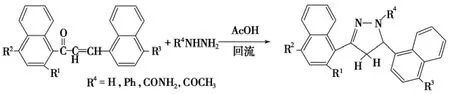
Scheme 11
β-萘基取代氨基查尔酮在少量冰醋酸的催化下与质量分数为90%的水合肼环化可生成1-乙酰基-3-(β-氨基萘)-5-取代芳基-2-吡唑啉,收率40%~50%[64].
将查尔酮溶解在乙醇中,逐滴加入肼,加热回流7h,吡唑啉的产率为44%~92%[65].Palaska等则用1,3-二苯基-2-丙烯-1-酮和肼直接在乙醇中回流4h后静置过夜,制得系列3,5-二苯基-2-吡唑啉,产率在75%以上.苯环3位甲氧基的引入使得吡唑啉有较好的抗沮丧活性[66].溶解在醋酸中的1,3-二芳基-2-丙烯-1-酮逐滴加入溶于乙醇的肼中,在120℃搅拌24h也可合成一系列取代的3,5-二苯基-2-吡唑啉,产率为52%~90%[67].
2004年,Rani等将1-吲哚基-3-芳基-2-丙烯-1-酮与质量分数为99%的水合肼混合在无水乙醇中,加入几滴冰醋酸回流6~7h,产物产率为25%~75%[68].
4 其他取代2-吡唑啉的合成
1,3-二芳基-2-丙烯-1-酮与肼在乙醇中回流1h可合成杀虫剂3,5-二苯基-1-苯氨羰基-2-吡唑啉(Scheme 12).吡唑啉环的5位被取代基取代时,不但能够提高其产率而且可以改善其杀虫活性[50].
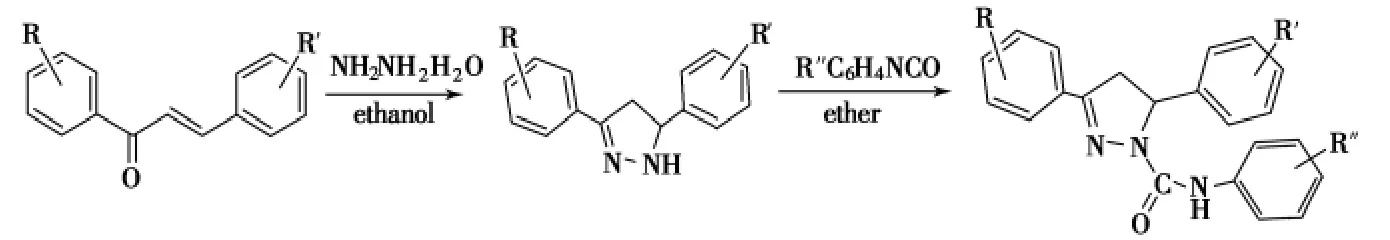
Scheme 12
将4-氯-2-苯基苯乙酮和甲醛反应后,与水合肼在丙醇中回流3h,可成功制得3-(4-氯苯基)-4-苯基吡唑啉,进而在无水乙醚中与4-氯苯基异氰酸酯缩合得到3-(4-氯苯基)-1-(4-氯苯基氨基甲酰基)-4-苯基-2-吡唑啉(Scheme 13).此类化合物可作为高效杀虫剂的先导化合物,用于对其他杀虫剂产生抗性的昆虫有很好的活性[70].
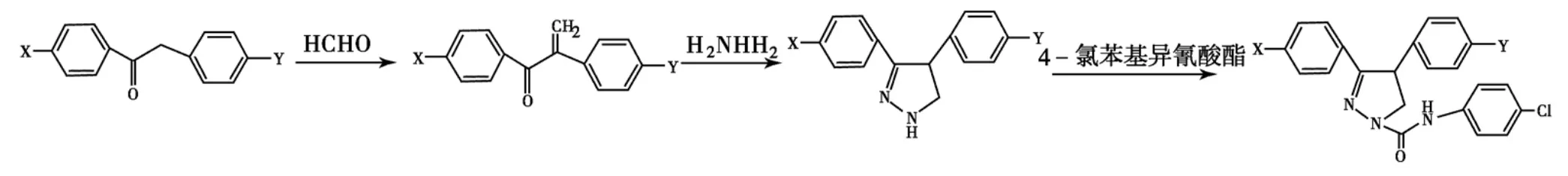
Scheme 13
2-肼基苯并咪唑和苄叉苯乙酮在乙二醇独乙醚溶液中回流3h,可以制得1-苯并咪唑基-3,5-苯基吡唑啉,产率72%,为偏绿光兰色荧光化合物.如果在吡唑啉环的1位引入的是苯并噻唑基,得到的是偏兰色的荧光化合物[71].
Budakoti等将1,3-二芳基-2-丙烯-1-酮、硫代氨基脲及氢氧化钠的混合物在乙醇中回流8h合成了1-硫代氨基甲酰基-3,5-二芳基-2-吡唑啉,产率8%~18%(Scheme 14)[72].而在同样条件下 Turan-Zitouni等可将产率提高到62%~82%[73].
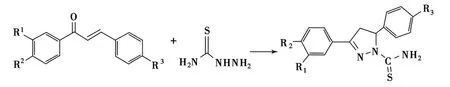
Scheme 14
在微波辐射下碱性氧化铝固载碳酸钾催化下,反应7~9min,1-氨基硫羰基-3,5-二苯基-2-吡唑啉的产率60%~85%[74].
3-苯甲叉基-6-氯苯并二氢吡喃-4-酮用无水乙醇溶解后加入苯肼盐酸盐,回流8h,放置过夜,得到苯并二氢吡喃并[4,3-c]-2-吡唑啉衍生物[75],均具有蓝色荧光性能.
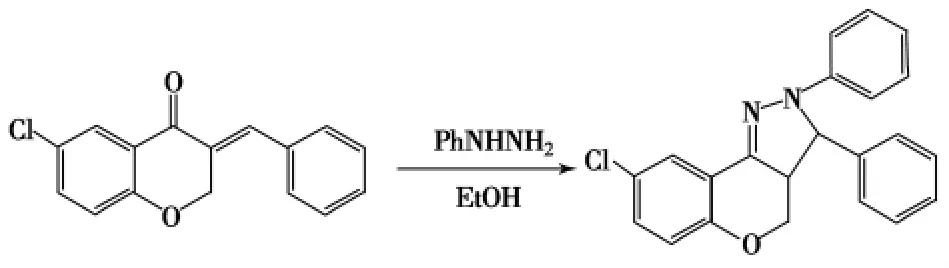
Scheme 15
Xie等[76]利用三氟甲磺酸催化,通过1,3偶极环加成合成了一系列取代吡唑啉,产率46%~91%(Scheme 16).
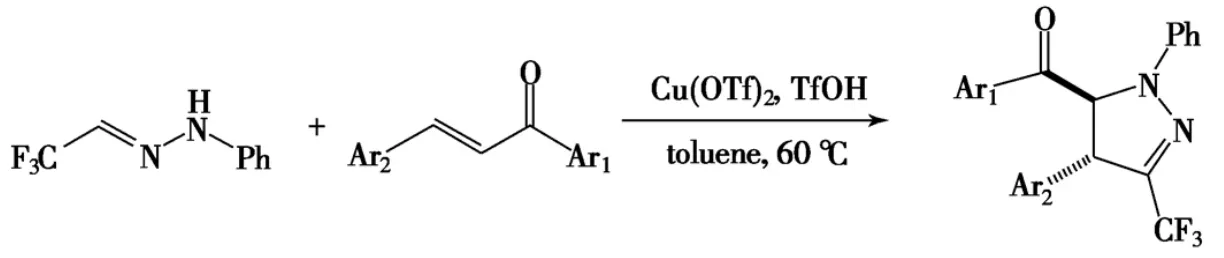
Scheme 16
Yar等在冰醋酸中合成了一系列1-酰基-2-吡唑啉,产率65%~90%[77],均具有较好的抗结核分枝杆菌H37Rv活性.在醋酸催化下,Yar等[78]将查尔酮与INH(异烟肼)在乙醇中反应8~14h,成功合成了1-位含有吡啶环的2-吡唑啉(Scheme 17),产率65%~94%,其中5-位苯环上带有卤素的产物抗结核分枝杆菌H37Rv活性比较好.
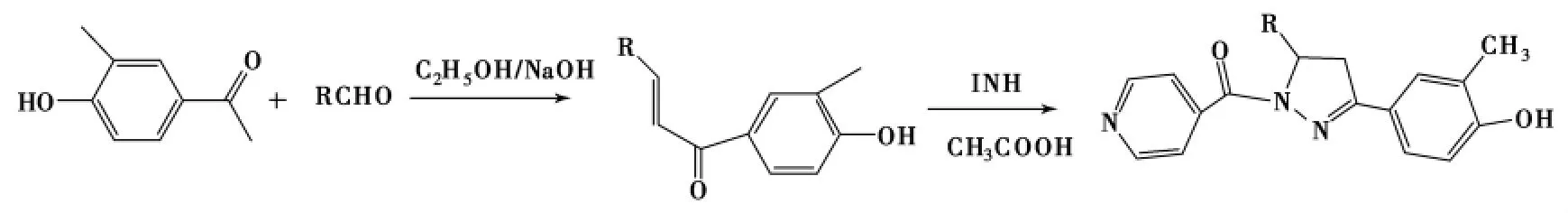
Scheme 17
2007年,Bai等[79]改用盐酸催化将查尔酮溶解于乙二醇独乙醚后加入苯肼,室温或加热搅拌2~24h,合成了4种典型的荧光染料化合物:1,3-二苯基-5-(4-氯苯基)-2-吡唑啉 (DCP),1,5-二苯基-3-联苯基-2-吡唑啉 (DBP),1,5-二苯基-3-(N-乙基咔唑-3-基)-2-吡唑啉(DEP),和1-苯基-3-(N-乙基咔唑-3-乙烯基)-5-(N-乙基咔唑-6-基)-2-吡唑啉(PEEP),产率40%~80%.
将查尔酮和水合肼在70℃的无水乙醇中先制得2-吡唑啉,然后用聚合物固载的碱处理可进一步合成多种N-取代的2-吡唑啉衍生物,产率65%~89%[80].另外,2-吡唑啉类化合物还可以由Mannich碱与氨基硫脲环化、二溴代查尔酮与芳氧基酰肼等[81-83]的方法来合成.
2-吡唑啉在杂环化学和有机化学中均占有重要的地位,因此此类化合物的合成研究对寻找新型抗菌剂和杀虫药物具有重要的理论意义及应用价值.寻求高效、廉价的催化体系和环境友好的合成方法仍将是有机合成工作者研究的重点.从满足环境的要求和降低有机转换的成本观点出发,将高聚物固载催化剂以及超声波和微波应用于2-吡唑啉衍生物的合成更具有前景.
[1]KHALI H Z,YANNI S A.Synthesis of new anilido-pyrazoline and isoxazoline derivatives[J].J Indian Chem Soc,1981,58(2):168-170.
[2]RAWAL A A,THAKOR V M,SHAH N M.Synthesis of some 1,3,5-triphenylpyrazolines and 3,5-diphenyl-cyclohexen-1-ones[J].J Indian Chem Soc,1963,40(4):323-326.
[3]BHATIA M S,SOOD R K.New heterocyclic ring containing phosphorus:synthesis of 4-chloro-1,4-dihydro-2h-naphth-[2,1-c][1,2]oxaphosphorin[J].Indian J Chem,1978,16B:638-645.
[4]MISHRIKY N,ASAAD F M,IBRAHIM Y A,et al.New 2-pyrazolines of anticipated molluscicidal activity[J].Pharmazie,1996,51:544-548.
[5]PROVOST P,MERHI Y.A dual lipoxygenase/cyclooxygenase inhibitor reduces mural platelet and neutrophil deposition and vasoconstriction after angioplasty injury in pigs[J].J Pharmacol Exp Ther,1996,277(1):17-21.
[6]TURAN-ZITOUNI G,CHEVALLET P,KILIC F S,et al.Synthesis of some thiazolyl-pyrazoline derivatives and preliminary investigation of their hypotensive activity[J].Eur J Med Chem,2000,35(6):635-641.
[7]HUSAIN M I,SHUKLA S.Synthesis and biological activity of 4-(3-aryl-4-exo-2-thioxothia-zolidin-5-ylimino)-3-methyl-1- (N,N-disubstituted amino-methyl)pyrazolin-5-ones[J].Ind J Chem,1986,25B:983-985.
[8]KRISHNA R,PANDE B R,BHARTHWAL S P,et al.Antiinflammatory and antiproteolytic properties of 1,3-disubstituted-5-(2-arylindol-3-yl)-DELTA 2-pyrazolines[J].Eur J Med Chem,1980,15(6):567-569.
[9]李光才.吡唑啉类荧光增白剂的合成研究[J].印染助剂,1999,16(4):11-14.
LI Guangcai.A study on synthesis of fluorescent bleaching agents of pyrazoline[J].Textile Auxiliaries,1999,16(4):11-14.
[10]BROSENBERGER P M,SCHEIN L B.Hole transport in 1-phenyl-3-((diethylamino)styryl)-5-(p-(diethylamino)pheyl)pyrazoline-doped[J].Polymers J Phys Chem,1994,98(1):233-239.
[11]RAIFORD L C,PETERSON W J.Identification of phenylhydrazones and isomeric pyrazolines obtained from chalcones[J].J Org Chem,1936,4:545-551.
[12]SACHCHAR S P,SINGH A K.Synthesis of some new fluorinated heteroaryl pyrazolines and isooxazolines as potential biocidal agents[J].J Indian Chem Soc,1985,LXII:142-146.
[13]PALASKA E,EROL D,DEMIRDAMAR R.Synthesis and antidepressant activities of some 1,3,5-triphenyl-2-pyrazolines[J].Eur J Chem,1996,31(1):43-47.
[15]OZDEMIR Z,KANDILCI H B,GUMUSEL B,et al.Synthesis and studies on antidepressant and anticonvulsant activities of some 3-(2-furyl)-pyrazoline derivatives[J].Eur J Med Chem,2007,42(3):373-379.
[16]王炳祥,刘玮炜,胡宏纹.1,3,5-三芳基吡唑类衍生物合成的新方法[J].高等学校化学学报,2003,24(4):648-650.
WANG Bingxiang,LIU Weiwei,HU Hongwen.A novel method for the synthesis of 1,3,5-triarylpyrazoles[J].Chemical Journal of Chinese University,2003,24(4):648-650.
[17]朴文香,姜日善.1,3,5-三苯基-2-吡唑啉衍生物的合成[J].延边大学学报:自然科学版,2009,35(2):160-163.
PIAO Wenxiang,JIANG Rishan.Synthesis of 1,3,5-triphenyl-2-pyrazoline[J].Journal of Yanbian University:Natural Science Edition,2009,35(2):160-163.
[18]马引民,李仲杰.芳呋喃吡唑啉的合成和波谱研究[J].高等学校化学学报,1990,11(9):967-971.
MA Yinmin,LI Zhongjie.Studies on the synthesis and spectra of arylfurylpyrazolines[J].Chemical Journal of Chinese University,1990,11(9):967-971.
[19]黎芳,解正峰,刘方明.5-(2-苯基-1,2,3-三唑基)-3-芳基吡唑啉衍生物的合成及其荧光性能[J].高等学校化学学报,2006,27(6):1058-1061.
LI Fang,XIE Zhengfeng,LIU Fangming.Syntheses and fluorescent property of 5-(2-phenyl-1,2,3-triazoly)-3-arylpyrazoline derivatives[J].Chemical Journal of Chinese University,2006,27(6):1058-1061.
[20]徐峰,刘方明,徐瑜,等.3-(2-苯基-1,2,3-三唑基-4-基)-5-芳基-4,4-二氢吡唑啉衍生物的合成[J].杭州师范学院学报:自然科学版 ,2007,6(1):40-50.
XU Feng ,LIU Fangming,XU Yu,et al.Syntheses of 3-(2-phenyl-1,2,3-triazole-4-yl)-5-aryl-4,4-dihydropyrazoline derivatives[J].Journal of Hangzhou Normal University:Natural Science Edition,2007,6(1):40-50.
[21]雷光东,卢志云,谢明贵.一种蓝色荧光材料的合成[J].化学研究与应用,2006,18(3):240-244.
LEI Guangdong,LU Zhiyun,XIE Minggui.Synthesis of a blue fluorescentmaterial[J].Chemical Research and Application,2006,18(3):240-244.
[22]雷光东,卢志云,朱卫国,等.有机荧光防伪材料的制备化学[J].化学研究与应用,1999,11(3):308-311.
LEI Guangdong,LU Zhiyun,ZHU Weiguo,et al.Preparaton of organic fluorescent materials for security stickers[J].Chemical Research and Application,1999,11(3):308-311.
[23]韩秀英,林培华,田美令,等.1,3-二苯基-5-(4-氯苯基)-2-吡唑啉的合成[J].山西大学学报:自然科学版,2003,26(4):327-329.
HAN Xiuying,LIN Peihua,TIAN Meiling,et al.Synthesis and application of 1,3-diphenyl-5-(4-chlorophenyl)-2-pyrazoline[J].Journal of Shanxi University:Naturnal Science Edition,2003,26(4):327-329.
[24]张耀谋,付方平,张志祥,等.1,3,5-三芳基-2-吡唑啉类化合物的合成及其光活化除草活性[J].农药学学报,2004,6(4):89-92.
ZHANG Yaomou,FU Fangping,ZHANG Zhixiang,et al.Synthesis and light-activated herbicidal activities of 1,3,5-tr iaryl-2-pyrazoline[J].Chinese Journal of Pesticide Science,2004,6(4):89-92.
[25]POWERS D G,CASEBIER D S,FOLAS D,et al.Automated parallel synthesis of chalcone-based screening libraries[J].Tetrahedron,1998(16),54:4085-4096.
[26]PRASAD Y R,RAO A L,MURALI K,et al.Synthesis and antidepressant activity of some 1,3,5-tripheyl-2-pyrazolines and 3-(2-hydroxynaphtahalen-1-yl)-1,5-diphenyl-2- pyrazolines[J].Bioorg Med Chem Lett,2005,15(22):5030-5034.
[27]钱峰,房建华,金伟,等.吡唑啉类化合物的合成及其光电导性能的研究[J].化学世界,1994(11):575-578.
QIAN Feng,FANG Jianhua,JIN Wei,et al.The synthesis of pyrazolines and study of photoconductivity[J].Chemical World,1994(11):575-578.
[28]EL-RAYYES N,AL-JOHARY A.synthesis of new pyrazolines[J].J Chem Eng Data,1985,30:500-502.
[29]杨青.1,3,5-三芳基-2-吡唑啉的合成[J].化学试剂,2001,23(1):48-54.
YANG Qing.Synthesis and structure of 1,3,5-triaryl-2-pyrazoline[J].Chemical Reagent,2001,23(1):48-54.
[30]刘志杰,张建恒,姜林.1-苯基-3-(3’-吲哚基)-5-取代苯基-2-吡唑啉的合成及 H'NMR和 MS中的取代基效应[J].高等学校化学学报,1991,12(1):39-43.
LIU Zhijie,ZHANG Jianheng,JIANG Lin.The synthesis of 1-phenyl-3-(3’-indolyl)-5-substituted phenyl-2-pyrazolines and their substituent effects in1H NMR,MS[J].Chemical Journal of Chinese University,1991,12(1):39-43.
[31] 王忠义,史海健,史好新.1,5-二芳基-3,4-二取代吡唑啉的合成[J].合成化学,1995,3(3):226-230.
WANG Zhongyi,SHI Haijian,SHI Haoxin.Study on synthesis of 1,5-diary1-3,4-disubstituted pyrazoline[J].Chinese Journal of Synthetic Chemistry S C,1995,3(3):226-230.
[32]刘天麟,谢建华,余盛良.具有生物活性的吡唑啉衍生物的合成[J].南开大学学报:自然科学,1999,32(1):101-104.
LIU Tianlin,XIE Jianhua,YU Shengliang.Synthesis of bioactive dihydropyrazole derivatives[J].Journal of Nankai U-niversity:Natural Science Edition,1999,32(1):101-104.
[33]刘天麟,谢建华,杨卓鸿.α-三唑基查尔酮与苯肼的加成-关环反应研究[J].有机化学,2000,20(6):900-904.
LIU Tianlin,XIE Jianhua,YANG Zhuohong.Study on the addition-cyclization ofα-triazolyl chalcone and phenylhydrazine[J].Chinese Journal of Organic Chemistry,2000,20(6):900-904.
[34]耿亮,雷鸣,王彦广.异噁唑基取代的1,2,4-噁二唑啉和吡唑啉类化合物的合成[J].有机化学,2005,25(6):690-695.
GENG Liang,LEI Ming,WANG Yanguang.Synthesis of isoxazolyl substituted 1,2,4-oxadiazolines and pyrazolines[J].Chinese Journal of Organic Chemistry,2005,25(6):690-695.
[35]李红亚,刘卉闵,吴国江,等.微波辐射下β-芳氧丙酸和色满酮的合成[J].河北大学学报:自然科学版,2008,28(4):399-402.
LI Hongya,LIU Huimin,WU Guojiang.Synthesis ofβ-aryloxypropionic acids and chroman-4-ones under microwave irradiation[J].Journal of Hebei University:Natural Science Edition,2008,28(4),399-402.
[36]刘秀英,郄录江,马志广,等.微波辐射无溶剂合成(E)-肉桂酸[J].河北大学学报:自然科学版,2001,21(4):389-390.
LIU Xiuying,QIE Lujiang,MA Zhiguang.(E)-Cinnamic acids synthesized in solvent free system under microwave irradiation[J].Journal of Hebei University:Natural Science Edition,2001,21(4):389-390.
[37]KIDWAI M,MISRA P.Ring closure reaction of chalcones using microwave technology[J].Synth Commun,1999,29(18):3237-3250.
[38]KIDWAI M,KUKREJA S,THAKUR R.K2CO3-mediated regioselective synthesis of isoxazoles and pyrazolines[J].Lett Org Chem,2006,3(1):135-139.
[39]廖勇,黄文海,胡永洲,等.微波辅助合成1,3-二苯基-2-吡唑啉[J].有机化学,2008,28(5):885-888.
LIAO Yong,HUANG Wenhai,HU Yongzhou,et al.Microwave-assisted synthesis of 1,3-diphenyl-2-pyrazolines[J].Chin J Org Chem,2008,28(5):885-888.
[40]LI Jitai,ZHANG Xiaohui,LIN Zhiping.An improved synthesis of 1,3,5-triaryl-2-pyrazolines in acetic acid aqueous solution under ultrasound irradiation[J].Beilstein J Org Chem,2007,3(1):13-16.
[41]LIN Zhiping,LI Jitai.An convenient and efficient protocol for the synthesis of 1,3,5-triaryl-2-pyrazolines in acetic acid under ultrasound[J].E J Chem,2012,9(1):267-271.
[42]LI Jitai,ZHAI Xinli,MENG Xiantao.Synthesis of 1,5-diaryl-3-arylethenyl-2- pyrazolines under ultrasound irradiation[J].Asian J Chem,2010,22(1):589-592.
[43]冼远芳,李东风,吴臣.1-(6-溴代)苯并噻唑基-3,5-二苯基吡唑啉衍生物的合成[J].吉林工学院学报:自然科学版,1996,17(2):37-42.
XIAN Yuanfang,LI Dongfeng,WU Chen.Synthesis of 1-(6-bromo)benzothiazoy1-3,5-biphenyl pyrazoline type compounds[J].Journal of Jihin Institute Technology:Natural Science Edition,1996,17(2):37-42.
[44]冼远芳,李东风,敖玉辉,等.1-苯并噻唑基-吡唑啉类荧光化合物的合成及其荧光光谱[J].光谱实验室,2004,21(3):457-460.
XIAN Yuanfang,LI Dongfeng,AO Yuhu,et al.Synthesis of benzothiazolyl-pyrazoline compound and their fluorescence property[J].Chinese Journal of Spectroscopy Laboratory,2004,21(3):457-460.
[45]冼远芳,李东风,李海东,等.吡唑啉类新型荧光化合物的合成及其红外光谱和荧光性能[J].光谱学与光谱分析,1998,118(15):543-546.
XIAN Yuanfang,LI Dongfeng,LI Haidong,et al.Synthesis of new pyrazoline fluorescent compounds and their IR spectra and fluorescence property[J].Spectroscopy and Spectral Analysis,1998,118(15):543-546.
[46]冼远芳,李东风,李长海,等.1-苯并噻唑基-3,5-二苯基吡唑啉衍生物的合成与荧光性[J].染料与染色,2003,40(6):314-318.
XIAN Yuanfang,LI Dongfeng,LI Changhai,et al.Synthesis of 1-benzothiazolyl-3,5-biphenyl pyrazolines and their fluorescence property[J].Dyestuffs and Coloration,2003,40(6):314-318.
[47]李东风,吴臣,于兵兵.苯并噻唑基吡唑啉荧光化合物研究进展[J].吉林工学院学报:自然科学版,1995,16(1):37-41.LI
Dongfeng,WU Chen,YU Bingbing.The present status of bezothiazolyl pyrazoline fluorescent compounds[J].Journal of Jilin Institute Technology:Natural Science Edition,1995,16(1):37-41.
[48]冼远芳,李东风,敖玉辉,等.荧光化合物苯并噻唑吡唑啉的合成[J].吉林化工学院学报:自然科学版,2003,20(4),51-53.
XIAN Yuanfang,LI Dongfeng,AO Yuhu,et al.Synthesis of benzothiazoyl-pyrazoline and its fluorescence property[J].Journal of Jilin Institute of Chemical Technology,2003,20(4):51-53.
[49]李东风,冼远芳,薛冬桦,等.1-(2-苯并噻唑基)-3,5-二芳基吡唑啉的合成与荧光性能[J].长春工业大学学报:自然科学版,2002,23(1):115-117.
LI Dongfeng,XIAN Yuanfang,XUE Donghua,et al.Synthesis and fluorescent property of 1-(2-benzothiazoly)-3,5-biarylpyrazoline compounds[J].Journal of Changchun University Techndogy:Natural Science Edition,2002,23(1):115-117.
[50]王进敏,李东风,王芳,等.5-呋喃基-1-苯并噻唑基吡唑啉类荧光化合物的合成及光谱分析[J].光谱学与光谱分析,2008,28(3):629-632.
WANG Jinmin,LI Dongfeng,WANG Fang,et al.Synthesis and spectrum of some 5-furanyl-1-benzothiazoyl pyrazoline compounds with fluorescence[J].Spectroscopy and Spectral Analysis,2008,28(3):629-632.
[51]刘秋君,高磊,李栋,等.新型吡唑啉类荧光增白剂的合成及性能研究[J].应用科技,2009,36(1),1-3.
LIU Qiujun,GAO Lei,LI Dong,et al.Synthesis and property study of novel pyrazoline fluorescent whitening agents[J].Application Science and Technology,2009,36(1),1-3.
[52]刘秋君,高磊,王雷,等.新型吡唑啉类荧光化合物的合成及光谱分析[J].光谱学与光谱分析,2009,29(10):2810-2814.
LIU Qiujun,GAO Lei,WANG Lei,et al.Synthesis and spectrum of novel pyrazoline fluorescent compounds[J].Spectroscopy and Spectral Analysis,2009,29(10):2810-2814.
[53]冼远芳,李东风,王宇明.新型蓝光吡唑啉荧光化合物的合成与红外光谱研究[J].光谱学与光谱分析,2008,28(7):1617-1620.
XIAN Yuanfang,LI Dongfeng,WANG Yuming.Synthesis of new blue pyrazoline fluorescent compounds and study of infrared spectroscopy[J].Spectroscopy and Spectral Analysis,2008,28(7):1617-1620.
[54]JI Shunjun,SHI Haibin.Synthesis and fluorescent property of some novel benzothiazoyl pyrazoline derivatives containing aromatic heterocycle[J].Dye Pigments,2006,70(3):246-250.
[55] BING Bian,JI Shunjun,SHI Haibin.Synthesis and fluorescent property of some novel bischromophore compounds containing pyrazoline and naphthalimide groups[J].Dye Pigments,2008,76(2):348-352.
[56]SHI Haibin,JI Shunjun,ZHANG Yong.Syntheses and crystal structures of pyrazoline derivants[J].Chin J Struct Chem,2005,24(5):586-590.
[57]LEVAI A.Synthesis of chlorinated 3,5-diaryl-2-pyrazolines by the reaction of chlorochalcones with hydrazines,ARKIVOC,2005(ix):344-352.
[58]齐传民,李玉兰.二环吡唑啉衍生物的合成及其抗炎活性[J].中国药物化学杂志,1997,7(2):88-92.
QI Chuanmin,LI Yulan.Synthesis and antiinflammatory activity of bicyclic pyrazoline derivatives[J].Chinese Journal of Medicinal Chemistry,1997,7(2):88-92.
[59]MANNA F,CHIMENTI F,BOLASCO A,et al.Inhibition of amine oxidases activity by 1-acetyl-3,5-diphenyl-4,5-dihydro-(1H)-pyrazole derivatives[J].Bioorg Med Chem Lett,2002,12(24):3629-3633.
[60]徐助雄,吴婧,沈健,等.1,5-二芳基-3-(2-羟基-4,6-二甲氧基苯基)-2-吡唑啉的合成及铜离子荧光探针行为[J].高等学校化学学报,2010,31(8):1570-1573.
XU Zhuxiong,WU Jing,SHEN Jian,et al.Synthesis of 1,5-diaryl-3-(2-hydroxyl-4,6-dimethoxylphenyl)-2-pyrazolines as fluorescent sensors for Cu2+[J].Chemical Journal of Chinese Universify,2010,31(8):1570-1573.
[61]杨安博,徐助雄,吴婧,等.1-乙酰基-3-(2-羟基-4,6-二甲氧基苯基)-5-苯基-2-吡唑啉的合成及锌离子探针的研究[J].高等学校化学学报,2010,31(7):1365-1368.
YANG Anbo,XU Zhuxiong,WU Jing,et al.Synthesis of 1-acetyl-3-(2-hydroxyl-4,6-dimethoxylphenyl)-5-phenyl-2-pyrazoline and studies on its zinc ion probe[J].Chemical Journal of Chinese Universify,2010,31(7):1365-1368.
[62]AZARIFAR D,SHAEBANZADEH M.Synthesis and characterization of new 3,5-dinaphthyl substituted 2-pyrazolines and study of their antimicrobial activity[J].Molecules,2002,7:885-895.
[63]AZARIFAR D,GHASEMNEJAD H.Microwave-assisted synthesis of some 3,5-arylated 2-pyrazolines[J].Molecules,2003,8:642-648.
[64]BANSAL E,SRIVASTAVA V K,KUMAR A.Synthesis and anti-inflammatory activity of 1-acetyl-5-substitutedaryl-3-(-aminonaphthyl)-2-pyrazolines and-(substituted aminoethyl)amidonaphthalenes[J].Eur J Med Chem,2001,36(1):81-92.
[65]ALI M A,SHAHARYAR M,SIDDIQUI A A.Synthesis,structural activity relationship and anti-tubercular activity of novel pyrazoline derivatives[J].Eur J Med Chem,2007,42(2):268-275.
[66]PALASKA E,AYTEMIR M,UZBAY I T,et al.Synthesis and antidepressant activities of some 3,5-diphenyl-2-pyrazolines[J].Eur J Med Chem,2001,36(6):539-543.
[67]CHIMENTI F,BIZZARRI B,MANNA F,et al.Synthesis and in vitro selective anti-Helicobacter pylori activity of pyrazoline derivatives[J].Bioorg Med Chem Lett,2005,15(3):603-607.
[68]RANI P,SRIVASTAVA V K,DUMAR A.Synthesis and antiinflammatory activity of heterocyclic indole derivatives[J].Eur J Med Chem,2004,39(5):449-452.
[69]HES RV,WELLINGA K,GROSSCURT A C.1-Phenylcarbamoyl-2-pyrazolines:a new class of insecticides.2.Synthesis and insecticidal properties of 3,5-dipheyl-1-pheylcarbamoyl-2-pyrazolines[J].Agric Food Chem,1978,26(4):915-918.
[70]李玉新.吡唑啉—一类新型高效杀虫剂[J].湖南化工,1997,27(3):8-12.
LI Yuxin.Pyrazoline-a new type of highly active insecticide[J].Hunan Chemical Industry,1997,27(3):8-12.
[71]冼远芳,李东风,李海东,等.吡唑林荧光化合物的合成与红外光谱研究[J].光谱学与光谱分析,2005,25(3):391-394.
XIAN Yuanfang,LI Dongfeng,LI Haidong,et al.Synthesis of byrazoline fluorescent compounds and studies by infrared spectroscopy[J].Spectroscopy and Spectral Analysis,2005,25(3):391-394.
[72]BUDAKOTI A,ABID M,AZAM A.Synthesis and antiamoebic activity of new 1-N-substituted thiocarbamoyl-3,5-diphenyl-2-pyrazoline derivatives and their Pd(Ⅱ)complexes[J].Eur J Med Chem,2006,41(1):63-70.
[73]TURAN-ZITOUNI G,CHEVALLET P,KILIC F S,et al.Synthesis of some thiazolyl-pyrazoline derivatives and preliminary investigation of their hypotensive activity[J].Eur J Med Chem,2000,35(6):635-641.
[74]PATEL V M,DESAI K R.Eco-friendly synthesis of fluorine-containing pyrazoline derivatives over potassium carbonate[J].ARKIVOC,2004(i):123-129.
[75]金慧娟,刘文博.2,3-二苯基苯并二氢吡喃并[4,3-c]-2-吡唑啉衍生物的合成及光学性质研究[J].化学试剂,2009,31(1):11-14.
JIN Huijuan,LIU Wenbo.Synthesis and fluorescent properties of 2,3-diphenylchromano[4,3-c]-2-pyrazoline derivatives[J].Chemcal Reagent,2009,31(1):11-14.
[76]XIE Haibo,ZHU Jiangtao,CHEN Zixian,et al.Reaction of a trifluoromethylatedN-monosubstituted hydrazone withα,β-ethenyl ketones:a novel synthesis of substituted pyrazolidines and pyrazolines[J].Synthesis,2011,(17):2767-2774.
[77]YAR M S,SIDDIQUI A A,ALI M A.Synthesis and evaluation of phenoxy acetic acid derivatives as a anti-mycobacterial agents[J].Bioorg Med Chem Lett,2006,16(17):4571-4574.
[78]YAR M S,SIDDIQUI A A,ALI M A,et al.Synthesis and in vitro antimycobacterial activity ofN'-nicotinoyl-3-(4’-hydroxy-3’-methylphenyl)-5-[(sub)phenyl]-2-pyrazolines[J].Bioorg Med Chem Lett,2006,16(15):3947-3949.
[79]BAI Guan,LI Junfen,LI Duxin,et al.Synthesis and spectrum characteristic of four new organic fluorescent dyes of pyrazoline compounds[J].Dyes and Pigments,2007,75(1):93-98.
[80]BAUER U,EGNER B J,NILSSON I,et al.Parallel solution phase synthesis ofN-substituted 2-pyrazoline libraries[J].Tetrahedron Lett,2000,41(15):2713-2717.
[81]ABID M,AZAM A.Synthesis,characterization and antiamoebic activity of 1-(thiazolo[4,5-b]quinoxaline-2-yl)-3-phenyl-2-pyrazoline derivatives[J].Bioorg Med Chem Lett,2006,16(10):2812-2816.
[82]BID M,AZAM A.Synthesis and antiamoebic activities of 1-N-substituted cyclised pyrazoline analogues of thiosemicarbazones[J].Bioorg Med Chem,2005,13(6):2213-2220.
[83]KARTHIKEYAN M S,HOLLA B S,KUMARI N S.Synthesis and antimicrobial studies on novel chloro-fluorine containing hydroxy pyrazolines[J].Eur J Med Chem,2007,42(1):30-36.
Progress in synthesis of 2-pyrazoline derivatives
LIN Zhi-ping,QIAO Feng-xia
(Department of Biology and Chemistry,Baoding University,Baoding 071000,China)
2-Pyrazoline compounds are extremely important aza-pentacyclic compounds in organic synthesis and other field.The progress in synthesis of pyrazoline derivatives with different substituents was reviewed in recent years.
2-pyrazoline;organic synthesis;review
O643.32
A
1000-1565(2012)03-0326-11
2012-02-01
河北省教育厅资助项目(Z2010101);保定学院资助项目(2009002)
蔺志平(1976-),女,河北灵寿人,保定学院讲师,理学博士,主要从事有机合成化学研究.
E-mail:linzhiping888999@126.com
梁俊红)
