Celastrol Inhibits Hypoxia-Inducible Factor-1αActivation in Hepatocellular Carcinoma Cells
2011-01-15LeeJungJoon
Lee Jung Joon
(Center for Molecular Cancer Research,Korea Research Institute of B ioscience and Biotechnology,Chungbuk 363-883,Korea)
Celastrol Inhibits Hypoxia-Inducible Factor-1αActivation in Hepatocellular Carcinoma Cells
Lee Jung Joon
(Center for Molecular Cancer Research,Korea Research Institute of B ioscience and Biotechnology,Chungbuk 363-883,Korea)
To investigate the effects of celastrol on activation of H IF-1αin hepatocellular carcinoma cells,exp lore the reasonable strategy of chemical hypoxia induced by CoCl2,through an HRE-luciferase cell based screen,we examined whether celastrol inhibited H IF-1αactivation.Furthermore,the effects of celastrol on exp ression level of H IF-1αtarget gene for vascular endothelial gro wth factor(VEGF)and the DNA-binding activity of H IF-1αwere also detected by Western blot,RT-PCR and EMSA respectively.The results sho wthat celastrol p revented exp ression of H IF-1αp rotein,target gene fo r VEGF and DNA-binding activity of H IF-1α upon CoCl2stimulation.Our data suggested that celastrolmay exhibit anticancer activity by inhibiting HIF-1α.
celastrol;H IF-1α;anticancer
1 Introduction
In hypoxia,several transcrip tion factors were induced to respond to the decreased oxygen level.Among these transcrip tion facto rs,hypoxia-inducible factor-1α(H IF-1α)is one of the most important factors that p lay a critical role in controlling oxygen delivery and metabolic adaptation to hypoxic conditions.H IF-1 is a heterodimer composed of H IF-1α and H IF-1β subunits, which are basic helix-loop-helix-PAS domain p roteins,only H IF-1αis regulated by the oxygen tension[1-2].The regulation of H IF-1αoccursat posttranslational level involvingmodifications of hydroxylation,acetylation and phosphorylation[3-6]. In no rmoxic conditions,specific
H IF-1αp rolyl-hydroxylase hydroxylates two p roline residues(p402 and p564) within the oxygen-dependent degradation domain of H IF-1α,resulting in H IF-1αrecognition by von Hippel-Lindau(V HL)ubiquitin ligase comp lex that targets H IF-1αfor degradation through 26Sp roteasome pathway[5-6]. Similarly,an asparaginyl hydroxylase termed as factor inhibiting H IF-1α hydroxylates the asparagines residue 803 within the transactivation domain of H IF-1α,abolishing its interaction with the transcrip tional co-activator p300/CBP[7].In response to physiological hypoxia,H IF-1αbecomes rapidly stabilized and is localized to the nucleus, where it binds to H IF-1βto fo rm the H IF-1 comp lex.H IF-1 specifically binds to a sho rt DNA sequence,5′-ACGTG-3′,kno wn as the hypoxia responsive element(HRE) within target genes.Upon binding,H IF-1 recruits the coactivato r cAMP-responsive element binding p rotein(CREB)-binding p rotein/p300 and various other p roteins to activate transcrip tion[8-9].
H IF-1 p lays a central role in tumo r p rogression and angiogenesis in vivo.Oncogenic activation(e.g.,Ha-ras,m yc,o r src)o r loss of tumor supp ressor function(e.g.,p53,PTEN,or VHL)is associated with H IF-1-mediated tumor p rogression[10].Exposure to a variety of gro wth facto rs has also been sho wn to increase H IF-1 activity in normoxic and hypoxic conditions.H IF-1αis overexp ressed in many human cancers and has been associated with tumo r aggressiveness,vascularity,treatment failure,and mo rtality[11-12].In addition,tumor gro wth and angiogenesis in xenograft tumo rs also depends on H IF-1 activity and the exp ression level of H IF-1α[13].A ll of these activitiesmake the H IF-1 transcrip tion factor an attractive target for the development of new anticancer therapeutics[2,12,14].
In our search fo r inhibito rs of H IF-1αactivation from natural p roducts,we identified celastrol,a quinone methide triterpenoid as a major component of active p rincip les in the methanol extract of the roots ofCelastrusorbiculatus, which has been effectively used in the treatment of autoimm une diseases,asthma,chronic inflammation,and neurodegenerative disease[15-18].U nder in vitro conditions,celastrol was found to inhibit cancer cell p roliferation and induce leukemic cell death.However,themechanism s responsible for antitumor activity of celastrol are largely unkno wn.In this study,we showed that celastrol inhibited hypoxia-induced H IF-1α activation. This compound rapidly do wn-regulates H IF-1αp rotein levels,and the exp ression of H IF-1αtarget gene such as vascular endothelial gro wth factor(VEGF), which is essential fo r tumo r gro wth.We also demonstrated that celastrol inhibited DNA-binding activity of H IF-1α.
2 Materialsand Methods
2.1 Cell Culture and Chem icals
Human hepatocellular carcinoma Hep3B cells were maintained in Dulbecco’s Modified Essential Medium (Invitrogen Co rpo ration,Carlsbad,CA,USA).This media was supp lemented with penicillin(100 units/mL)-strep tomycin(100 units/mL)(Invitrogen Corpo ration,Carlsbad,CA,USA)and 10%heat-inactivated fetal bovine serum (Hyclone,Logan,U T,USA).Celastrol was iso lated from dried root ofCelastrusorbiculatus.The purity of celastrol is more than 98%in HPLC analysis.
2.2 Transfection and Luciferase Reporter Assay
The ability of the compound to inhibit hypoxia inducible factor was determined by HRE-dependent reporter assay as p reviously described.In brief,at 50%—80%confluence,Hep3B cells, which were cotransfected with the vectors for p GL 3-HRE-Luciferase p lasmid containing six copies of HREs derived from the human VEGF gene and with pRL-CMV(Promega,Madison,W I,USA)using Lipofectamine p lus reagent(Invitrogen).Follo wing 48 h incubation,the cells were treated with various concentrations of celastrol and incubated for 12 h upon CoCl2stimulation.Luciferase assay was performed using Dual-luciferase reporter assay system acco rding to the instructionsof themanufacturer(Promega).Luciferase activity was determ ined in Microlumat p lus lum inometer(EG&G Berthold,Bad Wildbad,Germany)by injecting 100μL of assay buffer containing luciferin and measuring light emission for 10 seconds.The results were no rmalized to the activity ofrenillaexp ressed by cotransfected Rluc gene under the control of a constitutive p romo ter.Data were analyzed using ANOVA(Analysis of variance).
2.3 Measuremen t of Cell Viability
Cell viability was measured by a MTT[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay (Sigma-A ldrich).Briefly,untreated cells or treated cells with celastrol in a 96-well p late were incubated for 24 h follo wed by the addition of MTT to the cells.Op tical densities were determined on a microp late reader(Mo lecular Devices,Sunnyvale,CA,USA).
2.4 Western Blot Analysis
H IF-1αp rotein was analyzed in nuclear extracts p repared from cells using NE-PER reagent(Pierce,Rockfo rd,IL,USA),according to the instructionsof manufacturer.An aliquot of p rotein extracts was used to determine p rotein concentration by the Bradfo rd method.Thirtyμg of nuclear extract p rotein per lane was separated by SDS-polyacrylamide gels and follo wed by transferring to a polyvinylidene difluoride membrane(Millipo re,Bedfo rd,MA,USA).The membrane was blocked with 5%skim milk,and then incubated with the co rresponding antibody.Antibodies for H IF-1αand Topo-I was obtained from Santa Cruz Biotechnology(Santa Cruz,CA,USA).After binding of an app rop riate secondary antibody coup led to ho rseradish peroxidase,p roteins were visualized by enhanced chem ilum inescence acco rding to the instructions of the manufacturer(Amersham Pharmacia Biotec,Buckingham shire,U K).
2.5 RT-PCR Analysis
Total RNA from Hep3B cells was obtained using RNA Mini kit(Qiagen,Valencia,CA,USA).To tal RNA(2μg)was used to perfo rm reverse transcrip tion-PCR(RT-PCR)using RTPCR kit (Invitrogen,Carlsbad,Califo rnia,USA)according to themanufacturer’s p rotocol.The PCR p rimers for VEGF were 5′-GCTCTACCTCCACCA TGCCAA-3′(sense)and 5′-TGGAAGA TGTCCACCAGGGTC-3′ (antisense);for GAPDH were 5′-ACCACAGTCCA TGCCA TCAC-3′(sense)and 5′-TCCACCACC CTGTTGCTGTA-3′(antisense).The oligonucleotide sequences of the reaction p roductswere confirmed by sequencing.
2.6 Electrophoretic Mobility Shift Assay(EMSA)
The nuclear lysates was used for EMSA analysis. Briefly,the H IF-1αoligonucleotide(3.5pmol;Promega) was labeled with 10μCi of[γ-32P]A TP,in the p resence of 5 U of T4 polynucleotide kinase(Gibco-BRL)by incubation fo r 30min at 37 ℃in 10μL of kinase buffer.The reaction was term inated by the addition of 50 mMEDTA and centrifugation through a Sephadex G-25 co lum n(Amersham)to remove uninco rporated32P.For the binding reaction,nuclear p rotein extract(10μg)was incubated for 30 min at room temperature in a final volume of 10μL containing 0.03pmol of the32P-labeled H IF-1αoligonucleotide,40 mMHepes-KOH(p H 7.8),10%glycerol,1mMMgCl2,0.1mMD TT,and 1μg of poly(d I-dC).The reaction was stopped by the addition of gel loading dye,and the samp leswere subjected to electrophoresis on a nondenaturing 4%polyacrylamide gel in 0.5×Trisborate-EDTA buffer followed by autoradiography.
3 Resultsand Discussion
3.1 Identification of Celastrol as a HIF-1αInhibitor from a Cell-based Screen ing Assay
To identify a natural compound targeting the H IF-1α we generated a human hepatocellular carcinoma cell line Hep3B-HRE-Luc, which ex-p resses a hypoxia-responsive luciferase reporter gene.We used these cells to screen of 1 000 natural p roducts from the p lant extract bank of Korea Research Institute of Bioscience and Biotechnology.The screening of this library in Hep3BHRE-Luc cells led to the discovery of a quinone methide triterpenoid,celastrol,from a traditional Chinese medical p lantCelastrusorbiculatus.Follo wing 12 h treatment under CoCl2,celastrol disp layed a potent inhibito ry activity against hypoxia-induced luciferase reporter activity(Fig.1A).On the other hand,MTT assay indicated that celastrol did not sho wa significant cytotoxicity on the Hep3B cells up to 10μM(Fig.1B).
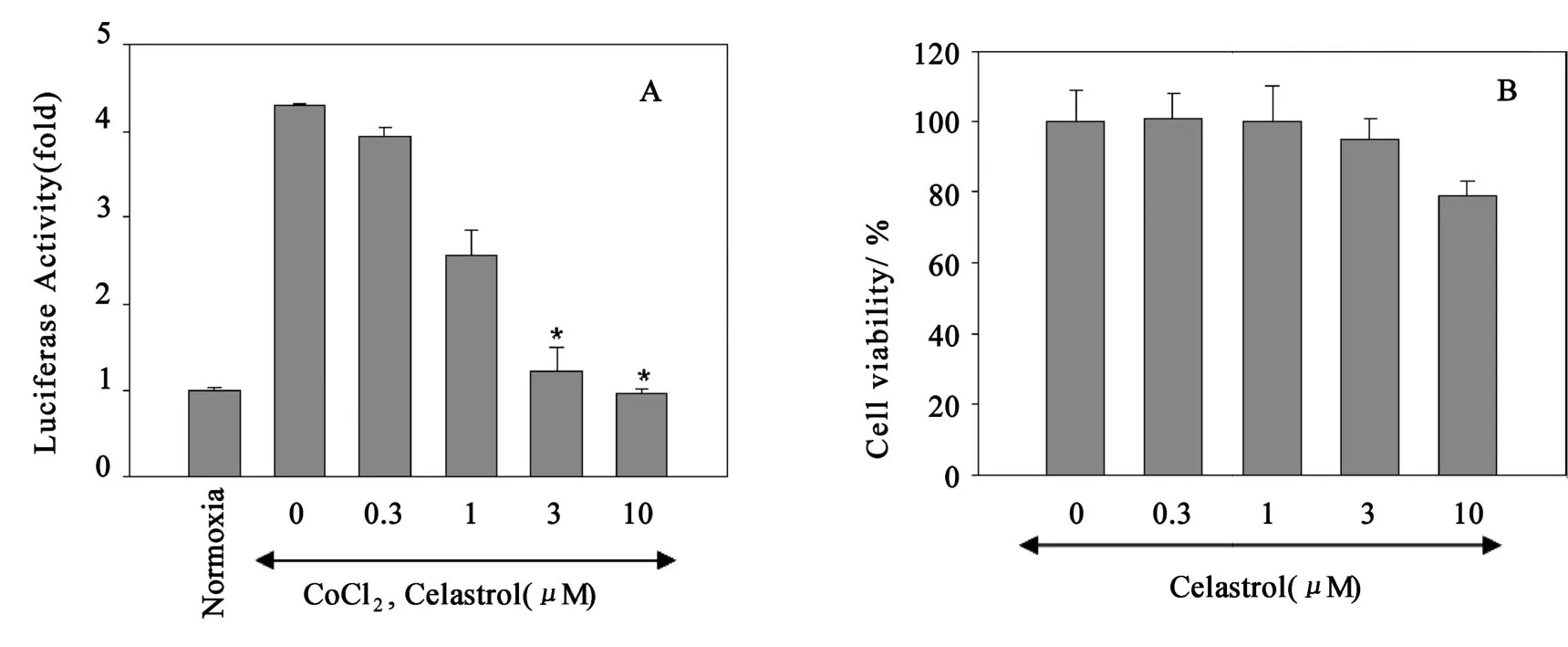
Fig.1 Effect of celastrol on HRE-mediated reporter gene expression under CoCl2 stimulation
3.2 Celastrol Decreases HIF-1αProtein Levels in a Dose-dependent Manner
To exp lore the underlying mechanism of celastrol activity,we investigated its effect on H IF-1α p ro tein levels.In Hep3B cells,H IF-1α p rotein is undetectable under normoxia, whereas it is stabilized in the p resence of CoCl2and becomes readily detectable by Western blotting.Follo wing 12 h treatment,celastrol exerted dose-dependent inhibition of H IF-1αp rotein levels induced by CoCl2in Hep3B cells, with comp lete abrogation at 10μM(Fig.2A),and had almost no effect on the levels of Topo-Ip roteins.
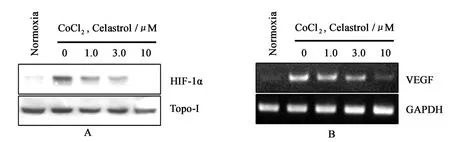
Fig.2 Effect of celastrol on the expression of HIF-1α and HIF-1α target gene
3.3 Celastrol Decreases Expression of HIF-1α Target Gene
To study whether the inhibition of H IF-1α would result in the decreased exp ression of its target genes,the exp ression of one kno wn H IF-1αtarget gene,VEGF,were analyzed.V EGF m RNA levels were measured by RT-PCR analysis in Hep3B cells.Treatment of the cells with celastrol resulted in a dose-dependent inhibition of VEGF m RNA exp ression(Fig.2B).The concentrations to inhibit the exp ression of H IF-1αtarget geneswere comparable with those of H IF-1αp rotein accumulation(Fig.2A and B).
3.4 Celastrol Inhibits DNA-binding Activity of HIF-1α
Since nuclear translocation of H IF-1αsubunit is a necessary step for H IF-1αactivation,we examined the effect of celastrolon the CoCl2-induced DNA-binding activity of H IF-1α.Hep3B cells were p retreated with celastrol fo r 8 h,and subsequently stimulated with CoCl2for 12 h.Nuclear extracts were analyzed fo r the DNA-binding activity of H IF-1α with EMSA analysis.Celastrol significantly inhibited DNA-binding activity of H IF-1α(Fig.3).

Fig.3 Effect of celastrol on DNA-binding activity of HIF-1α
[1]Wang G L,Jiang B H,Rue E A,et al.Hypoxiainducible Factor 1 is a Basic-helix-loop-helix-PAS Heterodimer Regulated by Cellular O2Tension[J].Proc Natl Acad Sci USA,1995,92:5510-5514.
[2]Semenza G L.Targeting H IF-1 fo r Cancer Therapy[J].Nat Rev Cancer,2003,3:721-732.
[3]Bruick R K,Mc Knight SL.A Conserved Family of Prolyl-4-hydroxylases That Modify H IF[J].Science,2001,294:1337-1340.
[4]Epstein A C,Gleadle JM,McNeill L A,et al.Elegans EGL-9 and Mammalian Homologs Define a Family of Dioxygenases That Regulate H IF by Prolyl Hydroxylation[J].Cell,2001,107:43-54.
[5]Ivan M,Kondo K,Yang H,et al.H IFalpha Targeted fo r VHL-mediated Destruction by Proline Hydroxylation:imp lications fo r O2Sensing[J].Sci-
ence,2001,292:464-468.
[6]Jaakkola P,Mole D R,Tian YM,et al.Targeting of H IF-alpha to the Von Hippel-Lindau Ubiquitylation Complex by O2-regulated Prolyl Hydroxylation[J].Science,2001,292:468-472.
[7]Lando D,Peet D J,Go rman J J,et al.FIH-1 is an Asparaginyl Hydroxylase Enzyme That Regulates the Transcrip tional Activity of Hypoxia-inducible Factor[J].Genes Dev,2002,16:1466-1471.
[8]A rany Z,Huang L E,Eckner R,et al.An Essential Role for p300/CBP in the Cellular Response to Hypoxia[J].Proc Natl Acad Sci USA,1996,93:12969-12973.
[9]Ebert B L,Bunn H F.Regulation of Transcrip tion by Hypoxia Requires a Multip rotein Comp lex That Includes Hypoxia-inducible Facto r 1,an Adjacent Transcrip tion Factor,and p300/CREB Binding Protein[J].Mol Cell Biol,1998,18:4089-4096.
[10]Bardos J I,Ashcroft M. Hypoxia-inducible Facto r-1 and Oncogenic Signalling[J].Bioessays,2004,26:262-269.
[11]Birner P,Schindl M,Obermair A,et al.Overexp ression of Hypoxia-inducible Factor 1alpha is a Marker fo r an Unfavo rable Prognosis in Earlystage Invasive Cervical Cancer[J].Cancer Res,2000,60:4693-4696.
[12]Giaccia A,Siim B G,Johnson R S.H IF-1 as a Target for Drug Development[J].Nat Rev Drug Discov,2003,2:803-811.
[13]Maxwell P H,Dachs G U,Gleadle J M,et al.Hypoxia-inducible Facto r-1 Modulates Gene Exp ression in Solid Tumo rs and Influences both Angiogenesis and Tumo r Gro wth[J].Proc Natl Acad Sci U SA,1997,94:8104-8109.
[14]Belozerov V E,Van Meir E G.Hypoxia Inducible Facto r-1:a Novel Target for Cancer Therapy[J].Anticancer D rugs,2005,16:901-909.
[15]Li H,Jia Y F,Pan Y,et al.Effect of Tripterine on Collagen-induced A rthritis in Rats[J].Acta Pharmalologica Sinica,1997,18:270-273.
[16]Xu X,Wu Z,Xu C,et al.Observation on Serum Anti-double Stranded DNA Antibodies of Tripterine in Systemic Lupus Erythematosus of(NZBxW)F1 Mice[J].Ann Rheum Dis,2003,62:377-378.
[17]Liu R L,Liu Z L,Li Q,et al.The Experimental Study on the Inhibito ry Effect of Trip terine on Airway Inflammation in Asthmatic Mice[J].Chinese Journalof Tubevculosis and Respiratory Diseases,2004,27:165-168.
[18]Pinna G F,Fio rucci M,Reimund J M,et al.Celastrol Inhibits p ro-inflammato ry Cytokine Secretion in Crohn’s Disease Biopsies[J].Biochem Biophys Res Commun,2004,322:778-786.
1004-4353(2011)02-0171-05
雷公藤红素对人肝癌细胞 Hep3B中HIF-1α表达的影响
李晶埈
(韩国生命工学研究院分子癌研究中心,忠北梧仓363-883)
为探讨雷公藤红素对人体肝癌细胞 Hep3B中缺氧诱导因子(H IF-1a)活化的影响,利用氯化钴(CoCl2)模拟肝癌细胞缺氧模式,采用双荧光素酶报告基因法检测雷公藤红素对 H IF-1α的转录活性,蛋白免疫印迹实验检测雷公藤红素对 H IF-1α蛋白质的表达水平,RT-PCR检测雷公藤红素对 H IF-1α下游靶基因血管内皮生长因子(VEGF)m RNA的表达水平,电泳泳动度移动分析(EMSA)检测雷公藤红素对 H IF-1αDNA的结合能力.结果显示:雷公藤红素呈剂量依赖性抑制了 H IF-1α蛋白质的表达水平,降低了肝癌细胞中VEGF m RNA的表达水平并有效抑制了 H IF-1α蛋白的DNA结合能力.这表明,雷公藤红素可以抑制肝癌细胞中H IF-1α的活性,这可能是其抗肿瘤的机理之一.
雷公藤红素;缺氧诱导因子;抗癌
2011-04-05
李晶埈(1949—),男,教授,韩国翰林院院士,研究方向为分子生物学.
R285
A
